Abstract
Polymeric micelles represent an effective delivery system for poorly water-soluble anticancer drugs. With small size (10–100 nm) and hydrophilic shell of PEG, polymeric micelles exhibit prolonged circulation time in the blood and enhanced tumor accumulation. In this review, the importance of rational design was highlighted by summarizing the recent progress on the development of micellar formulations. Emphasis is placed on the new strategies to enhance the drug/carrier interaction for improved drug-loading capacity. In addition, the micelle-forming drug-polymer conjugates are also discussed which have both drug-loading function and antitumor activity.
KEY WORDS: cancer therapy, drug-interactive domain, dual-functional carrier, micelles, targeted delivery
INTRODUCTION
Chemotherapeutic agents are typically water-insoluble, and their therapeutic outcome is compromised by the short circulation time and systemic toxicity. During the past century, tremendous efforts have been made to circumvent these limitations and improve the therapeutic benefit of anticancer therapeutics. This was originated from the concept of “magic bullet” that was proposed by Paul Ehrlich, recipient of the Nobel Prize for Physiology or Medicine in 1908, which suggests the benefit of targeted delivery of drug to the diseased cells. In the past decades, nanocarriers have emerged as an attractive research field in cancer therapy, including liposomes, micelles, and nanoparticles made of various materials. Polymeric micelles are extensively studied carriers for the delivery of poorly water-soluble drugs. By enhancing the aqueous solubility and prolonging the blood half-life of chemotherapeutic agents, the anticancer agents can passively accumulate in the tumor site through the leaky vasculature via the enhanced permeability and retention (EPR) effect (1,2). Compared with other drug carriers, micelles have the advantages of very small size (10–100 nm), which is critical for passive targeting to solid tumors, particularly the poorly vascularized tumors (3).
Based on the type of intermolecular forces driving the micelle formation, block copolymer micelles can be divided into several categories including hydrophobically assembled amphiphilic micelles, polyion-complex micelles, and micelles stemming from metal complexation (4). The hydrophobically assembled micelles usually consist of amphiphilic macromolecules that have distinct hydrophobic and hydrophilic domains, and the commonly used block segments of copolymers have been summarized (5). Upon exposure to aqueous medium, the amphiphilic molecules are spontaneously self-assembled into supramolecular core/shell structures, and water-insoluble drugs can be loaded into the hydrophobic cores.
Micelles have demonstrated a variety of shapes such as spheres, rods, vesicles, tubules, and lamellae depending on the relative length of hydrophobic/hydrophilic blocks as well as solvent environment (6–8). Morphology of micelles has significant impact on the pharmacokinetic properties of micelles. For example, worm-like filomicelles have shown ten times longer circulation time compared with the spherical counterpart made of similar material (9).
The most commonly used hydrophilic segment of micelles for drug delivery is poly (ethylene glycol) (PEG), with a molecular weight of 2–15 kDa. PEG is highly water-soluble, non-toxic and neutrally charged. PEG forms a hydrophilic corona on the surface of micelles which minimizes the nonspecific interaction with blood components and prolongs the circulation time. Besides PEG, other polymers including poly(N-vinyl pyrrolidone) (PVP) (10) and poly(N-isopropyl acrylamide) (pNIPAM) (11) have also been used as hydrophilic portion of micelles.
Most polymeric systems involve the use of polymers as the hydrophobic domain including polyesters such as poly(lactic acid) (PLA) and polyamides such as poly (l-lysine) (PLL) and poly (beta-amino ester). Biocompatibility and biodegradability are two important prerequisites in designing these micellar carriers for clinical application. Polyesters and polyamides undergo enzyme-catalyzed hydrolysis in vivo and thus are considered biodegradable. There are several polymer micelle systems that have been studied in clinical phase (12). For example, Genexol-PM, a polymeric micelle formulation of paclitaxel, was evaluated in a phase I study in patients with advanced malignancies with emphasis on pharmacokinetics evaluation (13). Phase II clinical trial of Genexol-PM was performed in patients with metastatic breast cancer (14) and advanced non-small-cell lung cancer (15). Some other micellar formulations also have their phase I clinical trial completed, including paclitaxel-incorporated micellar formulation, NK105 (16), pluronic polymer-bound doxorubicin (SP1049C) (17) and NK911, a micelle-encapsulated doxorubicin (18).
Another type of polymeric micelles that have been investigated involves the use of lipids as the hydrophobic core. For example, Torchilin’s group has synthesized several PEG-diacyllipid conjugates, in which the hydrophobic segments are lipids of various acyl chains such as phosphatidylethanolamine (PEG-PE) (19). Due to the strong hydrophobic interactions between the double acyl chains (20), the PEG-PE conjugates can form stable micelles with very low CMC value (~10−5 M) (21). These micelles can solubilize many types of poorly water-soluble drugs including paclitaxel (2), tamoxifen (2), porphyrin (2), camptothecin (22), and vitamin K3 (23). The PEG-PE micelles exhibit favorable stability, longevity in blood and tumor accumulation via the EPR effect (20,24). In contrast to liposomes (25), the small size of micelles enables them to effectively penetrate the vasculature of tumors, even for those with very low cutoff size (26).
Recently, Lam and colleagues have developed a series of micellar systems composed of PEG-cholic acid (CA) conjugates (27). A conjugate of eight CA molecules with one PEG5000 chain (PEG5K-CA8) was shown to load paclitaxel with high loading capacity (7.3 mg paclitaxel/mL) and a size of 20–60 nm (27). These paclitaxel-loaded PEG5K-CA8 micelles achieved improved antitumor effect and showed less toxicity in murine models of ovarian cancer compared to Taxol® and Abraxane® at equivalent paclitaxel doses (27). Phase I clinical trial of paclitaxel-loaded PEG5K-CA8 micelles demonstrated the superior anticancer efficacy and tolerance (28). In addition, compared to PEG5K-CA8 micelles, a similar micellar carrier PEG2K-CA4 was demonstrated to have higher doxorubicin loading capacity and more sustained drug release profile (28).
The last decade has seen significant progress in the development of various micellar systems for targeted delivery of anticancer agents (5,29,30). However, much improvement is still needed for the existing systems with respect to drug-loading capacity and formulation stability. Despite the structural differences among the reported micellar systems, most of them mainly rely on hydrophobic/hydrophobic interaction for drug incorporation into the hydrophobic cores. Such mechanism, while working well for some highly lipophilic drugs, may not provide sufficient drug/carrier interaction to effectively load other drugs. Various strategies have been proposed to introduce additional drug-interactive domains into the micellar system to improve the overall carrier/drug interaction. In addition, progress has been made in developing dual-functional carriers that demonstrate both delivery function and antitumor activity. The following two sections will summarize some of the works from our lab and other labs on developing improved micellar systems for anticancer agents.
MICELLES WITH BUILT-IN DRUG-INTERACTIVE DOMAIN AS IMPROVED DELIVERY SYSTEMS FOR ANTICANCER AGENTS
The drug-loading capacity of polymeric micelles is critically dependent on the compatibility between the drug and the micelle core (31). A series of studies have demonstrated that the drug-loading capacity of micelles can be greatly enhanced by optimizing chemical structures of the inner core segment for stronger drug/carrier interaction. A typical example of the strategy for enhanced compatibility between drug and block copolymer is the development of hydrotropic polymers. Hydrotropy refers to a phenomenon that the aqueous solubility of a poorly soluble compound is significantly enhanced by the presence of large amounts of a second solute, named hydrotrope (32). The hydrotropes aggregate only above a certain concentration, which is known as the minimal hydrotrope concentration (MHC) (33). Various studies have been carried out to elucidate the process of hydrotropic solubilization. Although the exact mechanism is not fully clarified, it may involve multiple non-covalent interactions including hydrophobic interaction, hydrogen bonding (34) as well as parallel stacking effect (35,36).
Most of the hydrotropes include an aromatic ring substituted by heteroatoms. The aromatic rings are basically hydrophobic, which can stack with each other or with the benzene rings in drug molecules (37). In addition, the polar groups may interact with drugs via hydrogen bonding (38). For example, nicotinamide, a typical hydrotrope, has been shown to form complexes with various hydrophobic drugs and enhance their water solubility (39). It has a pyridine ring that promotes the π-π stacking with drug molecules through its planarity (40). In addition, self-association of nicotinamide was shown to play a major role in the hydrotropic solubilization of riboflavin instead of complexation between the two species (32). Another example is N,N-diethylnicotinamide, which was shown to be the most effective hydrotrope for solubilizing paclitaxel among more than 60 structures tested (41). One drawback of hydrotropic solubilization is that high concentration of hydrotrope is usually required, which may cause various side effects. This is due to the high MHC of usual hydrotropes of about 1 M, compared to the critical micelle concentration (CMC) of typical micelles at 10−2–10−3 M (42). To overcome this barrier, hydrotropic polymers have been developed which can dramatically enhance the local concentration of hydrotropes. Park’s group has synthesized PEG block copolymers with hydrotropes linked with the hydrophobic domain of polymeric micelles, which appeared to be a versatile strategy to enhance the water solubility of various hydrophobic drugs of different structures (43,44). The key factors that affect the performance of hydrotropic polymers include the polymer backbone, type and orientation of hydrotropic moieties and the spacer groups connecting the backbone and hydrotropes. The review by Ooya et al. provides a comprehensive summary of the studies regarding the structure-activity relationship of hydrotropic polymers (36).
Besides hydrotropic effect, additional non-covalent interactions have been employed to improve drug-loading in polymeric micelles. For example, modification of poly(ethylene glycol)-poly(beta-benzyl l-aspartate) (PEG-PBLA) block copolymer with benzyl ester on the PBLA chain enhanced the loading efficiency and stability of camptothecin-loaded micelles (45). Incorporation of hydrogen bonding urea-functional groups into block copolymers led to decreased CMC and improved stability of doxorubicin-loaded micelles (46). Similarly, the stability of micelles was enhanced with increased number of acid/urea groups in the micelles, which was due to improved hydrogen bonding between the carrier molecules and acid-amine ionic interaction between the drug and carrier (47). In addition, the loading of indomethacin and ibuprofen into polymeric micelles was dramatically improved by the acid-base interaction between hydrophobic segments of micelles and guest molecules containing carboxylic acid groups (48). Furthermore, Kataoka’s group has shown that the encapsulation of cisplatin into polymeric micelles was facilitated by metal-ligand coordination (49,50). Inclusion of aromatic end groups (e.g. benzoyl and naphthoyl) has also been shown to improve the loading of paclitaxel into mPEG750-b-oligo(epsilon-caprolactone)5-based oligomeric micelles (51).
We have recently demonstrated that the introduction of Fmoc as a “drug-interactive domain” can also significantly improve the drug-loading capacity of both emulsion and lipid-core micellar system (52). A series of PEGylated lipopeptide surfactants were designed and constructed to solubilize a synthetic antioxidant, JP4-039. Several ε-Boc lysine derivatives with various protective groups at α-NH2 position were tested for their ability to solubilize JP4-039, among which the α-Fmoc-ε-Boc lysine was shown to be the most potent one (52). Incorporation of this drug-interactive motif Fmoc into drug-loaded emulsion led to significant increase in the formulation stability. We then designed another polymer-based micelle system, in which the Fmoc motifs were located at the interfacial region of lipopeptide surfactants with PEG5K as the headgroup and two oleoyl chains as the core-forming segment (53) (Fig. 1). The PEG5K-(Fmoc-OA)2 exhibited lower CMC value and significantly improved loading capacity for paclitaxel compared with an analogue without Fmoc motifs. The paclitaxel-loaded PEG5K-(Fmoc-OA)2 micelles showed increased anticancer effect over Taxol in vitro and in vivo. In addition, seven other drugs were effectively loaded into PEG5K-(Fmoc-OA)2 micelles, which suggests the utility and versatility of this platform for a broad range of drugs with different structures (53). Although the exact mechanism of carrier/drug interaction is not fully understood, the hydrophobic interaction and π-π stacking effect possibly contribute to the compatibility between the drug and carrier (Fig. 1).
Fig. 1.
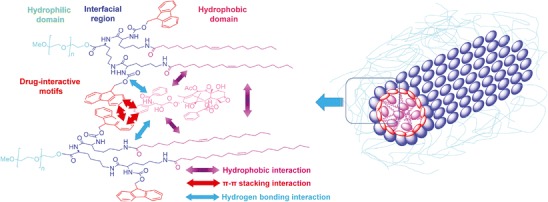
Structure of PEG5000-Lys-(α-Fmoc-ε-oleoyl lysine)2 and the postulated modes of carrier/drug and carrier/carrier interactions. Taken with permission from (53)
PEG-DRUG CONJUGATES AS DUAL-FUNCTION CARRIERS FOR CANCER TARGETED DELIVERY
Rationale of Combination Therapy Using Polymer-Drug Conjugates as Carriers
As discussed above, improved drug-loading capacity, stability and tumor-specific distribution can be achieved by various strategies. However, most of the polymeric materials for drug delivery are “inert” and lack of therapeutic activity. In addition, the use of large amounts of carrier materials may impose safety concerns (54). Since Ringsdorf (55) proposed the concept of “polymeric prodrug” in 1975, the utility of polymer-drug conjugates in clinical therapy has been well demonstrated. Interestingly, the conjugate of hydrophobic drug with hydrophilic polymers might be self-assembled into micelles, which can be potentially useful for the loading of another therapeutic molecule. Drug encapsulation with a biologically active carrier is an attractive strategy as it represents a unique form of combination. Combination therapy with multiple agents working at several signaling pathways at the same time could not only lead to maximized anticancer effect but also help to overcome the drug resistance (56). For example, the combination of PGA-paclitaxel conjugate with cisplatin (57) or carboplatin (58) has shown improved therapeutic benefits or reduced toxicity in Phase I clinical trial. In addition, the anticancer effect of N-(2-hydroxypropyl)methacrylamide (HPMA) copolymer-doxorubicin conjugate in combination with HPMA copolymer-mesochlorin e6 was shown to be more efficacious than either conjugate alone (59).
In contrast to the common combination regimen, drug-loading with bioactive carriers ensures the simultaneous arrival of multiple therapeutic agents at the same targeting site, and thus represents a promising strategy for better therapeutic outcome. These dual-function carriers not only deliver the drug to tumor site but also are biologically effective, which either enhances the therapeutic effect (60) or reduce the toxicity caused by the incorporated drug (61).
However, micellar systems based on drug-polymer conjugates are rarely used for the physical incorporation of another hydrophobic molecule. The following section summarizes some of the recent works from us and others, which demonstrate that such a strategy is not only feasible but also effective.
PEG-Vitamin E Conjugates as Dual-Function Carriers for Cancer-Targeted Delivery
d-α-tocopheryl polyethylene glycol (PEG) 1,000 succinate (TPGS) is a PEG-derivatized natural vitamin E which has been approved by FDA as a safe pharmaceutical adjuvant for drug formulation. In recent years, the application of TPGS in drug formulations has been extensively studied, such as emulsifier in poly (lactic-co-glycolic acid) (PLGA) nanoparticles (62), solubilizer and permeation enhancer (63), TPGS-based liposomes (64), copolymers (65), and nanocrystal (66). By inhibiting the function of P-glycoprotein (P-gp), TPGS also helps to overcome the multidrug resistance (67) and enhance the oral bioavailability of anticancer drugs (68). In addition, TPGS-doxorubicin conjugate was developed as a prodrug for enhanced therapeutic effect (69).
TPGS forms micelles in aqueous solution, which were used for the dispersion of functional nanostructures such as carbon nanotubes (70,71), fullerenes (71), and iron oxide (72). However, with a relatively high critical micelle concentration (CMC) value of 0.2 mg/mL (73), TPGS micelles are not stable and easily dissociated upon dilution by plasma after intravenous injection. Therefore, TPGS is usually used together with other excipients to form mixed micelles. For example, PEG-phosphatidylethanolamine (PEG-PE) was mixed with TPGS at a molar ratio of 2:1 for the loading of camptothecin (CPT), which increased the CPT solubility by at least 50% compared to PEG-PE micelles without TPGS (74). Similarly, mixed micelles of 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-1,000] (DSPE-PEG) with TPGS were prepared to encapsulate an anticancer agent, 17-allyamino-17-demethoxygeldanamycin (17-AAG) (75), resulting in controlled drug release and improved cytotoxicity compared with the free drug. In addition, TPGS also forms mixed micelles with Pluronic P105 (76), Pluronic P123 (77), and Pluronic F127/poly(butyl cyanoacrylate) (PBCA) (78). Compared with free drug or micelles without TPGS, those micelles showed improved solubility of hydrophobic anticancer drugs and increased cytotoxicity against MCF-7, MCF-7/ADR and HepG2 cell lines.
To further facilitate the use of TPGS as a micellar formulation, several strategies have been developed to decrease its CMC. Feng’s group has conjugated one tocopheryl succinate molecule with a PEG2K chain to generate TPGS2K for the delivery of docetaxel, which showed much lower CMC value compared with traditional TPGS (60). This improvement has enabled the formation of stable drug-loaded TPGS micelles without the help of other polymers or lipids. Another benefit of longer PEG chain is to further decrease the nonspecific uptake of TPGS2K micelles by RES. The study by Wang et al. showed that a conjugate of PEG2K with two vitamin E molecules exhibited further reduced CMC of 1.14 μg/mL (79), compared to that of TPGS2K (21.9 μg/mL) and TPGS (200 μg/mL) (60). Importantly, PEG2K-Vitamin E2 well retained the intrinsic activity of TPGS in inhibiting the activity of P-gp. Doxorubicin-loaded TPGS2K micelles showed greater cytotoxicity and tumor inhibitory effect than doxorubicin formulated in conventional TPGS (79). In light of this information, we have recently developed four PEG/vitamin E conjugates that differ in PEG molecular weight (PEG2K vs PEG5K) and the molar ratio of PEG/vitamin E (1/1 vs 1/2), and their paclitaxel loading capacity was subsequently compared (80). Our data have shown that among all the four conjugates, PEG5K conjugate with two vitamin E molecules (PEG5K-VE2) showed the lowest CMC value, with highest loading capacity and stability. All the four conjugates retained the P-gp inhibition activity of TPGS. Delivery of paclitaxel via PEG5K-VE2 led to significantly improved antitumor activity compared with the commercial formulation Taxol® and other paclitaxel micellar formulations (Fig. 2).
Fig. 2.
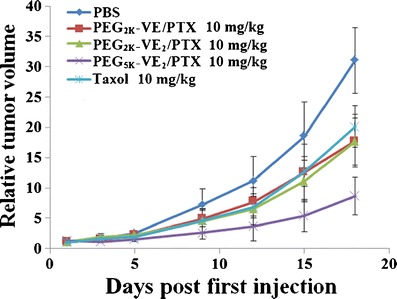
Enhanced antitumor activity of PTX formulated in PEG5K-VE2 micelles. BALB/c mice were inoculated s.c. with 4T1.2 cells (2 × 105 cells/mouse). Five days later, mice received various treatments on days 1, 3, 5, 9, 12, and tumor growth was monitored and plotted as relative tumor volume (mm3). P < 0.02 (PEG5K-VE2/PTX vs. Taxol, PEG2K-VE/PTX or PEG2K-VE2/PTX). N = 5. Taken with permission from (80)
PEG-Derivatized Embelin as a Nanocarrier for the Delivery of Paclitaxel
Embelin is an alkyl-substituted hydroxyl benzoquinone natural product discovered from the Japanese Ardisia Herb (Herba Ardisiae Japonicae) (81). Embelin was shown to possess a broad spectrum of biological activities including antidiabetic (82), anti-inflammatory (83), and hepato-protective effects (84). In addition, embelin exhibits antitumor activity in many types of cancers such as breast (85), colon (86), prostate (87), and pancreatic cancer (88). Through computational structure-based computer screening, Wang et al. (81) discovered that embelin is a potent inhibitor of X-linked inhibitor of apoptosis protein (XIAP), which partially explained its anticancer mechanism. XIAP is over-expressed in various types of cancer cells (89), particularly in drug-resistant cancer cells (90), while it plays a minimal role in normal cells. Inhibition of XIAP has been demonstrated as an effective approach to selectively inhibit the growth of cancer cells (91). Embelin also inhibits NF-κB activation, which mediates the downregulation of several genes including surviving, XIAP, IAP1/2, TRAF1, cFLIP, Bcl-2 and Bcl-xL (92).
Bearing a long lipophilic chain, embelin is extremely hydrophobic and water-insoluble. In an attempt to explore the PEG modification as an approach to increase its water solubility, we have found that PEG-derivatized embelin forms micelles in aqueous solution (93). This is not a surprise considering the structural similarity between embelin and vitamin E (Fig. 3a–b). Interestingly, the antitumor activity of embelin was well retained after coupling with PEG chain (93). In addition, the PEG-embelin micelles are highly efficient in solubilizing various types of anticancer agent such as paclitaxel (93,94). Furthermore, PEG-embelin, at nanomolar range, showed synergistic effect with paclitaxel in several cancer cell lines tested (93). In vitro and in vivo studies have shown that the conjugate with two embelin molecules linked with one PEG chain is significantly more effective for loading paclitaxel than the conjugate with a 1:1 molar ratio of PEG and embelin. In addition, the PEG-embelin conjugates with longer PEG chain (PEG5K) were shown to be more advantageous compared with the counterparts with shorter PEG chain (PEG3.5K) (93,94). Near-infrared fluorescence (NIRF) imaging of PC-3 xenograft-bearing mice showed that PEG5K-EB2 micelles were selectively accumulated at tumor site with minimal distribution in major organs including liver and spleen (Fig. 4) (94). Delivery of paclitaxel via PEG5K-embelin2 micelles leads to superior antitumor activity compared to Taxol in murine models of breast and prostate cancers (94).
Fig. 3.
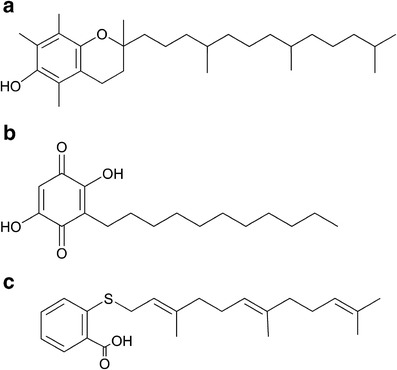
Chemical structures of vitamin E (a), embelin (b) and FTS (c)
Fig. 4.
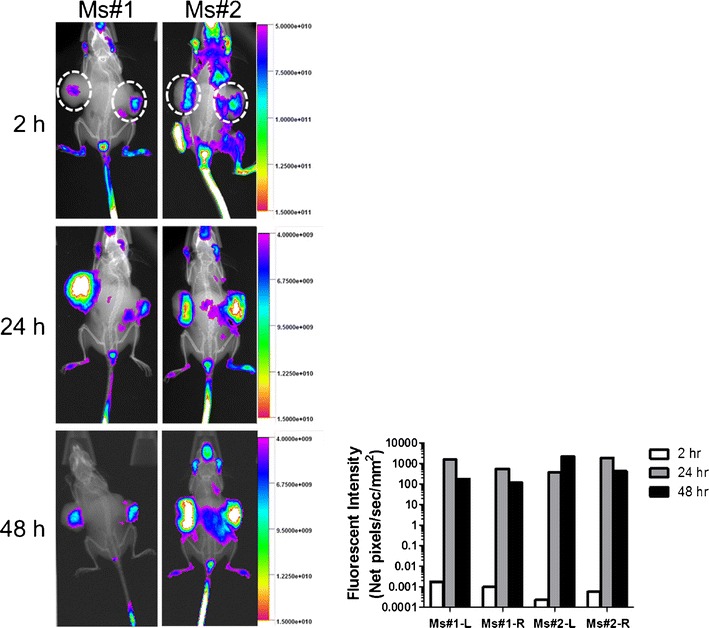
In vivo NIRF imaging over time as indicated in prostate cancer. PC-3 xenograft-bearing mice at 2, 24, 48 h following i.v. injection of PEG5K-EB2 micelles co-loaded with PTX and DiD. Taken with permission from (94)
PEG-Farnesylthiosalicylate (PEG-FTS) Conjugate Micelles for the Delivery of Paclitaxel
S-trans,trans-farnesylthiosalicylic acid (Salirasib, or FTS) is a synthetic antagonist of Ras protein. By disrupting the anchorage of Ras on cell membrane (95), FTS is designed to inhibit Ras-dependent growth of cancer cells. Approximately 20% to 30% of human tumors express permanently active oncogenic Ras (96), and the mutationally activated Ras was most commonly found in adenocarcinomas of the pancreas (90%), colon (50%), lung (30%), thyroid tumors (50%), and myeloid leukemia (30%) (97,98). Accordingly, FTS exhibited potent antitumor effect in various tumors such as pancreatic cancer (95), colon cancer (99), melanoma (100), and neurofibromatosis (101). In addition, Ras inhibitors demonstrated synergistic effect with other chemotherapeutics, which validated their application in combination therapy (102). For example, treatment of the resistant SW480 cells with FTS dramatically enhanced the sensitivity to gemcitabine and led to improved inhibition of tumor growth in vivo (103).
Similar to vitamin E and embelin, FTS is also a hydrophobic small molecule with a long hydrophobic chain and a functional group (–COOH) that can be readily used for further modification (Fig. 3c). The biological activity and chemical structure of FTS has prompted us to design the PEG-derivatized FTS conjugate as another dual-function micellar drug carrier (104). A labile ester linkage was used to facilitate the release of FTS and disassembly of the drug-loaded micelles following intracellular delivery to tumor cells. Our data have shown that the PEG-FTS2 readily forms micelles in aqueous solution with a CMC of 0.68 μM. Paclitaxel can be efficiently loaded into those micelles, which are spherical in morphology with a uniform size of 20–30 nm. Ras protein downregulation (Fig. 5) and cytotoxicity of PEG-FTS2 were comparable to free FTS as shown in 4T1.2 and HCT-116 cancer cell lines. The antitumor activity of paclitaxel-loaded PEG-FTS2 micelles was shown to be significantly higher than that of Taxol in a syngeneic murine breast cancer model (104).
Fig. 5.
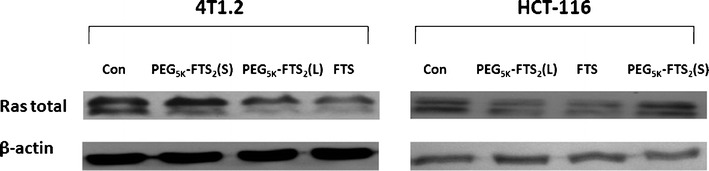
Effects of FTS, PEG5K-FTS2 (L), and PEG5K-FTS2 (S) on total Ras expression by western blot analysis. Cells grown in medium containing 5% FBS were treated with 0.1% DMSO (control), PEG5K-FTS2 with liable linkage (L), PEG5K-FTS2 with stable linkage (S), and free FTS (at a FTS concentration of 10 μM), respectively, for 48 h. Total cell lysate was subjected to western blot analysis. Anti-Ras antibody was used to determine the total Ras levels in the cells. Taken with permission from (104)
Other Drug-Polymer Conjugate Micelles for the Delivery of a Hydrophobic Chemotherapeutics
Several other micellar systems have been studied, which are based on polymer-drug conjugates such as polymer-curcumin and polymer-adriamycin conjugates. Curcumin is a natural polyphenol compound with promising anticancer application (105,106). It was reported that curcumin blocks NF-κB pathway (107) and in turn, induces apoptosis and inhibits the function of protein kinase C, epidermal growth factor receptor tyrosine kinase, and HER-2 (108,109). Curcumin has shown antitumor activity against various types of cancers including those of breast (110), colon (111), prostate (112,113), kidney (114), liver (115), lymphoid and myeloid tissues (116), and melanoma (117). In spite of all the anticancer activities, the potential application of curcumin is hindered by its poor water solubility and limited bioavailability. Furthermore, high drug dose is required for a desired therapeutic outcome due to its relatively low potency. Therefore, nano-sized delivery systems with high loading capacity and tumor-specific distribution represent an attractive strategy to address these issues.
In an attempt to increase the solubility of curcumin, various formulations have been developed such as cyclodextrin (118), nanoparticles (119), microparticles (120) and nano-sized complex (121). Polymer-curcumin conjugates were also synthesized as polymeric prodrugs. For example, Safavy et al. synthesized two conjugates including curcumin-PEG750 and curcumin-PEG3.5K (122). Both conjugates exhibited enhanced water solubility and cytotoxicity against several human cancer cell lines in comparison with free curcumin (122). To further increase the drug content in the nanoparticles, Tang et al. synthesized a curcumin prodrug by attaching it with two short oligo (ethylene glycol) (Curc-OEG) chains. Beta-thioester bond was applied which can be selectively cleaved intracellularly by glutathione and esterase to release the drug (123). With a curcumin loading content of 25.3 wt.%, the Curc-OEG conjugate formed stable nanoparticles in aqueous solution and exhibited dramatic anticancer effect in vitro and in vivo without causing significant toxicity (123). Curcumin-polymer conjugates were also synthesized with hyaluronic acid (124) and polyvinylpyrrolidone (125). Most recently, Yang et al. (126) demonstrated that the covalent curcumin-polymer conjugates can be further used to physically encapsulate additional curcumin. Curcumin was loaded into the polymeric micelles, which were synthesized by attaching multiple curcumin molecules to the hydrophobic poly (lactic acid) (PLA) backbone of PEG-PLA copolymer. Such micelles exhibited ten times lower CMC value and dramatically enhanced curcumin loading capacity (approximately fivefold) compared to traditional mPEG-PLA micelles (126).
Yokoyama et al. has reported that adriamycin (ADR)-conjugated polyethylene glycol–poly(aspartic acid) block copolymers (PEG-P[Asp(ADR)]) form micelles in aqueous solution and exhibited dramatic antitumor activity in vivo (127–129). However, the ratio of chemically/physically entrapped adriamycin was not analyzed, and certain amounts of adriamycin derivatives were incorporated in the micelles as impurities, which may cause side effects (130). The micelle preparation method was then improved which enabled the determination of this ratio and reduced the amounts of impurities (130,131). In addition, Yang et al. (132) developed a dual-drug system in which doxorubicin was chemically linked to the PLA end of polyethylene glycol-b-poly lactic acid (PEG-b-PLA). This conjugate was mixed with RGD-PEG-b-PLA, PEG-b-PLA and an antivascular agent combretastatin A4 to prepare the micelles. This dual-drug system significantly enhanced cellular uptake of the drug by B16-F10 cells and human umbilical vein endothelial cells, and achieved significant antitumor effect with increased lifespan of tumor bearing mice.
CONCLUSION AND FUTURE DIRECTIONS
Polymeric micelles have been extensively studied over the last decade as versatile and efficient drug delivery systems for cancer therapy. The design of polymer structure is increasingly sophisticated to improve the drug-loading capacity, tumor-specific uptake as well as anticancer effect. Various strategies have been developed to increase the drug/carrier interaction of polymeric micelles to maximize the drug-loading capacity, such as hydrotropic polymers and Fmoc-conjugated surfactants. In addition, several biologically active carriers have been developed as dual-functional carriers to improve the anticancer effect. More systematic studies on the structure-activity relationship of polymeric micellar systems are needed to better understand the mechanism of drug/carrier interaction and the effect of polymer structure on the drug-loading capacity. In addition, computational modeling may offer help in the tailored design of a polymeric carrier for each drug. These studies shall lead to the development of further improved micellar systems to advance the treatment of cancers.
Acknowledgments
This work was supported in part by NIH grants, RO1CA174305, R21CA173887, and R01GM102989.
References
- 1.Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–88. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- 2.Gao Z, Lukyanov AN, Singhal A, Torchilin VP. Diacyllipid-polymer micelles as nanocarriers for poorly soluble anticancer drugs. Nano Lett. 2002;2(9):979–82. doi: 10.1021/nl025604a. [DOI] [Google Scholar]
- 3.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 4.Gaucher G, Dufresne MH, Sant VP, Kang N, Maysinger D, Leroux JC. Block copolymer micelles: preparation, characterization and application in drug delivery. J Control Release. 2005;109(1–3):169–88. doi: 10.1016/j.jconrel.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 5.Sutton D, Nasongkla N, Blanco E, Gao J. Functionalized micellar systems for cancer targeted drug delivery. Pharm Res. 2007;24(6):1029–46. doi: 10.1007/s11095-006-9223-y. [DOI] [PubMed] [Google Scholar]
- 6.Choucair A, Eisenberg A. Control of amphiphilic block copolymer morphologies using solution conditions. Eur Phys J E Soft Matter. 2003;10(1):37–44. doi: 10.1140/epje/e2003-00002-5. [DOI] [PubMed] [Google Scholar]
- 7.Yu Y, Zhang L, Eisenberg A. Morphogenic effect of solvent on crew-cut aggregates of amphiphilic diblock copolymers. Macromolecules. 1998;31(4):1144–54. doi: 10.1021/ma971254g. [DOI] [Google Scholar]
- 8.Shen H, Zhang L, Eisenberg A. Multiple pH-induced morphological changes in aggregates of polystyrene-block-poly(4-vinylpyridine) in DMF/H2O mixtures. J Am Chem Soc. 1999;121(12):2728–40. doi: 10.1021/ja983712m. [DOI] [Google Scholar]
- 9.Geng Y, Dalhaimer P, Cai S, Tsai R, Tewari M, Minko T, et al. Shape effects of filaments versus spherical particles in flow and drug delivery. Nat Nanotechnol. 2007;2(4):249–55. doi: 10.1038/nnano.2007.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benahmed A, Ranger M, Leroux JC. Novel polymeric micelles based on the amphiphilic diblock copolymer poly(N-vinyl-2-pyrrolidone)-block-poly(D, L-lactide) Pharm Res. 2001;18(3):323–8. doi: 10.1023/A:1011054930439. [DOI] [PubMed] [Google Scholar]
- 11.Chung JE, Yokoyama M, Aoyagi T, Sakurai Y, Okano T. Effect of molecular architecture of hydrophobically modified poly(N-isopropylacrylamide) on the formation of thermoresponsive core-shell micellar drug carriers. J Control Release. 1998;53(1–3):119–30. doi: 10.1016/S0168-3659(97)00244-7. [DOI] [PubMed] [Google Scholar]
- 12.Kim S, Shi Y, Kim JY, Park K, Cheng JX. Overcoming the barriers in micellar drug delivery: loading efficiency, in vivo stability, and micelle-cell interaction. Expert Opin Drug Deliv. 2010;7(1):49–62. doi: 10.1517/17425240903380446. [DOI] [PubMed] [Google Scholar]
- 13.Kim TY, Kim DW, Chung JY, Shin SG, Kim SC, Heo DS, et al. Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies. Clin Cancer Res. 2004;10(11):3708–16. doi: 10.1158/1078-0432.CCR-03-0655. [DOI] [PubMed] [Google Scholar]
- 14.Lee KS, Chung HC, Im SA, Park YH, Kim CS, Kim SB, et al. Multicenter phase II trial of Genexol-PM, a Cremophor-free, polymeric micelle formulation of paclitaxel, in patients with metastatic breast cancer. Breast Cancer Res Treat. 2008;108(2):241–50. doi: 10.1007/s10549-007-9591-y. [DOI] [PubMed] [Google Scholar]
- 15.Kim DW, Kim SY, Kim HK, Kim SW, Shin SW, Kim JS, et al. Multicenter phase II trial of Genexol-PM, a novel Cremophor-free, polymeric micelle formulation of paclitaxel, with cisplatin in patients with advanced non-small-cell lung cancer. Ann Oncol. 2007;18(12):2009–14. doi: 10.1093/annonc/mdm374. [DOI] [PubMed] [Google Scholar]
- 16.Hamaguchi T, Kato K, Yasui H, Morizane C, Ikeda M, Ueno H, et al. A phase I and pharmacokinetic study of NK105, a paclitaxel-incorporating micellar nanoparticle formulation. Br J Cancer. 2007;97(2):170–6. doi: 10.1038/sj.bjc.6603855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Danson S, Ferry D, Alakhov V, Margison J, Kerr D, Jowle D, et al. Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP1049C) in patients with advanced cancer. Br J Cancer. 2004;90(11):2085–91. doi: 10.1038/sj.bjc.6601856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura Y, Hamaguchi T, Ura T, Muro K, Yamada Y, Shimada Y, et al. Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin. Br J Cancer. 2004;91(10):1775–81. doi: 10.1038/sj.bjc.6602204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Trubetskoy VS, Torchilin VP. Use of polyoxyethylene-lipid conjugates as long-circulating carriers for delivery of therapeutic and diagnostic agents. Adv Drug Deliv Rev. 1995;16(2–3):311–20. doi: 10.1016/0169-409X(95)00032-3. [DOI] [Google Scholar]
- 20.Lukyanov AN, Torchilin VP. Micelles from lipid derivatives of water-soluble polymers as delivery systems for poorly soluble drugs. Adv Drug Deliv Rev. 2004;56(9):1273–89. doi: 10.1016/j.addr.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 21.Musacchio T, Laquintana V, Latrofa A, Trapani G, Torchilin VP. PEG-PE micelles loaded with paclitaxel and surface-modified by a PBR-ligand: synergistic anticancer effect. Mol Pharm. 2009;6(2):468–79. doi: 10.1021/mp800158c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mu L, Chrastina A, Levchenko T, Torchilin VP. Micelles from poly(ethylene glycol) phosphatidyl ethanolamine conjugates (PEG-PE) as pharmaceutical nanocarriers for poorly soluble drug camptothecin. J Biomed Nanotechnol. 2005;1(2):190–5. doi: 10.1166/jbn.2005.030. [DOI] [Google Scholar]
- 23.Wang J, Mongayt DA, Lukyanov AN, Levchenko TS, Torchilin VP. Preparation and in vitro synergistic anticancer effect of vitamin K3 and 1,8-diazabicyclo[5,4,0]undec-7-ene in poly(ethylene glycol)-diacyllipid micelles. Int J Pharm. 2004;272(1–2):129–35. doi: 10.1016/j.ijpharm.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Lukyanov AN, Gao Z, Mazzola L, Torchilin VP. Polyethylene glycol-diacyllipid micelles demonstrate increased acculumation in subcutaneous tumors in mice. Pharm Res. 2002;19(10):1424–9. doi: 10.1023/A:1020488012264. [DOI] [PubMed] [Google Scholar]
- 25.Parr MJ, Masin D, Cullis PR, Bally MB. Accumulation of liposomal lipid and encapsulated doxorubicin in murine Lewis lung carcinoma: the lack of beneficial effects by coating liposomes with poly(ethylene glycol) J Pharmacol Exp Ther. 1997;280(3):1319–27. [PubMed] [Google Scholar]
- 26.Weissig V, Whiteman KR, Torchilin VP. Accumulation of protein-loaded long-circulating micelles and liposomes in subcutaneous Lewis lung carcinoma in mice. Pharm Res. 1998;15(10):1552–6. doi: 10.1023/A:1011951016118. [DOI] [PubMed] [Google Scholar]
- 27.Xiao K, Luo J, Fowler WL, Li Y, Lee JS, Xing L, et al. A self-assembling nanoparticle for paclitaxel delivery in ovarian cancer. Biomaterials. 2009;30(30):6006–16. doi: 10.1016/j.biomaterials.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xiao K, Luo J, Li Y, Lee JS, Fung G, Lam KS. PEG-oligocholic acid telodendrimer micelles for the targeted delivery of doxorubicin to B-cell lymphoma. J Control Release. 2011;155(2):272–81. doi: 10.1016/j.jconrel.2011.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torchilin VP. Micellar nanocarriers: pharmaceutical perspectives. Pharm Res. 2007;24(1):1–16. doi: 10.1007/s11095-006-9132-0. [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Lee H, Allen C. Formulation of drugs in block copolymer micelles: drug loading and release. Curr Pharm Des. 2006;12(36):4685–701. doi: 10.2174/138161206779026263. [DOI] [PubMed] [Google Scholar]
- 31.Latere Dwan’Isa JP, Rouxhet L, Preat V, Brewster ME, Arien A. Prediction of drug solubility in amphiphilic di-block copolymer micelles: the role of polymer-drug compatibility. Pharmazie. 2007;62(7):499–504. [PubMed] [Google Scholar]
- 32.Coffman R, Kildsig D. Hydrotropic solubilization—mechanistic studies. Pharm Res. 1996;13(10):1460–3. doi: 10.1023/A:1016011125302. [DOI] [PubMed] [Google Scholar]
- 33.Bauduin P, Renoncourt A, Kopf A, Touraud D, Kunz W. Unified concept of solubilization in water by hydrotropes and cosolvents. Langmuir. 2005;21(15):6769–75. doi: 10.1021/la050554l. [DOI] [PubMed] [Google Scholar]
- 34.Kim JY, Kim S, Papp M, Park K, Pinal R. Hydrotropic solubilization of poorly water-soluble drugs. J Pharm Sci. 2010;99(9):3953–65. doi: 10.1002/jps.22241. [DOI] [PubMed] [Google Scholar]
- 35.Cui Y. Parallel stacking of caffeine with riboflavin in aqueous solutions: the potential mechanism for hydrotropic solubilization of riboflavin. Int J Pharm. 2010;397(1–2):36–43. doi: 10.1016/j.ijpharm.2010.06.043. [DOI] [PubMed] [Google Scholar]
- 36.Ooya T, Lee S, Huh K, Park K. Hydrotropic nanocarriers for poorly soluble drugs. In: Mozafari MR, editor. Nanocarrier technologies. Netherlands: Springer; 2006. pp. 51–73. [Google Scholar]
- 37.Landauer J, McConnell H. A study of molecular complexes formed by aniline and aromatic nitrohydrocarbons. J Am Chem Soc. 1952;74(5):1221–4. doi: 10.1021/ja01125a025. [DOI] [Google Scholar]
- 38.Charman W, Lai C, Craik D, Finnin B, Reed B. Self-association of nicotinamide in aqueous-solution: N.M.R. studies of nicotinamide and the mono- and di-methyl-substituted amide analogs. Aust J Chem. 1993;46(3):377–85. doi: 10.1071/CH9930377. [DOI] [Google Scholar]
- 39.Rasool AA, Hussain AA, Dittert LW. Solubility enhancement of some water-insoluble drugs in the presence of nicotinamide and related compounds. J Pharm Sci. 1991;80(4):387–93. doi: 10.1002/jps.2600800422. [DOI] [PubMed] [Google Scholar]
- 40.Sanghvi R, Evans D, Yalkowsky SH. Stacking complexation by nicotinamide: a useful way of enhancing drug solubility. Int J Pharm. 2007;336(1):35–41. doi: 10.1016/j.ijpharm.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, Lee SC, Acharya G, Chang CJ, Park K. Hydrotropic solubilization of paclitaxel: analysis of chemical structures for hydrotropic property. Pharm Res. 2003;20(7):1022–30. doi: 10.1023/A:1024458206032. [DOI] [PubMed] [Google Scholar]
- 42.da Silva RC, Spitzer M, da Silva LHM, Watson L. Investigations on the mechanism of aqueous solubility increase caused by some hydrotropes. Thermochim Acta. 1999;328(1–2):161–7. doi: 10.1016/S0040-6031(98)00637-6. [DOI] [Google Scholar]
- 43.Huh KM, Lee SC, Cho YW, Lee J, Jeong JH, Park K. Hydrotropic polymer micelle system for delivery of paclitaxel. J Control Release. 2005;101(1–3):59–68. doi: 10.1016/j.jconrel.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 44.Kim JY, Kim S, Pinal R, Park K. Hydrotropic polymer micelles as versatile vehicles for delivery of poorly water-soluble drugs. J Control Release. 2011;152(1):13–20. doi: 10.1016/j.jconrel.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 45.Opanasopit P, Yokoyama M, Watanabe M, Kawano K, Maitani Y, Okano T. Block copolymer design for camptothecin incorporation into polymeric micelles for passive tumor targeting. Pharm Res. 2004;21(11):2001–8. doi: 10.1023/B:PHAM.0000048190.53439.eb. [DOI] [PubMed] [Google Scholar]
- 46.Kim SH, Tan JP, Nederberg F, Fukushima K, Colson J, Yang C, et al. Hydrogen bonding-enhanced micelle assemblies for drug delivery. Biomaterials. 2010;31(31):8063–71. doi: 10.1016/j.biomaterials.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 47.Yang C, Attia AB, Tan JP, Ke X, Gao S, Hedrick JL, et al. The role of non-covalent interactions in anticancer drug loading and kinetic stability of polymeric micelles. Biomaterials. 2012;33(10):2971–9. doi: 10.1016/j.biomaterials.2011.11.035. [DOI] [PubMed] [Google Scholar]
- 48.Giacomelli C, Schmidt V, Borsali R. Specific interactions improve the loading capacity of block copolymer micelles in aqueous media. Langmuir. 2007;23(13):6947–55. doi: 10.1021/la700337s. [DOI] [PubMed] [Google Scholar]
- 49.Yokoyama M, Okano T, Sakurai Y, Suwa S, Kataoka K. Introduction of cisplatin into polymeric micelle. J Control Release. 1996;39(2–3):351–6. doi: 10.1016/0168-3659(95)00165-4. [DOI] [Google Scholar]
- 50.Nishiyama N, Kato Y, Sugiyama Y, Kataoka K. Cisplatin-loaded polymer-metal complex micelle with time-modulated decaying property as a novel drug delivery system. Pharm Res. 2001;18(7):1035–41. doi: 10.1023/A:1010908916184. [DOI] [PubMed] [Google Scholar]
- 51.Carstens MG, de Jong PH, van Nostrum CF, Kemmink J, Verrijk R, de Leede LG, et al. The effect of core composition in biodegradable oligomeric micelles as taxane formulations. Eur J Pharm Biopharm. 2008;68(3):596–606. doi: 10.1016/j.ejpb.2007.08.014. [DOI] [PubMed] [Google Scholar]
- 52.Gao X, Huang Y, Makhov AM, Epperly M, Lu J, Grab S, et al. Nanoassembly of surfactants with interfacial drug-interactive motifs as tailor-designed drug carriers. Mol Pharm. 2013;10(1):187–98. doi: 10.1021/mp300319m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang P, Lu J, Huang Y, Zhao W, Zhang Y, Zhang X, et al. Design and evaluation of a PEGylated lipopeptide equipped with drug-Interactive motifs as an improved drug carrier. AAPS J. 2014;16(1):114–24. doi: 10.1208/s12248-013-9536-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Croy SR, Kwon GS. Polymeric micelles for drug delivery. Curr Pharm Des. 2006;12(36):4669–84. doi: 10.2174/138161206779026245. [DOI] [PubMed] [Google Scholar]
- 55.Ringsdorf H. Structure and properties of pharmacologically active polymers. J Polym Sci Pol Sym. 1975;51(1):135–53. doi: 10.1002/polc.5070510111. [DOI] [Google Scholar]
- 56.Broxterman HJ, Georgopapadakou NH. Anticancer therapeutics: “Addictive” targets, multi-targeted drugs, new drug combinations. Drug Resist Updat. 2005;8(4):183–97. doi: 10.1016/j.drup.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 57.Verschraegen CF, Skubitz K, Daud A, Kudelka AP, Rabinowitz I, Allievi C, et al. A phase I and pharmacokinetic study of paclitaxel poliglumex and cisplatin in patients with advanced solid tumors. Cancer Chemother Pharmacol. 2009;63(5):903–10. doi: 10.1007/s00280-008-0813-8. [DOI] [PubMed] [Google Scholar]
- 58.Langer CJ, O’Byrne KJ, Socinski MA, Mikhailov SM, Lesniewski-Kmak K, Smakal M, et al. Phase III trial comparing paclitaxel poliglumex (CT-2103, PPX) in combination with carboplatin versus standard paclitaxel and carboplatin in the treatment of PS 2 patients with chemotherapy-naive advanced non-small cell lung cancer. J Thorac Oncol. 2008;3(6):623–30. doi: 10.1097/JTO.0b013e3181753b4b. [DOI] [PubMed] [Google Scholar]
- 59.Shiah JG, Sun Y, Kopečková P, Peterson CM, Straight RC, Kopeček J. Combination chemotherapy and photodynamic therapy of targetable N-(2-hydroxypropyl)methacrylamide copolymer–doxorubicin/mesochlorin e6-OV-TL 16 antibody immunoconjugates. J Control Release. 2001;74(1–3):249–53. doi: 10.1016/S0168-3659(01)00325-X. [DOI] [PubMed] [Google Scholar]
- 60.Mi Y, Liu Y, Feng SS. Formulation of docetaxel by folic acid-conjugated D-alpha-tocopheryl polyethylene glycol succinate 2000 (Vitamin E TPGS(2K)) micelles for targeted and synergistic chemotherapy. Biomaterials. 2011;32(16):4058–66. doi: 10.1016/j.biomaterials.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 61.Dong Y, Feng SS. Poly(d, l-lactide-co-glycolide)/montmorillonite nanoparticles for oral delivery of anticancer drugs. Biomaterials. 2005;26(30):6068–76. doi: 10.1016/j.biomaterials.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 62.Mu L, Feng SS. Vitamin E TPGS used as emulsifier in the solvent evaporation/extraction technique for fabrication of polymeric nanospheres for controlled release of paclitaxel (Taxol) J Control Release. 2002;80(1–3):129–44. doi: 10.1016/S0168-3659(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 63.Yu L, Bridgers A, Polli J, Vickers A, Long S, Roy A, et al. Vitamin E-TPGS increases absorption flux of an HIV protease inhibitor by enhancing its solubility and permeability. Pharm Res. 1999;16(12):1812–7. doi: 10.1023/A:1018939006780. [DOI] [PubMed] [Google Scholar]
- 64.Muthu MS, Kulkarni SA, Xiong J, Feng SS. Vitamin E TPGS coated liposomes enhanced cellular uptake and cytotoxicity of docetaxel in brain cancer cells. Int J Pharm. 2011;421(2):332–40. doi: 10.1016/j.ijpharm.2011.09.045. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Feng SS. Nanoparticles of poly(lactide)/vitamin E TPGS copolymer for cancer chemotherapy: synthesis, formulation, characterization and in vitro drug release. Biomaterials. 2006;27(2):262–70. doi: 10.1016/j.biomaterials.2005.05.104. [DOI] [PubMed] [Google Scholar]
- 66.Liu Y, Huang L, Liu F. Paclitaxel nanocrystals for overcoming multidrug resistance in cancer. Mol Pharm. 2010;7(3):863–9. doi: 10.1021/mp100012s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dintaman JM, Silverman JA. Inhibition of P-glycoprotein by D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS) Pharm Res. 1999;16(10):1550–6. doi: 10.1023/A:1015000503629. [DOI] [PubMed] [Google Scholar]
- 68.Varma MV, Panchagnula R. Enhanced oral paclitaxel absorption with vitamin E-TPGS: effect on solubility and permeability in vitro, in situ and in vivo. Eur J Pharm Sci. 2005;25(4–5):445–53. doi: 10.1016/j.ejps.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 69.Cao N, Feng SS. Doxorubicin conjugated to D-alpha-tocopheryl polyethylene glycol 1000 succinate (TPGS): conjugation chemistry, characterization, in vitro and in vivo evaluation. Biomaterials. 2008;29(28):3856–65. doi: 10.1016/j.biomaterials.2008.05.016. [DOI] [PubMed] [Google Scholar]
- 70.Xu H, Abe H, Naito M, Fukumori Y, Ichikawa H, Endoh S, et al. Efficient dispersing and shortening of super-growth carbon nanotubes by ultrasonic treatment with ceramic balls and surfactants. Adv Powder. 2010;21(5):551–5. doi: 10.1016/j.apt.2010.02.011. [DOI] [Google Scholar]
- 71.Yan A, Von Dem Bussche A, Kane AB, Hurt RH. Tocopheryl polyethylene glycol succinate as a safe, antioxidant surfactant for processing carbon nanotubes and fullerenes. Carbon. 2007;45(13):2463–70. doi: 10.1016/j.carbon.2007.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chandrasekharan P, Maity D, Yong CX, Chuang KH, Ding J, Feng SS. Vitamin E (D-alpha-tocopheryl-co-poly(ethylene glycol) 1000 succinate) micelles-superparamagnetic iron oxide nanoparticles for enhanced thermotherapy and MRI. Biomaterials. 2011;32(24):5663–72. doi: 10.1016/j.biomaterials.2011.04.037. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z, Tan S, Feng SS. Vitamin E TPGS as a molecular biomaterial for drug delivery. Biomaterials. 2012;33(19):4889–906. doi: 10.1016/j.biomaterials.2012.03.046. [DOI] [PubMed] [Google Scholar]
- 74.Mu L, Elbayoumi TA, Torchilin VP. Mixed micelles made of poly(ethylene glycol)–phosphatidylethanolamine conjugate and d-α-tocopheryl polyethylene glycol 1000 succinate as pharmaceutical nanocarriers for camptothecin. Int J Pharm. 2005;306(1–2):142–9. doi: 10.1016/j.ijpharm.2005.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chandran T, Katragadda U, Teng Q, Tan C. Design and evaluation of micellar nanocarriers for 17-allyamino-17-demethoxygeldanamycin (17-AAG) Int J Pharm. 2010;392(1–2):170–7. doi: 10.1016/j.ijpharm.2010.03.056. [DOI] [PubMed] [Google Scholar]
- 76.Gao Y, Li LB, Zhai G. Preparation and characterization of Pluronic/TPGS mixed micelles for solubilization of camptothecin. Colloids Surf B: Biointerfaces. 2008;64(2):194–9. doi: 10.1016/j.colsurfb.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 77.Zhao L, Shi Y, Zou S, Sun M, Lil L, Zhail G. Formulation and in vitro evaluation of quercetin loaded polymeric micelles composed of pluronic P123 and D-a-tocopheryl polyethylene glycol succinate. J Biomed Nanotechnol. 2011;7(3):358–65. doi: 10.1166/jbn.2011.1298. [DOI] [PubMed] [Google Scholar]
- 78.Wang J, Wang R, Li LB. Preparation and properties of hydroxycamptothecin-loaded nanoparticles made of amphiphilic copolymer and normal polymer. J Colloid Interface Sci. 2009;336(2):808–13. doi: 10.1016/j.jcis.2009.04.080. [DOI] [PubMed] [Google Scholar]
- 79.Wang J, Sun J, Chen Q, Gao Y, Li L, Li H, et al. Star-shape copolymer of lysine-linked di-tocopherol polyethylene glycol 2000 succinate for doxorubicin delivery with reversal of multidrug resistance. Biomaterials. 2012;33(28):6877–88. doi: 10.1016/j.biomaterials.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 80.Lu J, Huang Y, Zhao W, Chen Y, Li J, Gao X, et al. Design and characterization of PEG-derivatized vitamin E as a nanomicellar formulation for delivery of paclitaxel. Mol Pharm. 2013;10(8):2880–90. doi: 10.1021/mp300729y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nikolovska-Coleska Z, Xu L, Hu Z, Tomita Y, Li P, Roller PP, et al. Discovery of embelin as a cell-permeable, small-molecular weight inhibitor of XIAP through structure-based computational screening of a traditional herbal medicine three-dimensional structure database. J Med Chem. 2004;47(10):2430–40. doi: 10.1021/jm030420+. [DOI] [PubMed] [Google Scholar]
- 82.Bhandari U, Jain N, Pillai KK. Further studies on antioxidant potential and protection of pancreatic beta-cells by Embelia ribes in experimental diabetes. Exp Diabetes Res. 2007;2007:15803. doi: 10.1155/2007/15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chitra M, Sukumar E, Suja V, Devi CS. Antitumor, anti-inflammatory and analgesic property of embelin, a plant product. Chemotherapy. 1994;40(2):109–13. doi: 10.1159/000239181. [DOI] [PubMed] [Google Scholar]
- 84.Singh D, Singh R, Singh P, Gupta RS. Effects of embelin on lipid peroxidation and free radical scavenging activity against liver damage in rats. Basic Clin Pharmacol Toxicol. 2009;105(4):243–8. doi: 10.1111/j.1742-7843.2009.00429.x. [DOI] [PubMed] [Google Scholar]
- 85.Li Y, Li D, Yuan S, Wang Z, Tang F, Nie R, et al. Embelin-induced MCF-7 breast cancer cell apoptosis and blockade of MCF-7 cells in the G2/M phase via the mitochondrial pathway. Oncol Lett. 2013;5(3):1005–9. doi: 10.3892/ol.2012.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dai Y, Qiao L, Chan KW, Yang M, Ye J, Ma J, et al. Peroxisome proliferator-activated receptor-gamma contributes to the inhibitory effects of Embelin on colon carcinogenesis. Cancer Res. 2009;69(11):4776–83. doi: 10.1158/0008-5472.CAN-08-4754. [DOI] [PubMed] [Google Scholar]
- 87.Danquah M, Li F, Duke CB, 3rd, Miller DD, Mahato RI. Micellar delivery of bicalutamide and embelin for treating prostate cancer. Pharm Res. 2009;26(9):2081–92. doi: 10.1007/s11095-009-9903-5. [DOI] [PubMed] [Google Scholar]
- 88.Mori T, Doi R, Kida A, Nagai K, Kami K, Ito D, et al. Effect of the XIAP inhibitor Embelin on TRAIL-induced apoptosis of pancreatic cancer cells. J Surg Res. 2007;142(2):281–6. doi: 10.1016/j.jss.2007.03.068. [DOI] [PubMed] [Google Scholar]
- 89.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6(5):1796–803. [PubMed] [Google Scholar]
- 90.Berezovskaya O, Schimmer AD, Glinskii AB, Pinilla C, Hoffman RM, Reed JC, et al. Increased expression of apoptosis inhibitor protein XIAP contributes to anoikis resistance of circulating human prostate cancer metastasis precursor cells. Cancer Res. 2005;65(6):2378–86. doi: 10.1158/0008-5472.CAN-04-2649. [DOI] [PubMed] [Google Scholar]
- 91.Vogler M, Walczak H, Stadel D, Haas TL, Genze F, Jovanovic M, et al. Small molecule XIAP inhibitors enhance TRAIL-induced apoptosis and antitumor activity in preclinical models of pancreatic carcinoma. Cancer Res. 2009;69(6):2425–34. doi: 10.1158/0008-5472.CAN-08-2436. [DOI] [PubMed] [Google Scholar]
- 92.Ahn KS, Sethi G, Aggarwal BB. Embelin, an inhibitor of X chromosome-linked inhibitor-of-apoptosis protein, blocks nuclear factor-kappaB (NF-kappaB) signaling pathway leading to suppression of NF-kappaB-regulated antiapoptotic and metastatic gene products. Mol Pharmacol. 2007;71(1):209–19. doi: 10.1124/mol.106.028787. [DOI] [PubMed] [Google Scholar]
- 93.Huang Y, Lu J, Gao X, Li J, Zhao W, Sun M, et al. PEG-derivatized embelin as a dual functional carrier for the delivery of paclitaxel. Bioconjug Chem. 2012;23(7):1443–51. doi: 10.1021/bc3000468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu J, Huang Y, Zhao W, Marquez RT, Meng X, Li J, et al. PEG-derivatized embelin as a nanomicellar carrier for delivery of paclitaxel to breast and prostate cancers. Biomaterials. 2013;34(5):1591–600. doi: 10.1016/j.biomaterials.2012.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Weisz B, Giehl K, Gana-Weisz M, Egozi Y, Ben-Baruch G, Marciano D, et al. A new functional Ras antagonist inhibits human pancreatic tumor growth in nude mice. Oncogene. 1999;18(16):2579–88. doi: 10.1038/sj.onc.1202602. [DOI] [PubMed] [Google Scholar]
- 96.Grewal T, Koese M, Tebar F, Enrich C. Differential regulation of RasGAPs in cancer. Genes Cancer. 2011;2(3):288–97. doi: 10.1177/1947601911407330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Bos JL. Ras oncogenes in human cancer: a review. Cancer Res. 1989;49(17):4682–9. [PubMed] [Google Scholar]
- 98.Downward J. Targeting RAS, signalling pathways in cancer therapy. Nat Rev Cancer. 2003;3(1):11–22. doi: 10.1038/nrc969. [DOI] [PubMed] [Google Scholar]
- 99.Halaschek-Wiener J, Wacheck V, Schlagbauer-Wadl H, Wolff K, Kloog Y, Jansen B. A novel Ras antagonist regulates both oncogenic Ras and the tumor suppressor p53 in colon cancer cells. Mol Med. 2000;6(8):693–704. [PMC free article] [PubMed] [Google Scholar]
- 100.Jansen B, Schlagbauer-Wadl H, Kahr H, Heere-Ress E, Mayer BX, Eichler H, et al. Novel Ras antagonist blocks human melanoma growth. Proc Natl Acad Sci U S A. 1999;96(24):14019–24. doi: 10.1073/pnas.96.24.14019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barkan B, Starinsky S, Friedman E, Stein R, Kloog Y. The Ras inhibitor farnesylthiosalicylic acid as a potential therapy for neurofibromatosis type 1. Clin Cancer Res. 2006;12(18):5533–42. doi: 10.1158/1078-0432.CCR-06-0792. [DOI] [PubMed] [Google Scholar]
- 102.Blum R, Kloog Y. Tailoring Ras-pathway–inhibitor combinations for cancer therapy. Drug Resist Updat. 2005;8(6):369–80. doi: 10.1016/j.drup.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 103.Gana-Weisz M, Halaschek-Wiener J, Jansen B, Elad G, Haklai R, Kloog Y. The Ras inhibitor S-trans, trans-farnesylthiosalicylic acid chemosensitizes human tumor cells without causing resistance. Clin Cancer Res. 2002;8(2):555–65. [PubMed] [Google Scholar]
- 104.Zhang X, Lu J, Huang Y, Zhao W, Chen Y, Li J, et al. PEG-farnesylthiosalicylate conjugate as a nanomicellar carrier for delivery of paclitaxel. Bioconjug Chem. 2013;24(3):464–72. doi: 10.1021/bc300608h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res. 2003;23(1A):363–98. [PubMed] [Google Scholar]
- 106.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shishodia S, Amin HM, Lai R, Aggarwal BB. Curcumin (diferuloylmethane) inhibits constitutive NF-kappaB activation, induces G1/S arrest, suppresses proliferation, and induces apoptosis in mantle cell lymphoma. Biochem Pharmacol. 2005;70(5):700–13. doi: 10.1016/j.bcp.2005.04.043. [DOI] [PubMed] [Google Scholar]
- 108.Aggarwal BB, Shishodia S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem Pharmacol. 2006;71(10):1397–421. doi: 10.1016/j.bcp.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 109.Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer. 2005;41(13):1955–68. doi: 10.1016/j.ejca.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 110.Holy JM. Curcumin disrupts mitotic spindle structure and induces micronucleation in MCF-7 breast cancer cells. Mutat Res. 2002;518(1):71–84. doi: 10.1016/S1383-5718(02)00076-1. [DOI] [PubMed] [Google Scholar]
- 111.Jiang MC, Yang-Yen HF, Yen JJ, Lin JK. Curcumin induces apoptosis in immortalized NIH 3T3 and malignant cancer cell lines. Nutr Cancer. 1996;26(1):111–20. doi: 10.1080/01635589609514468. [DOI] [PubMed] [Google Scholar]
- 112.Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, et al. Curcumin [1,7-Bis(4-hydroxy-3-methoxyphenyl)-1–6-heptadine-3,5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-κB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther. 2007;321(2):616–25. doi: 10.1124/jpet.106.117721. [DOI] [PubMed] [Google Scholar]
- 113.Holy J. Curcumin inhibits cell motility and alters microfilament organization and function in prostate cancer cells. Cell Motil Cytoskeleton. 2004;58(4):253–68. doi: 10.1002/cm.20012. [DOI] [PubMed] [Google Scholar]
- 114.Woo JH, Kim YH, Choi YJ, Kim DG, Lee KS, Bae JH, et al. Molecular mechanisms of curcumin-induced cytotoxicity: induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome C and inhibition of Akt. Carcinogenesis. 2003;24(7):1199–208. doi: 10.1093/carcin/bgg082. [DOI] [PubMed] [Google Scholar]
- 115.Yoysungnoen P, Wirachwong P, Bhattarakosol P, Niimi H, Patumraj S. Effects of curcumin on tumor angiogenesis and biomarkers, COX-2 and VEGF, in hepatocellular carcinoma cell-implanted nude mice. Clin Hemorheol Microcirc. 2006;34(1–2):109–15. [PubMed] [Google Scholar]
- 116.Anuchapreeda S, Limtrakul P, Thanarattanakorn P, Sittipreechacharn S, Chanarat P. Inhibitory effect of curcumin on WT1 gene expression in patient leukemic cells. Arch Pharm Res. 2006;29(1):80–7. doi: 10.1007/BF02977473. [DOI] [PubMed] [Google Scholar]
- 117.Odot J, Albert P, Carlier A, Tarpin M, Devy J, Madoulet C. In vitro and in vivo anti-tumoral effect of curcumin against melanoma cells. Int J Cancer. 2004;111(3):381–7. doi: 10.1002/ijc.20160. [DOI] [PubMed] [Google Scholar]
- 118.Yallapu MM, Jaggi M, Chauhan SC. beta-Cyclodextrin-curcumin self-assembly enhances curcumin delivery in prostate cancer cells. Colloids Surf B: Biointerfaces. 2010;79(1):113–25. doi: 10.1016/j.colsurfb.2010.03.039. [DOI] [PubMed] [Google Scholar]
- 119.Anand P, Nair HB, Sung B, Kunnumakkara AB, Yadav VR, Tekmal RR, et al. Design of curcumin-loaded PLGA nanoparticles formulation with enhanced cellular uptake, and increased bioactivity in vitro and superior bioavailability in vivo. Biochem Pharmacol. 2010;79(3):330–8. doi: 10.1016/j.bcp.2009.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 120.Shahani K, Panyam J. Highly loaded, sustained-release microparticles of curcumin for chemoprevention. J Pharm Sci. 2011;100(7):2599–609. doi: 10.1002/jps.22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang F, Koh GY, Jeansonne DP, Hollingsworth J, Russo PS, Vicente G, et al. A novel solubility-enhanced curcumin formulation showing stability and maintenance of anticancer activity. J Pharm Sci. 2011;100(7):2778–89. doi: 10.1002/jps.22512. [DOI] [PubMed] [Google Scholar]
- 122.Safavy A, Raisch KP, Mantena S, Sanford LL, Sham SW, Krishna NR, et al. Design and development of water-soluble curcumin conjugates as potential anticancer agents. J Med Chem. 2007;50(24):6284–8. doi: 10.1021/jm700988f. [DOI] [PubMed] [Google Scholar]
- 123.Tang H, Murphy CJ, Zhang B, Shen Y, Sui M, Van Kirk EA, et al. Amphiphilic curcumin conjugate-forming nanoparticles as anticancer prodrug and drug carriers: in vitro and in vivo effects. Nanomedicine (Lond) 2010;5(6):855–65. doi: 10.2217/nnm.10.67. [DOI] [PubMed] [Google Scholar]
- 124.Manju S, Sreenivasan K. Conjugation of curcumin onto hyaluronic acid enhances its aqueous solubility and stability. J Colloid Interface Sci. 2011;359(1):318–25. doi: 10.1016/j.jcis.2011.03.071. [DOI] [PubMed] [Google Scholar]
- 125.Manju S, Sreenivasan K. Synthesis and characterization of a cytotoxic cationic polyvinylpyrrolidone-curcumin conjugate. J Pharm Sci. 2011;100(2):504–11. doi: 10.1002/jps.22278. [DOI] [PubMed] [Google Scholar]
- 126.Yang R, Zhang S, Kong D, Gao X, Zhao Y, Wang Z. Biodegradable polymer-curcumin conjugate micelles enhance the loading and delivery of low-potency curcumin. Pharm Res. 2012;29(12):3512–25. doi: 10.1007/s11095-012-0848-8. [DOI] [PubMed] [Google Scholar]
- 127.Yokoyama M, Okano T, Sakurai Y, Ekimoto H, Shibazaki C, Kataoka K. Toxicity and antitumor activity against solid tumors of micelle-forming polymeric anticancer drug and its extremely long circulation in blood. Cancer Res. 1991;51(12):3229–36. [PubMed] [Google Scholar]
- 128.Yokoyama M, Kwon GS, Okano T, Sakurai Y, Ekimoto H, Okamoto K, et al. composition-dependent in vivo antitumor activity of adriamycin-conjugated polymeric micelle against murine colon adenocarcinoma 26. Drug Deliv. 1993;1(1):11–9. doi: 10.3109/10717549309031336. [DOI] [Google Scholar]
- 129.Yokoyama M, Kwon GS, Okano T, Sakurai Y, Seto T, Kataoka K. Preparation of micelle-forming polymer-drug conjugates. Bioconjug Chem. 1992;3(4):295–301. doi: 10.1021/bc00016a007. [DOI] [PubMed] [Google Scholar]
- 130.Yokoyama M, Okano T, Sakurai Y, Kataoka K. Improved synthesis of adriamycin-conjugated poly (ethylene oxide)-poly (aspartic acid) block copolymer and formation of unimodal micellar structure with controlled amount of physically entrapped adriamycin. J Control Release. 1994;32(3):269–77. doi: 10.1016/0168-3659(94)90237-2. [DOI] [Google Scholar]
- 131.Yokoyama M, Fukushima S, Uehara R, Okamoto K, Kataoka K, Sakurai Y, et al. Characterization of physical entrapment and chemical conjugation of adriamycin in polymeric micelles and their design for in vivo delivery to a solid tumor. J Control Release. 1998;50(1–3):79–92. doi: 10.1016/S0168-3659(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 132.Yang T, Wang Y, Li Z, Dai W, Yin J, Liang L, et al. Targeted delivery of a combination therapy consisting of combretastatin A4 and low-dose doxorubicin against tumor neovasculature. Nanomedicine. 2012;8(1):81–92. doi: 10.1016/j.nano.2011.05.003. [DOI] [PubMed] [Google Scholar]


