Abstract
Hydrogen sulfide (H2S) is having many potential pharmacological and physiological actions which reported that therapeutically useful concentration is low (100–160 μM) and a higher concentration could be toxic. Most of its donors produce it on coming into contact with water. All of these problems could be solved by a controlled-release delivery system which does not utilize water in any of its development steps. Therefore, 12 sustained release formulations were prepared by dissolving sodium hydrogen sulfide (NaHS)—a model H2S donor—in polymer solutions, prepared by dissolving polymers (consisted of either polylactide (PLA) or polylactide co-glycolide (PLGA), containing free carboxylic acid or capped allyl ester end group) in a mixture of benzyl benzoate (BB) and benzyl alcohol (BA). The formulation was injected in simulated tear fluid (STF) from which samples were withdrawn at specified times and assayed for NaHS content. We found decrease in burst and overall release with increase in polymer concentration from 10 to 20% w/v. The formulations containing free end group showed significant (p < 0.05) reduction of burst release (11% vs 21%). However, the overall release or the average amount released per hour was found to be significantly (p < 0.05) increased for formulations containing polymers with free end group than those with capped end group. A sustained level of H2S was found to be maintained for 72 h which should be further increased to a month to make it a viable H2S donor delivery system in addition to investigating toxicity profile specifically for the purpose of subconjunctival ocular delivery.
KEY WORDS: controlled release, hydrogen sulfide, hydrogen sulfide donor, in situ gel forming, phase sensitive, smart polymer
INTRODUCTION
The role of hydrogen sulfide (H2S) gas as a noxious, industrial, and environmental toxicant has been recognized for centuries (1). However, in the past decade, this gas has emerged as a significant signaling molecule that regulates several physiological and pathophysiological processes, including learning and memory, inflammation, reproduction, and the regulation of blood pressure (2–5). The circulating H2S levels in healthy humans and animals are estimated at 10–100 μM with up to 160 μM reported in brain tissue (6–8). The biosynthesis of H2S is accomplished by the activity of the cytosolic pyridoxol 5′-phosphate-dependent sulfuration pathway enzymes, cystathionine β-synthase (CBS), and cystathionine γ-lyase (CGL), on L-cysteine and homocysteine (9–11). Both CBS and CGL enzymes are expressed in several mammalian tissues such as brain, cardiovascular and respiratory systems, liver, kidney, and eye (12). Moreover, aberrant expression and/or activity of these enzymes in humans is implicated in the pathogenesis of several disorders, including sulfur-related metabolic disorders (1) and Alzheimer’s disease (13), affirming the significant physiological role for H2S in the body (14).
There are ample evidences which support the pharmacological actions of H2S in mammalian central nervous system (CNS) and peripheral tissues. It is reported to modulate hippocampal neurotransmission and memory in rat CNS (2,15) and exert a neuroprotective role on neurons (16,17). Human-cultured neuron cells are found to be protected from peroxynitrite-induced oxidative stress by H2S via KATP channel-dependent and free radical scavenging mechanisms (18,19). The ability of H2S to confer neuroprotection in neurons opens up a promising perspective for its possible role in the protection from neurodegenerative diseases such as Parkinson’s and Alzeimer’s disease (20,21).
In the cardiovascular system, H2S elicits cardioprotection in an irreversible ischemia/reperfusion injury animal model and exerts a vasorelaxant effect in various vascular tissues (22). Moreover, its beneficial effect in the cardiovascular system is comparable to that of nitric oxide (NO) without the deleterious production of reactive oxygen species (ROS) typical of NO (17). Contrary to NO, H2S acts as a scavenger of the ROS (23,24) suggesting that its cardiovascular-protective actions are superior to those exhibited by NO donors.
In mammalian ocular tissues, it modulates sympathetic neurotransmission and acts as a smooth muscle relaxant in the bovine and porcine iris–ciliary bodies (25). In the posterior segment of the eye, it regulates cyclic adenosine monophosphate (cAMP) production and prejunctional excitatory neurotransmission in porcine and bovine retina (26). Moreover, ACS67, an H2S-releasing molecule, demonstrated a significant ocular hypotensive activity in glaucomatous rabbits and an elevation of reduced glutathione levels (27) which suggest a potential therapeutic application for H2S donors in ocular hypertension and neuropathies.
Glaucoma is an ocular neuropathy that is characterized by elevated intraocular pressure (IOP), progressive degeneration of retinal neurons, and corresponding irreversible deficits in visual function. While current medication therapies target reduction in IOP, it is conceivable that therapeutic strategies that simultaneously exert ocular hypotensive and retinal neuroprotective action would present a superior therapeutic approach to management of glaucoma. Thus, the ability of H2S donors to lower IOP and exert a neuroprotective action to retinal neurons opens up the possibility for a new, multifaceted approach to glaucoma therapy.
Taken together, H2S donors offer potential therapeutic alternatives in multiple conditions. However, these potential therapeutic applications for H2S cannot be translated into clinical practice without an efficient method to deliver this gasotransmitter at a controlled rate sufficient to maintain a low sustained level of H2S. Part of the delivery challenges presented by H2S includes its gaseous nature, poor thermal and aqueous stability, short half-life, and potential toxicity when present in excess (28) through a compromised mitochondrial respiration (7). A review of the literature reveals that, so far, no studies have focused on the formulation and delivery aspects of H2S and its donors. In most cases, H2S is administered either by direct exposure to the animal through inhalation or via site-specific injections of donor solution (29,30).
The potential clinical use of both methods of application is limited because they require frequent multiple administrations to achieve endogenous therapeutic concentrations. Moreover, the necessity of higher initial dose associated with such delivery methods may increase the risk of adverse effects. A sustained-release delivery system of H2S donor would overcome these obstacles. Therefore, in the present study, we investigated the ability of a smart polymer-based delivery system for H2S donor that can instantaneously form a gel in situ upon subcutaneous or subconjunctival injection to release H2S at a controlled rate sufficient to maintain its therapeutically required sustained level.
MATERIALS AND METHODS
Chemicals
All the polymers (Resomer® R202 S, R202 H, RG502 H, and RG502 S named polymer 1, 2, 3, and 4, respectively) ranging in intrinsic viscosity (IV) from 0.19 to 0.32 dl/g were purchased from Boehringer Ingelheim (Petersburg, VA, USA) and used as obtained without any purification. Lysozyme (Cat. #L6876) and Micrococcus lysodeikticus ATCC #4698 (Cat. #M3770) were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). Benzyl benzoate (BB) and benzyl alcohol (BA) were purchased from Acros Organics, Fair Lawn, NJ, USA. All other chemical used were of analytical reagent quality.
Preparation of Standard Sodium Hydrogen Sulfide Solution
Simulated tear fluid (STF) has been used to mimic in vivo environment for the release of ocularly administered drugs (31). Standard NaHS solution was prepared by dissolving its specific amount (0.028 g) in STF (q.s. 1 L) maintained at a temperature of 4°C. The composition of STF solution was as follows: sodium chloride (6.80 g), sodium bicarbonate (2.2 g), calcium chloride · 2H2O (0.08 g), and potassium chloride (1.40 g) in ultrapure deionized water q.s. 1 L (32). The pH of the resulting solution was adjusted to 7.4 and its osmolarity was measured by using Osmometer (Micro-Osmette, Model 5004).
Preparation of Mixed Diamine Reagent
Two milliliters of N,N-diethyl-p-phenylenediamine sulfate and 3 g of ferric chloride (FeCl3.6H2O) were dissolved in 50 ml of 50% (v/v) hydrochloric acid maintained at 10°C or below (33). The resulting mixed diamine reagent was stored in a dark bottle in the refrigerator.
Determination of Sodium Hydrogen Sulfide
Mixed diamine reagent (0.06 ml) was added to 3 ml of standard NaHS solution maintained at 4°C, parafilmed, shaken vigorously, and set aside for 10 min. The intensity of the resultant colored solution was determined by measuring absorbance at 671 nm using PharmSpec UV-1700 UV-Visible Spectrophotometer (Shimadzu, USA).
A standard curve was obtained by plotting the absorbance obtained above vs. concentrations of NaHS solutions. The concentration of NaHS in the samples was determined by inputting the absorbance in regression equation representing the standard curve. The method was validated by determining the precision for inter- and intraday variability and its specificity.
Preparation of Polymer Solution
Specific amount of polymers 1–4 (Table I) were added to solvent mixtures of benzyl benzoate (BB) and benzyl alcohol (BA) in a glass vial and were placed in a shaking water bath (37°C, 35 rpm) for 24 h. This period of time was sufficient for complete dissolution of polymers which was confirmed by visual inspection of an appearance of a clear transparent solution. The injectability of the polymer solutions was determined by their easy flow through a 25-gauge needle.
Table I.
Determination of kinetics of release of sodium hydrogen sulfide
| Formulation | Release rate constants | ||
|---|---|---|---|
| Zero order (mg h−1) | First order (h−1) | Higuchi model (mg cm−2 h−1/2) | |
| Formulation 1 | 0.9237 ± 0.0109 | 0.8772 ± 0.0103 | 0.9773 ± 0.0171 |
| Formulation 2 | 0.9443 ± 0.0093 | 0.8677 ± 0.0087 | 0.9886 ± 0.0128 |
| Formulation 3 | 0.9572 ± 0.0084 | 0.9095 ± 0.0076 | 0.9835 ± 0.0073 |
| Formulation 4 | 0.8875 ± 0.0104 | 0.8971 ± 0.0054 | 0.9843 ± 0.0113 |
| Formulation 5 | 0.9378 ± 0.0078 | 0.8725 ± 0.0093 | 0.9741 ± 0.0159 |
| Formulation 6 | 0.9702 ± 0.0091 | 0.9364 ± 0.0137 | 0.9865 ± 0.0122 |
| Formulation 7 | 0.9539 ± 0.0088 | 0.8946 ± 0.0097 | 0.9915 ± 0.0101 |
| Formulation 8 | 0.9604 ± 0.0081 | 0.8769 ± 0.0082 | 0.9835 ± 0.0136 |
| Formulation 9 | 0.9742 ± 0.0117 | 0.8696 ± 0.0069 | 0.9744 ± 0.0164 |
| Formulation 10 | 0.8624 ± 0.0119 | 0.9543 ± 0.0114 | 0.9791 ± 0.0147 |
| Formulation 11 | 0.8833 ± 0.0073 | 0.9723 ± 0.0133 | 0.9834 ± 0.0097 |
| Formulation 12 | 0.8889 ± 0.0063 | 0.978 ± 0.0078 | 0.9747 ± 0.0135 |
Preparation of Sodium Hydrogen Sulfide Formulations
Twelve different formulations varying in polymer concentration, polymer composition, and end groups were prepared following the steps shown in Fig. 1. Concentration of NaHS was kept constant at 0.4% (w/v) in all these formulations. Sodium hydrogen sulfide was added to the polymer solutions and sonicated for 1 min at 10 W, resulting into a clear drug-polymer solution.
Fig. 1.
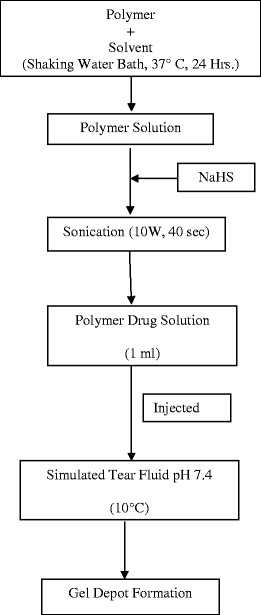
Steps involved in preparation of drug–polymer solution
Homogeneity of Sodium Hydrogen Sulfide in the Formulation
Three samples were collected from different regions of the formulation kept in a capped glass vial: one sample each from the top, middle, and bottom of the vial. These samples were analyzed for NaHS content by following the method described earlier for its determination. The homogeneity or lack of homogeneity was determined by comparison of the relative standard deviations of NaHS contents determined in the samples obtained from different regions of the formulation. The difference in standard deviations ≤5% was interpreted as an indicator of homogenous dispersion of NaHS in the formulation.
Determination of In Vitro Release Profile for Sodium Hydrogen Sulfide
One milliliter of polymer-NaHS solution was added to 15 ml of STF in a capped glass vials. The vials were placed in a refrigerator at a temperature of 4°C. One milliliter aliquot of the release media was withdrawn at specific time intervals and replaced with equal volume of fresh STF solution. The aliquot samples collected at specific time points were assayed for NaHS contents which were plotted against time to calculate the rate of release of NaHS from the formulation. The NaHS concentration in the released samples was corrected for sample withdrawal to account for ideality of sink condition (34).
Data Analysis
Statistical comparisons were made for significant differences among release profiles of NaHS from formulations 1–12 using analysis of variance (ANOVA). The level of significance used was p < 0.05.
Precision is a measure of the consistency and reproducibility of the method. A precise method gives us close values for repeated measurements of the same sample under similar conditions. Precision of the method for quantification of NaHS was determined by calculating relative standard deviation (RSD) values for both inter- and intraday variations using Eq. (1):
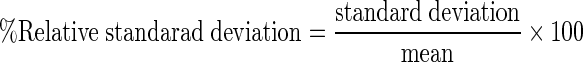 |
1 |
The accuracy of NaHS quantitation method was evaluated by comparing the estimated concentration with the known concentration using Eq. (2):
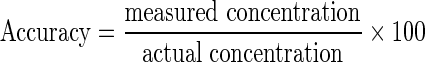 |
2 |
The concentration of NaHS in the releasing media was corrected for sampling withdrawal effects by using the following Eq. (3) (34):
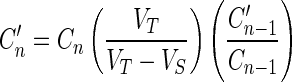 |
3 |
where, C′n is the corrected concentration of the nth sample, Cn is the measured concentration of NaHS in the nth sample, Cn−1 the measured concentration of NaHS in the (n−1)th sample, VT the total volume of the receiver fluid, and VS the volume of the sample drawn.
The release kinetics of NaHS was modeled by using the mathematical equations for zero-order, first-order, and Higuchi’s square root model which are shown below in Eqs. (4), (5), and (6), respectively:
-
Zero Order
where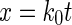
4 - x
- amount of NaHS released during a specific time period
- t
- time period of release
- k0
- zero-order rate constant
The plot of x vs. t would be a straight line with a slope equal to k0.
-
First Order
where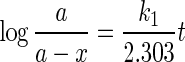
5 - a
- amount of NaHS incorporated in the formulation
- x
- amount of NaHS released during a specific time period
- t
- time period of release
- k1
- first-order rate constant
The plot of
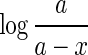 vs. t would be a straight line with a slope equal to
vs. t would be a straight line with a slope equal to 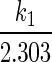 .
. -
Higuchi Square Root Model
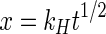
6 where- x
- amount of NaHS released during a specific time period
- t
- time period of release
- kH
- Higuchi constant
The plot of x vs. t1/2 would be a straight line with a slope equal to kH.
Rate constants were calculated using the above mathematical equations, and their correlation with specific time periods were calculated using linear regression analysis.
RESULTS AND DISCUSSION
Preparation of Sodium Hydrogen Sulfide Solution
All the solutions of NaHS were prepared in STF to mimic its release in ocular environment. The osmolality of STF was found out to be 297 ± 3 mOsmol/kg which is approximately equal to its theoretical value (300 mOsmol/kg) as well as standard value of human tear (299.3 ± 6.0 mOsmol/kg) (35). The initial pH of STF was found to be 7.8 which was adjusted to 7.4 using 0.1 N HCl. The use of STF can help correlate the in vitro release with potential in vivo release since the ion composition, tonicity, and pH values of the STF correspond to the values of actual human tear fluid (36). The long-term goal of this study is to develop a controlled release delivery system for H2S donors capable of maintaining its sustained level in anterior segment of eye on subconjunctival injection. Therefore, STF was used as releasing media for determining in vitro release profiles instead of phosphate-buffered saline solution which is the most-often-used releasing media.
Standard NaHS solution was prepared by adding its accurately weighed specified amount in STF stored at 4°C. Hydrogen sulfide is generated instantaneously on dissolution of NaHS in water following the reaction as shown below in Eq. (7):
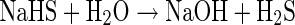 |
7 |
Similarly, other H2S donors also react with water to produce H2S (Fig. 2a) which would be lost to environment easily due to its gaseous nature. Therefore, it is critical to capture the H2S gas evolved in the releasing media itself for the accuracy of quantitative data generated as a result of assaying STF-based releasing media for H2S evolved from its donors. Hence, an important consideration in such studies is the temperature at which the solution of the H2S donor (in this case NaHS solution) as well as releasing media are maintained and stored to allow maximum solubility of H2S and minimize its escape into the environment.
Fig. 2.
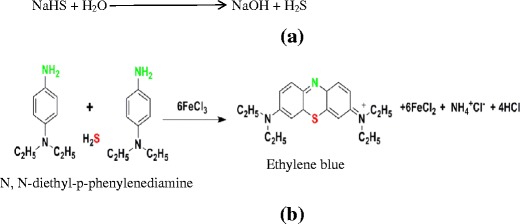
a Production of H2S from its model donor NaHS due to exposure to water. b Schematic representation of formation of ethylene blue by the reaction between hydrogen sulfide and the diamine reagent
Temperature has a significant effect on the solubility of H2S in water as the solubility at 4°C is almost twofold compared to the solubility at normal room temperature (176 mM at 4°C vs. 100 mM at 25°C) (37). Therefore, the standard solutions were maintained at 4°C to ensure the maximum solubility of hydrogen sulfide in water and thereby prevent its loss if any to the environment.
Quantification of Sodium Hydrogen Sulfide
The amount of NaHS in released samples were determined by a modified methylene blue method that is a well-established analytical method used for measuring low concentrations of sulfides in aqueous solutions (38). This is based on formation of a colored compound, methylene blue, due to the reaction of sulfide (S2−) with an acidified solution of N,N-dimethyl-p-phenylenediamine (DMPD) in the presence of the oxidizing agent ferric chloride (FeCl3). The amount of methylene blue produced, an indication of amount of sulfides present in the medium, is determined by measuring its absorbance at wavelength (671 nm).
A variation of this method is the ethylene blue method in which the reagent DMPD has been replaced by N,N-diethyl-p-phenylenediamine (DEPD) (Fig. 2b). The reaction involves a 2:1 stoichiometry of reagent to sulfide. This reaction is highly specific for sulfide at low concentration (39). The mixed diamine reagent was kept with NaHS and kept for 10 min, which was a sufficient time for the completion of the reaction resulting in formation of ethylene blue-colored complex.
The use of diethyl instead of methyl group in DEPD offers a variety of advantages such as (1) it is a less toxic substance compared to its parent compound, (2) ethylene blue has a high molar absorptivity (87,700 mol−1 L cm−1) than methylene blue (71,090 mol−1 L cm−1), and (3) aqueous solutions of methylene blue show a deviation from Beer’s law due to the formation of its dimers and trimers, while ethylene blue has a lower tendency to aggregate (40).
H2S is a strong reducing agent, and can spontaneously oxidize to sulfur dioxide or elemental sulfur as shown in Eqs. 8 and 9, respectively:
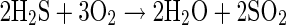 |
8 |
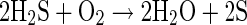 |
9 |
Thus, oxidation of sulfide can occur due to exposure to air when preparing and using standard solutions of sulfide during laboratory studies. The air oxidation of H2S in aqueous solution is a slow reaction, so at pH values below 6, where H2S is the dominant species, there is little oxidation of H2S. As the pH of the solution increases above pH 6, the concentration of the hydrogen sulfide ion, SH−, increases which readily undergoes oxidation in air (41). The immediate product of oxidation of HS− is sulfur, seen as a pale yellow or white precipitate in solutions of H2S. Therefore, in order to prevent the oxidation of hydrogen sulfide, acidified solution of diamine reagent was used that kept the pH of the reaction medium below pH 2 throughout the reaction.
At room temperature, a saturated solution of H2S in water has concentration of 0.11 mol L−1, while at 4°C the concentration is 0.19 mol L−1 (42). Thus, a special emphasis is also given to the temperature of the releasing medium in order to ensure solubilization of all of H2S produced. Therefore, the amount of NaHS in 1 mL polymer solution injected in 15 mL releasing media was kept equal to 0.711 × 10−3 mol, while the maximum solubilization capacity of the media was 2.82 × 10−3 mol (i.e., about four times). Moreover, the concentrations of standard solutions of NaHS were not more than 100 μmol/L.
Preparation of Polymer Solutions
The various polymers used in this study were Resomer R type 202 S, 202 H, RG type 502 H, and 502 S, which were named as polymers 1–4, respectively (Table II). These polymers were either PLA or PLGA and ended with either carboxylic acid or alkyl ester end group. These polymers were approved by FDA for their biocompatibility and biodegradability in a parenteral formulation. Moreover, the inherent viscosities of all the polymers used were in the ranges of 0.16–0.24 dL/g (43). The polymer solubility was investigated in a range of solvents consisting of varying amounts of BB and BA. A mixture of BB and BA provides an easy tool to manipulate and obtain a solvent system of optimal hydrophilicity or hydrophobicity suitable for solubilizing a particular polymer. Our objective was to find the solvent systems exhibiting maximum solubility yet injectable through 25-gauge needle.
Table II.
Characteristics of the Different Polymers Used for the Preparation of Formulations
| Polymers | Trade name | Composition | End groups | Hydrophilicity | Solvent systems (BB:BA) (% v/v) |
Solubility (% w/v) | Injectable concentration (% v/v) |
|---|---|---|---|---|---|---|---|
| Polymer 1 | Resomer R type 202 S | PLA | Ester | Low | 100:0 70:30 |
27 | 20 |
| Polymer 2 | Resomer R type 202 H | PLA | Acid | High | 70:30 | 20 | 20 |
| Polymer 3 | Resomer RG type 502 H | PLGA | Acid | High | 30:70 | 20 | 20 |
| Polymer 4 | Resomer R type 502 S | PLGA | Ester | Low | 100:0 70:30 |
27 | 20 |
BA benzyl alcohol, BB benzyl benzoate, PLA poly(dl-lactide), PLGA poly(dl-lactide-co-glycolide)
Polymers 1 and 4 were found to dissolve well in solvent system containing BB alone; however, polymers 2 and 3 were not found to be soluble which might be due to their relative hydrophilicity provided by the presence of acidic end groups (Fig. 3). Polymers 1 and 4 were relatively hydrophobic in nature due to the presence of ester end groups (Fig. 3) and therefore were soluble in BB. The maximum solubility of polymer 1 was 27% (w/v), but for polymer 4 it was 20% (w/v) in BB. This may be due to the polymer composition. Polymer 4 consisted of PLA and PGA (50:50), but polymer 1 entirely consisted of PLA only (Table II and Fig. 3). Consequently, polymer 4 could be expected to be less hydrophobic than polymer 1. Hence, it solubilizes to a lesser extent (20%) in comparison to polymer 1 (27%) in BB, a more hydrophobic solvent than BA (44).
Fig. 3.
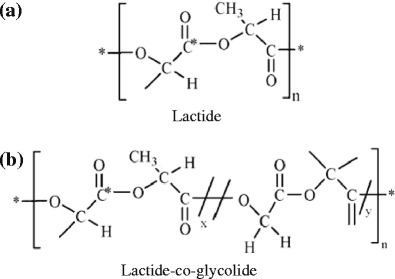
Structures of polymers used. a Structure of polymer 1 (*OCH2CH3), 2 (*OH), and b structure of polymer 3 (*OH), and 4 (*OCH2CH3) where * and n represent the end group and polymer chain length, respectively
Polymer 2 demonstrated greater solubility in the solvent system containing mixture of BB and BA (70:30), while polymer 3 favored the two solvents in a ratio of 30:70 which too can be explained on the basis of polymer composition. Polymer 3 consists of PGA in addition to the PLA but polymer 2 is composed of PLA only (Table II and Fig. 3). Since, GA is relatively hydrophilic than PLA; polymer 3 prefers a solvent system containing greater fraction of less hydrophobic solvent component BA to get dissolved.
Preparation of Delivery System
Twelve different formulations were prepared using polymers 1–4 (Table II) differing in composition and end groups (Table III). The solvent system used was a mixture of BB and BA in the ratio of 70:30 for all the polymers except polymer 3 which was dissolved in a solvent system consisting of BB:BA in the ratio of 30:70. Using a mixture of solvents instead of a single solvent provided an easy way of manipulating net hydrophobicity/hydrophilicity of a formulation for obtaining a desired optimum release profile (45). Concentration of NaHS was kept constant at 0.8% (w/v) in all these formulations which was selected to achieve an average release profile capable of maintaining a sustained level of H2S in the micromolar range for longer period of time. The micromolar concentrations of H2S have shown therapeutic potential in glaucoma or other ocular diseases (46). Intrinsic viscosity of all the polymers was equal to about 0.20 dl/g which is required for easy injectability (47). Polymers 3 and 4 consist of PLA and PGA in a 50:50 ratio, whereas, polymers 1 and 2 are composed of PLA only. Thus, formulations 1–12 enabled us to investigate the effect of formulation parameters such as polymer end groups, composition, and polymer concentration on the in vitro release profile of NaHS.
Table III.
The composition of sustained release formulations of NaHS
| Formulations | Polymers (end groups) | Polymer concentration (% w/v) | NaHS concentration (% w/v) |
|---|---|---|---|
| Formulation 1 | PLA (ester); polymer 1 | 10 | 0.4 |
| Formulation 2 | 15 | 0.4 | |
| Formulation 3 | 20 | 0.4 | |
| Formulation 4 | PLA (acid); polymer 2 | 10 | 0.4 |
| Formulation 5 | 15 | 0.4 | |
| Formulation 6 | 20 | 0.4 | |
| Formulation 7 | PLGA (acid); polymer 3 | 10 | 0.4 |
| Formulation 8 | 15 | 0.4 | |
| Formulation 9 | 20 | 0.4 | |
| Formulation 10 | PLGA (Ester); polymer 4 | 10 | 0.4 |
| Formulation 11 | 15 | 0.4 | |
| Formulation 12 | 20 | 0.4 |
Moreover, all the formulations studied were containing 0.8% w/v NaHS with 1 mL injected into the releasing media. The theoretically calculated maximum amounts of H2S produced from such formulations was equivalent to 0.143 mM H2S which were always less than its maximum aqueous solubility.
Homogeneity of NaHS in formulation
The relative standard deviation for experimental drug loads from the different regions of the gel formulation was found to be 6.7% (n = 3) indicating the homogeneity of the delivery system. The formulations were prepared by uniformly dispersing the drug in the polymer solutions. The drug itself might not be completely soluble in the solvent system that is used to solubilize the polymer. Hence, an important consideration in the characterization of the formulation is the determination of the homogeneity of the drug in the formulation which is crucial for release of NaHS at a controlled rate so that a low and safe therapeutic level of H2S can be sustained for an extended period of time. Homogeneity ensures the uniform dispersion of the drug throughout the polymer solution and helps account for the interferences due to sedimentation and particle size distribution.
In Vitro Release Studies
The percent cumulative release of NaHS was measured for 12 formulations at specific time points from 0 through 96 h (Figs. 4, 5, 6, and 7) and evaluated for burst release as well as rate of release because these parameters would play an important role in the build-up and sustainment of H2S level in vivo.
Fig. 4.
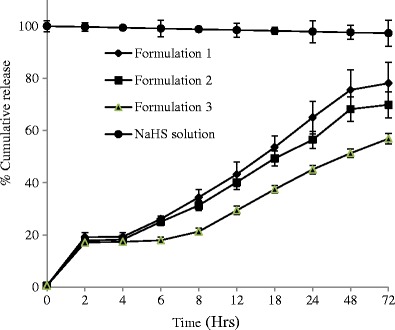
Effect of concentration on release of NaHS from formulations consisted of polymer type 1 (n = 3) and maintained at 4°C
Fig. 5.
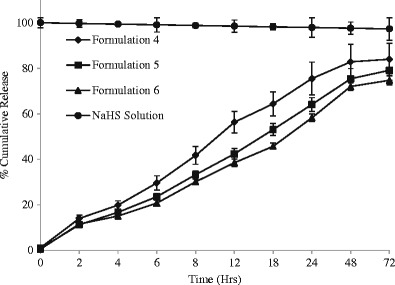
Effect of concentration on release of NaHS from formulations consisted of polymer type 2 (n = 3) and maintained at 4°C
Fig. 6.
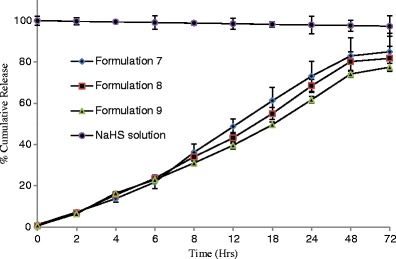
Effect of concentration on release of NaHS from formulations consisted of polymer type 3 (n = 3) and maintained at 4°C
Fig. 7.
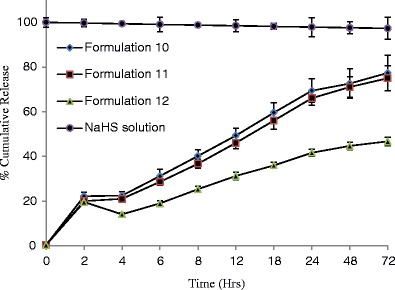
Effect of concentration on release of NaHS from formulations consisted of polymer type 4 (n = 3) and maintained at 4°C
The burst release is reported to be controlled by the rate of gelation. The higher the rate of gelation, the greater would be the burst release and the more hydrophobic (or less hydrophilic) a formulation is, the sooner it would form the gel depot in situ (48). The burst release from different formulations ranged from 6.68 to 27.09% (Table IV). Formulations 7–9 are relatively less hydrophobic than other formulations because it is consisted of PLGA-based polymer 3 ending in carboxylic acid; therefore, they showed lowest burst release in the range of 5.77–7.08% which decreases with increase of polymer concentrations (Table IV).
Table IV.
Burst release from various formulations
| Formulations | Burst release (%) |
|---|---|
| Formulation 1 | 27.09 ± 1.35 |
| Formulation 2 | 24.76 ± 1.28 |
| Formulation 3 | 25.95 ± 0.90 |
| Formulation 4 | 15.27 ± 0.99 |
| Formulation 5 | 14.29 ± 0.94 |
| Formulation 6 | 14.33 ± 0.93 |
| Formulation 7 | 7.08 ± 0.33 |
| Formulation 8 | 6.68 ± 0.58 |
| Formulation 9 | 5.72 ± 0.34 |
| Formulation 10 | 23.33 ± 1.11 |
| Formulation 11 | 22.95 ± 0.71 |
| Formulation 12 | 21.56 ± 1.17 |
The highest burst release was observed for formulations 1–3 consisted of PLA-based polymer 1 containing allyl ester as end group. This can be explained on the basis of the rate of gelation and hydrophobic nature of polymer (PLA only with ester end groups) and less hydrophilic nature of the solvent system comprising of 30% BA. The water-insoluble hydrophobic polymer dissolved in water-miscible solvent system (although BB and BA are organic solvents, their 1 mL mixture was miscible in 15 mL STF) undergoes fast phase separation quickly forming a gel. Due to the fast separation, large pores and water-accessible channels were formed on gel surface and in the gel core which explain the higher burst release of NaHS from formulations 1–3 than rest of the formulations studied (49).
The release pattern for all the formulations from 1 to 12 were analyzed to determine release kinetics by fitting the release data into zero-order, first-order, and Higuchi model as shown in Table I. Release profiles of NaHS showed best fit for Higuchi kinetics (r2 ≥ 0.97) followed by first order (r2 ≥ 0.87) and zero order (r2 ≥ 0.86). Higuchi model best describes the drug release from a heterogeneous polymer matrix as in the case of some transdermal systems (50). Higuchi kinetics takes into consideration certain hypotheses such as the concentration of drug in the formulation is much higher than its solubility in the formulation, the drug was uniformly dispersed throughout the polymer solution, drug particles are much smaller than system thickness, perfect sink conditions are always maintained in the release environment, and drug diffusivity is constant (51).
The fit of Higuchi model to the release from our formulations can be explained on the basis of the solubility of NaHS in polymeric solution. The drug is insoluble in the solvents used to dissolve the polymer so the concentration in which the drug was dispersed in the formulation could have been much higher compared to its solubility in the formulation.
The size of NaHS (molecular weight, 56 Da) particles could be smaller in comparison to the pore size of the polymer matrix, so the drug could have easily diffused through these pores into the release media. This is corroborated by our observation of no significant effect on the release rate of NaHS from formulations containing higher polymer concentrations. This can be explained on the basis of smaller drug size vis-a-vie polymer network pore size. Although, with the increase in polymer concentration, there may be a decrease in the pore size, but it may have been, still, large enough for the small-sized drug molecule to diffuse through polymer mesh network easily. Also, the loading concentration of NaHS (0.143 mM) was so chosen that even if all drugs gets released at once, it would be soluble in the release media; thus, ensuring the maintenance of sink conditions.
The PLGA 50:50 polymers have shown to control drug release for over 1–6 months where the size of drugs was immensely greater than NaHS (52). The release of drug from PLA and PLGA matrices generally follows three main phases: burst release, diffusion and chain scission, and biodegradation and mass loss (53). In our study, majority of drug was released within 4 days which suggests that drug diffusion may be the predominant mechanism for drug release. The drug diffusion may be from the surface of the polymer and from the pores and channels formed during the phase inversion of polymer formulation into a soft porous gel depot (49). However, in case of formulations 7–9, a significantly faster drug release was observed (the total release period was 72 h.), this can be explained on the basis of increase in the percentage of BA in the solvent system that increased the hydrophilicity of the polymer. The more hydrophilic formulation tends to gel slowly and thus makes a more leaky gel (48).
The releasing media was maintained at 4°C to prevent the loss of H2S which would not be a case in vivo where it would be being used up immediately after being produced. The polymers used in this study is degraded via hydrolysis which could happen at greater rate at elevated temperature of 37°C creating some additional diffusion pathways as well as widening the existing pathway resulting in a faster release rate than obtained in this study.
CONCLUSIONS
Formulations 1–12 were found to control the release of NaHS for up to 72 h which followed Higuchi square root model of release kinetics. The release of NaHS from formulations was predominantly controlled by drug diffusion which was a result of small size of the drug molecule in comparison to the polymeric diffusion network mesh size and high solubility of drug in the release media capable of maintaining sink condition throughout the period of release study. Phase-sensitive smart polymer-based in situ gel forming system could be an efficient and safe method for delivering hydrogen sulfide donor at a controlled rate for maintaining a low sustained level of H2S for extended period of time after administering subcutaneously in systemic circulation or subconjunctivally into the eye. Type and composition of polymer as well solvent, polymer end group, use of cross-linkers, and hydrogen sulfide donors can be further manipulated for achieving a therapeutically relevant release profile for providing H2S at the target site.
Eye drops containing 0.07% BA has been applied every 8 h for 22 months by patients in a clinical study of the effect on cataracts which was well tolerated by most of the patients. This activity is attributed to mainly its antioxidant activity in addition to its stabilizing effect on lens membrane integrity and a stimulating effect on Na-K ATPase and membrane sodium pump (54). On the other hand, BB is reported to be irritating to the eyes on direct contact, but no damage has been reported from it (55). Subconjunctival administration is an emerging and, at present, intensely investigated ocular delivery route for which there is paucity of toxicity data. The ocular toxicity of the solvents (BA and BB) used in this study should be evaluated before translating the finding of this study in any future subconjunctival administration. These solvents could be replaced with other solvents such as N,N dimethylformamide (DMF) or dimethyl sulfoxide (DMSO) which are reported to be used in ocular dosage forms. A drop containing 25% DMF applied to rabbit eye had no effect, while 50% was slightly irritant (56). A 10–30% solution of DMSO was well tolerated when applied in eye drops or by subconjunctival injection (55,57). A 5% DMSO solution injected into the anterior chamber did not damage the aqueous outflow system (58). Polylactide-based nanoparticles suspended in DMSO was injected intravitreally for targeting retina without any toxic effect (59).
Although, this study showed, as a proof of concept, that phase-sensitive smart polymer-based was able to sustain the release of NaHS for 72 h, the future direction of this study should include ways to extend the period of sustain release through a month so that subconjunctival or subcutaneous administration could be a viable and convenient delivery route for H2S donors. Furthermore, the frequency of subconjunctival administration would also depend on the elimination/excretion of polymer degradation products from the administration site. Therefore, the degradation and elimination profile of the polymers from the administration site should be included in any future study.
References
- 1.Wang R. Physiological implications of hydrogen sulfide: a whiff exploration that blossomed. Physiol Rev. 2012;92:791–896. doi: 10.1152/physrev.00017.2011. [DOI] [PubMed] [Google Scholar]
- 2.Abe K, Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci. 1996;16:1066–1071. doi: 10.1523/JNEUROSCI.16-03-01066.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doeller JE, Isbell TS, Benavides G, Koenitzer J, Patel H, Patel RP, et al. Polarographic measurement of hydrogen sulfide production and consumption by mammalian tissues. Anal Biochem. 2005;341:40–51. doi: 10.1016/j.ab.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 4.Lowicka E, Beltowski J. Hydrogen sulfide (H2S)—the third gas of interest for pharmacologists. Pharmacol Rep. 2007;9:4–24. [PubMed] [Google Scholar]
- 5.Szabo C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6:917–935. doi: 10.1038/nrd2425. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin LR, Francom D, Dieken FP, Taylor JD, Warenycia MW, Reiffenstein RJ, et al. Determination of sulfide in brain tissue by gas dialysis/ion chromatography: postmortem studies and two case reports. J Anal Toxicol. 1989;13:105–109. doi: 10.1093/jat/13.2.105. [DOI] [PubMed] [Google Scholar]
- 7.Warenycia MW, Goodwin LR, Benishin CG, Reiffenstein RJ, Francom DM, Taylor JD, et al. Acute hydrogen sulfide poisoning. Demonstration of selective uptake of sulfide by the brainstem by measurement of brain sulfide levels. Biochem Pharmacol. 1989;38:973–981. doi: 10.1016/0006-2952(89)90288-8. [DOI] [PubMed] [Google Scholar]
- 8.Hu LF, Lu M, Hon Wong PT, Bian JS. Hydrogen sulfide: neurophysiology and neuropathology. Antioxid Redox Signal. 2011;15:405–419. doi: 10.1089/ars.2010.3517. [DOI] [PubMed] [Google Scholar]
- 9.Stipanuk MH, Beck PW. Characterization of the enzymic capacity for cysteine desulphhydration in liver and kidney of the rat. Biochem J. 1982;206:267–277. doi: 10.1042/bj2060267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Erickson PF, Maxwell IH, Su LJ, Baumann M, Glode LM. Sequence of cDNA for rat cystathionine gamma-lyase and comparison of deduced amino acid sequence with related Escherichia coli enzymes. Biochem J. 1990;269:335–340. doi: 10.1042/bj2690335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swaroop M, Bradley K, Ohura T, Tahara T, Roper MD, Rosenberg LE, et al. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J Biol Chem. 1992;267:11455–11461. [PubMed] [Google Scholar]
- 12.Wang R. Hydrogen sulfide: the third gasotransmitter in biology and medicine. Antioxid Redox Signal. 2010;12:1061–1064. doi: 10.1089/ars.2009.2938. [DOI] [PubMed] [Google Scholar]
- 13.Fiorucci S, Distrutti E, Cirino G, Wallace JL. The emerging roles of hydrogen sulfide in the gastrointestinal tract and liver. Gastroenterology. 2006;131:259–271. doi: 10.1053/j.gastro.2006.02.033. [DOI] [PubMed] [Google Scholar]
- 14.Gong QH, Shi XR, Hong ZY, Pan LL, Liu XH, Zhu YZ. A new hope for neurodegeneration: possible role of hydrogen sulfide. J Alzheimers Dis. 2011;2:173–182. doi: 10.3233/JAD-2011-110128. [DOI] [PubMed] [Google Scholar]
- 15.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 16.Tygi N, Moshal KS, Sen U, Vacek TP, Kumar M, Highes WM, Jr, et al. H2S protects against methionine-induced oxidative stress in brain endothelial cell. Antioxid Redox Signal. 2009;11:25–33. doi: 10.1089/ars.2008.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whiteman M, Cheung NS, Zhu YZ, Chu SH, Siau JL, Wong BS, et al. Hydrogen sulphide: a novel inhibitor of hypochlorous acid-mediated oxidative damage in the brain. Biochem Byophys Res Commun. 2005;326:794–798. doi: 10.1016/j.bbrc.2004.11.110. [DOI] [PubMed] [Google Scholar]
- 18.Kimura Y, Dargusch R, Schubert D, Kimura H. Hydrogen sulfide protects HT22 neuronal cells from oxidative stress. Antioxid Redox Signal. 2006;8:661–670. doi: 10.1089/ars.2006.8.661. [DOI] [PubMed] [Google Scholar]
- 19.Kimura Y, Kimura H. Hydrogen sulfide protects neurons from oxidative stress. J Fed Ame Soc Exep Bio. 2004;18:1165–1167. doi: 10.1096/fj.04-1815fje. [DOI] [PubMed] [Google Scholar]
- 20.Eto K, Asad T, Arima K, Makifuchi T, Rimura H. Brain hydrogen sulfide is severely decreased in Alzheimer’s disease. Biochem Biophys Res Commun. 2002;293:1485–1488. doi: 10.1016/S0006-291X(02)00422-9. [DOI] [PubMed] [Google Scholar]
- 21.Lee M, Schwab C, Yu S, McGeer E, McGeer P. Astrocytes produce the anti-inflammatory and neuroprotective agent hydrogen sulphide. Neurobiol Aging. 2009;30:1523–1534. doi: 10.1016/j.neurobiolaging.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Liu YH, Lu M, Hu LF, Wong PT, Webb GD, Bian JS. Hydrogen sulfide in the mammalian cardiovascular system. Antioxid Redox Signal. 2012;17:141–185. doi: 10.1089/ars.2011.4005. [DOI] [PubMed] [Google Scholar]
- 23.Whiteman M, Armstrong JS, Chu SH, Siau JL, Cheung NS, Halliwell B, et al. The novel neuromodulator hydrogen sulfide: an endogenous peroxynitrite “scavenger”. J Neurochem. 2004;90:765–768. doi: 10.1111/j.1471-4159.2004.02617.x. [DOI] [PubMed] [Google Scholar]
- 24.Lefer DJ. A new gaseous signaling molecule emerges: cardioprotective role of hydrogen sulfide. Proc Natl Acad Sci. 2007;46:17907–17908. doi: 10.1073/pnas.0709010104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohia SE, Opere CA, Monjok EM, Kouamou G, Leday AM, Njie-Mbye YF. Role of hydrogen sulfide production in inhibitory action of l-cysteine on isolated porcine irides. Curr Eye Res. 2010;35:402–407. doi: 10.3109/02713680903576716. [DOI] [PubMed] [Google Scholar]
- 26.Njie-Mbye YF, Bongmba OY, Onyema CC, Chitnis A, Kulkarni M, Opere CA, et al. Effect of hydrogen sulfide on cyclic AMP production in isolated bovine and porcine neural retinae. Neurochem Res. 2010;35:487–494. doi: 10.1007/s11064-009-0085-7. [DOI] [PubMed] [Google Scholar]
- 27.Perrino E, Uliva C, Lanzi C, Soldato PD, Masini E, Sparatore A. New prostaglandin derivative for glaucoma treatment. Bioorg Med Chem Let. 2009;19:1639–1642. doi: 10.1016/j.bmcl.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Kasparek MS, Linden DR, Kreis ME, Sarr MG. Gasotransmitters in the gastrointestinal tract. Surgery. 2007;143:455–459. doi: 10.1016/j.surg.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang R. The gasotransmitter role of hydrogen sulfide. Antiox Red Sig. 2003;5:493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionin gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tayel SA, El-Nabarawi MA, Tadros MI, Abd-Elsalam WH. Promising ion-sensitive in situ ocular nanoemulsion gels of terbinafine hydrochloride: design, in vitro characterization and in vivo estimation of the ocular irritation and drug pharmacokinetics in the aqueous humor of rabbits. Int J Pharm. 2013;443:293–305. doi: 10.1016/j.ijpharm.2012.12.049. [DOI] [PubMed] [Google Scholar]
- 32.Cohen S, Lobel E, Trevgoda A, Peled Y. A novel in situ-forming ophthalmic drug delivery system from alginates undergoing gelation in the eye. J Control Rel. 1997;44:201–208. doi: 10.1016/S0168-3659(96)01523-4. [DOI] [Google Scholar]
- 33.Cline JD. Spectrophotometric determination of hydrogen sulfide in natural waters. Limnol Oceanogr. 1969;14:454–458. doi: 10.4319/lo.1969.14.3.0454. [DOI] [Google Scholar]
- 34.Hayton WL, Chen T. Correction of perfusate concentration for sample removal. J Pharm Sci. 1982;71:820–821. doi: 10.1002/jps.2600710726. [DOI] [PubMed] [Google Scholar]
- 35.Craig JP, McNeill R, Tomlinson A. Comparison of salinity and osmolality of human tears, poster# 50 (VB-303) Optom Vis Sci. 1995;72:133–134. doi: 10.1097/00006324-199512001-00218. [DOI] [PubMed] [Google Scholar]
- 36.Hägerström H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci. 2000;9:301–309. doi: 10.1016/S0928-0987(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 37.Carroll JJ, Mather AE. The solubility of hydrogen sulphide in water from 0 to 90°C and pressures to 1 MPa. Geochim Cosmochim Acta. 1989;53:1163–1170. doi: 10.1016/0016-7037(89)90053-7. [DOI] [Google Scholar]
- 38.Kirsten WJ. On the ethylene blue reaction and its use for determination of sulphide. Mikrochim Acta. 1978;11:403–409. doi: 10.1007/BF01197092. [DOI] [Google Scholar]
- 39.Lindsay SS, Baedecker MJ. Determination of aqueous sulfide in contaminated and natural water using the methylene blue method. Ground-water contamination : field methods : a symposium / sponsored by ASTM committees D-19 on Water and D-18 on Soil and Rock, Cocoa Beach, FL, 1986; 349–57.
- 40.Martelli A, Testai L, Breschi MC, Blandizzi C, Virdis A, Taddei S, et al. Hydrogen sulphide: novel opportunity for drug discovery. Med Res Rev. 2012;32:1093–1130. doi: 10.1002/med.20234. [DOI] [PubMed] [Google Scholar]
- 41.Hughes MN, Centelles MN, Moore KP. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Bio Med. 2009;47:1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 42.http://www.engineeringtoolbox.com/gases-solubility-water-d_1148.html. Accessed 16 Oct 2013.
- 43.Chaubal MV, Kipp J, Rabinow B. Excipient selection and criteria for injectable dosage forms. In: Katdare A, Chaubal MV, editors. Excipient development for pharmaceutical, biotechnology, and drug delivery systems. New York: CRC Press; 2006. pp. 271–290. [Google Scholar]
- 44.Merck Index, 13th Ed., Merck & Co., Inc., Whitehouse, NJ, 2001; 1132.
- 45.Al-Tahami K, Singh J. Smart polymer based delivery systems for peptides and proteins. Rec Pat Drug Del Form. 2007;1:65–71. doi: 10.2174/187221107779814113. [DOI] [PubMed] [Google Scholar]
- 46.Ohia SE, Opere CA, Zhan G, Monjok E, Kulkami K, Kouamou GE. Use of hydrogen sulfide in the treatment of eye diseases. 2012;US Patents 8092838.
- 47.Chitkara D, Shikanov A, Kumar N, Domb AJ. Biodegradable injectable in situ depot‐forming drug delivery systems. Macromol Biosci. 2006;6:977–990. doi: 10.1002/mabi.200600129. [DOI] [PubMed] [Google Scholar]
- 48.Chhabra S, Sachdeva V, Singh S. Influence of end groups on in vitro release and biological activity of lysozyme from a phase-sensitive smart polymer-based in situ gel forming controlled release drug delivery system. Int J Pharm. 2007;342:72–77. doi: 10.1016/j.ijpharm.2007.04.034. [DOI] [PubMed] [Google Scholar]
- 49.Graham P, Brodbeck K, McHugh A. Phase inversion dynamics of PLGA solutions related to drug delivery. J Control Rel. 1999;58:233–245. doi: 10.1016/S0168-3659(98)00158-8. [DOI] [PubMed] [Google Scholar]
- 50.Higuchi T. Rate of release of medicaments from ointment bases containing drugs in suspension. J Pharm Sci. 1961;50:874–875. doi: 10.1002/jps.2600501018. [DOI] [PubMed] [Google Scholar]
- 51.Dash S, Murthy PN, Nath L, Chowdhury P. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 2010;67:217–223. [PubMed] [Google Scholar]
- 52.Wang L, Venkatraman S, Kleiner L. Drug release from injectable depots: two different in vitro mechanisms. J Control Rel. 2004;99:207–216. doi: 10.1016/j.jconrel.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 53.Chen S, Singh J. Controlled delivery of testosterone from smart polymer solution based systems: in vitro evaluation. Int J Pharm. 2005;295:183–190. doi: 10.1016/j.ijpharm.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 54.Testa M, Iuliano G, Morton P, Longoni A. Topical benzyl alcohol reduces cataract surgery need: two long-term double blind studies. J Ocul Pharmacol. 1987;3(3):211–225. doi: 10.1089/jop.1987.3.211. [DOI] [PubMed] [Google Scholar]
- 55.Grant WM, Schuman JS. Toxicology of the eye: effects on the eyes and visual system from chemicals, drugs, metals and minerals, plants, toxins, and venoms; also, systemic side effects from eye medications. 4. Springfield: Thomas; 1993. p. 575. [Google Scholar]
- 56.Massmann W. Toxicological investigations on dimethylformamide. Br J Ind Med. 1956;13(1):51–54. doi: 10.1136/oem.13.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hanna C, Fraunfelder FT, Meyer SM. Effects of dimethyl sulfoxide on ocular inflammation. Ann Ophthalmol. 1977;9(1):61–65. [PubMed] [Google Scholar]
- 58.Kaufman PL, Erickson KA. Cytochalasin B and D dose-outflow facility response relationships in the cynomolgus monkey. Invest Ophthalmol Vis Sci. 1982;23(5):646–650. [PubMed] [Google Scholar]
- 59.Bourges JL, Gautier SE, Delie F, Bejjani RA, Jeanny JC, Gurny R, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44(8):3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]


