Abstract
The main objective of this investigation was to study the feasibility of developing a vaginal bioadhesive microbicide using a SRI’s proprietary two-polymer gel platform (SR-2P). Several formulations were prepared with different combinations of temperature-sensitive polymer (Pluronic® F-127) and mucoadhesive polymer (Noveon® AA-1), producing gels of different characteristics. Prototype polymeric gels were evaluated for pH, osmolality, buffering capacity, and viscosity under simulated vaginal semen dilutions, and bioadhesivity using ex vivo mini pig vaginal tissues and texture analyzer. The pH of the polymeric gel formulations ranged from 5.1 to 6.4; the osmolality varied from 13 to 173 mOsm. Absolute viscosity ranged from 513 to 3,780 cPs, and was significantly reduced (1.5- to 3-fold) upon incubation with simulated vaginal and semen fluid mixture. Among the tested gels (indicated in the middle row as a molar ratio of a mixture of Noveon vs. Pluronic), only SR-2P retained gel structure upon dilution with simulated fluids and mild simulated coital stress. The pH of the SR-2P gel was maintained at about 4.6 in simulated vaginal fluid and also showed high peak force of adhesion in mini pig vaginal tissue. Furthermore, SR-2P gel caused no or only minimal irritation in a mouse vaginal irritation model. The results of this preliminary study demonstrated the potential application of SR-2P gel as a vaginal microbicide vehicle for delivery of anti-HIV agents.
Figure.

Two-polymer bioadhesive vaginal placebo gel (SR-2P); and safety profile in mice
KEY WORDS: bioadhesivity, HIV, HSV, microbicide, sexually transmitted infections, vaginal gel
INTRODUCTION
The most prevalent sexually transmitted diseases are AIDS and herpes simplex virus (HSV) infection, which are caused by two opportunistic pathogens and lead to progressive failure of the immune system (1). Globally, over 33 million individuals are infected with HIV, and AIDS continues to date to be the most destructive known pandemic. HSV-2 infection is also a global epidemic, with nearly 20% of all sexually active adults being carriers; about 80% of HIV-infected adults are coinfected with HSV-2 (2). Over the past few decades, specifically in the developing nations, women have represented the fastest growing demographic of the HIV/AIDS pandemic. Reports in the literature suggest that biological and socioeconomic vulnerability makes women at higher risk for HIV (3,4). The current methods of preventing HIV infection, such as abstinence, condoms, and monogamy, are frequently ineffective and often outside a woman’s control (5). The failure of existing methods of HIV prevention contributes to women’s greater susceptibility to HIV infections. Furthermore, failures of the existing prevention approaches and the rapid surge of infection rates strongly indicate the need for woman-controlled preventive strategies. One of the most promising prophylactic woman-controlled strategies is the use of topical vaginal microbicides to protect women against male-to-female sexual transmission of HIV. Microbicides may function as a physical barrier or deliver antiviral agents singly or in combination. The success of 1.0 wt.% tenofovir gel in the CAPRISA 004 microbicide trial is a historic milestone in the field of microbicides and reinforces the hope for preventive strategies in the fight against HIV (6). The gel used in that trial was composed of 40 mg of 9-[(R)-2-phosphonomethoxy) propyl] adenine monohydrate (6) in a solution of purified water with edetate disodium, citric acid, glycerin, methylparaben, propylparaben, and hydroxyethycellulose (HEC). The placebo gel used in the trial was the “universal” HEC placebo gel, which has been shown to have minimal anti-HIV activity (6). We hypothesize that our patented bioadhesive formulation (7), composed of two FDA-approved polymers, Pluronic® F-127 (polyoxyethylene polyoxypropylene co-polymer, polaxomer) and Noveon® AA-1 (polyacryclic acid), will [1] be a more efficacious platform for delivery of the antiviral drugs tenofovir and acyclovir, [2] offer protection against viral infection, and [3] have a long residence time because it will form micelle-like particles that adhere to the vaginal wall. The epithelium of the vagina contains glycogen, which is broken down by enzymes and bacteria into lactic acid to maintain low vaginal pH between 4 and 5, which is inhospitable to pathogens (8). A patented mucoadhesive formulation platform developed at SRI International (Menlo Park, CA) showed promise as a vaginal bioadhesive (7). This proprietary formulation (denoted as “SR-2P”; Fig. 1) offers many advantages as a potential bioadhesive microbicide gel candidate. A microbicide is a vaginal formulation containing antiviral agents. Unlike a solution dosage form, which is easily washed off, a mucoadhesive gel formulation, containing antiviral agents, on application to vaginal mucosa results in longer drug exposure. The overall goal of this investigation was to identify a lead bioadhesive gel candidate from several prototype gel compositions suitable for loading of anti-HIV/HSV agents. Towards this objective, various prototype gels were prepared and pH, osmolality, buffering capacity, viscosity changes, and bioadhesivity were studied under simulated physiological conditions. The vaginal irritation potential of the SR-2P were studied, in a mouse model developed earlier at SRI International (9).
Fig. 1.

Novel bioadhesive vaginal gel (denoted as SR-2P) platform technology developed at SRI International (Menlo Park, CA)
MATERIALS AND METHODS
Materials
The pH-sensitive polymer, polycarbophil USP (Noveon® AA-1) was received as a gift sample from Lubrizol (Wickliffe, OH). Another temperature-sensitive polymer, poloxamer 407 NF (Pluronic® F-127), in addition to glacial acetic acid, lactic acid, bovine serum albumin, zinc chloride, magnesium chloride, calcium hydroxide, monosodium dihydrogen phosphate, sodium hydroxide, disodium monohydrogen phosphate, potassium hydroxide, glucose, fructose, urea, potassium chloride and trisodium citrate, carboxymethylcellulose, nonoxynol, and benzalkonium chloride, was purchased from Spectrum Chemicals Manufacturing Company (Gardena, CA).
Methods
Polymeric Composition of the Prototype Gels
The prototype placebo gels had two compartments: compartment A, containing Noveon® AA-1, and compartment B, containing Pluronic® F-127, as the polymer base. A double-barrel syringe model was used to mix the contents of the two compartments, and extrude the combination as a gel, as shown in Fig. 2.
Fig. 2.
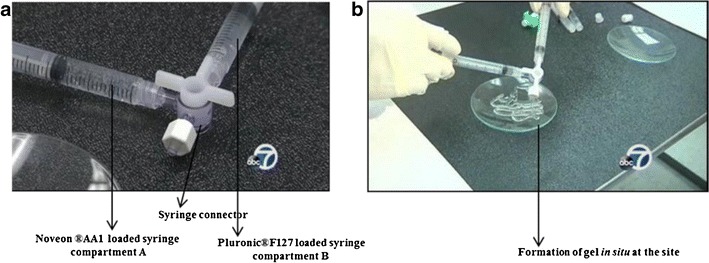
Double-barrel syringe device for preparation of prototype placebo gels. a Loading of aqueous solutions of polymers into compartment A and B connected by using syringe connector; b formation of in situ gel at the site upon mixing of two-polymer solutions in dual syringes
Preparation of Noveon® AA-1 Solution—Compartment A
Polymeric solutions of Noveon® AA-1 (from 0.25 to 15% w/w) were prepared by dispersing the required amounts gradually (to avoid aggregation and clump formation) into a beaker containing deionized water, and stirred with a laboratory paddle mixer (Framo-Gerätetechnik, Model-LR20®, Eisenbach, Baden-Wurttemberg) at 850 rpm for 15 min. The solution was allowed to equilibrate at 4°C for 30 min to ensure complete hydration of the polymer. The pH of the solution was adjusted to 4.8 ± 0.2 with 10 N NaOH. After pH adjustment, the final weight of the solution was adjusted to 100 g with deionized water. The polymeric solutions were allowed to equilibrate 24 h in refrigerator prior to being used in experiments, and were used within a week of preparation.
Preparation of Pluronic® F-127 Solution—Compartment B
Polymeric solutions of Pluronic® F-127 (from 0.5 to 15% w/w) were prepared by dispersing the required amounts gradually into a beaker containing deionized water, and stirred with a paddle mixer (Framo-Gerätetechnik, Model-LR20®, Eisenbach, Baden-Wurttemberg) at 300 rpm for 15 min. The solution was allowed to equilibrate at 4°C for 30 min to ensure complete hydration of the polymer. After complete dissolution, the solutions were allowed to equilibrate for 24 h in refrigerator prior to use in experiments, and were used within a week of preparation.
Preparation of Preliminary Prototype Bioadhesive Placebo Gels
Towards the optimization of SR-2P composition, several preliminary prototype bioadhesive placebo combination solutions were prepared by mixing different ratios of Noveon® AA-1 from syringe A and Pluronic® F-127 from syringe B in a connected double syringe (Fig. 2). Nominal thumb pressure was sufficient for initial mixing of the two solutions, as viscosity only increases gradually.
Characterization and Optimization of Placebo Gels
Gels were analyzed for pH, buffering capacity, osmolality, bioadhesivity, and gel matrix integrity on dilution with simulated fluids. The vaginal and semen simulant fluids were prepared as per the composition of previously reported methods (10). From these ten placebo gel compositions, only SR-2P gel was tested further at simulated physiological conditions of dilution and shear stress (Table I), since only SR-2P showed desired characteristics of acceptable pH, viscosity, and osmolality. Furthermore, SR-2P withstood dissolution in vaginal fluid simulant, and a mixture of semen and vaginal fluid simulants. Triplicate samples of placebo gel formulations were used for each test.
Table I.
Summary of Characteristics of Prototype Placebo Gel Formulations*
| Formulation ID | Pluronic® F-127 (% w/w) | Noveon® AA-1 (% w/w) | pH at t = 0 | Viscosity at t = 0 (cPs)* | Osmolality at t = 0 (mOsm) | Viscosity in VFS (1:1) (cPs) after 2 h of contact time | Viscosity in VFS and SFS (1:5) (cPs) after 2 h of contact time |
|---|---|---|---|---|---|---|---|
| 15987-16-3 | 0.5 | 0.5 | 5.1 ± 1.5 | 513 ± 3 | 20 ± 1 | Complete dissolution of gel | Complete dissolution of gel |
| 15987-16-1 | 0.9 | 0.5 | 5.3 ± 1.3 | 1,803 ± 10 | 16 ± 2 | Complete dissolution of gel | Complete dissolution of gel |
| 15987-14-1 | 1 | 0.5 | 5.3 ± 2.3 | 1,679 ± 38 | 34 ± 5 | Complete dissolution of gel | Complete dissolution of gel |
| 15987-17-3 | 1.87 | 0.5 | 5.8 ± 1.2 | 1,385 ± 15 | 33 ± 3 | Complete dissolution of gel | Complete dissolution of gel |
| 15987-18-2 | 3.74 | 0.5 | 6.1 ± 3.2 | 1,096 ± 13 | 22 ± 4 | Complete dissolution of gel | Complete dissolution of gel |
| 15987-18-1 | 0.9 | 1 | 5.6 ± 1.7 | 1,563 ± 33 | 13 ± 1 | 616 ± 13 | Complete dissolution of gel |
| 15987-17-1 | 1.87 | 1 | 5.8 ± 3.2 | 1,775 ± 18 | 29 ± 2 | 99 ± 1.52 | Complete dissolution of gel |
| 15987-17-2 | 3.75 | 1 | 6.2 ± 2.7 | 1,351 ± 29 | 29 ± 4 | 298 ± 6 | Complete dissolution of gel |
| 15987-15-1 | 15 | 1 | 6.4 ± 1.5 | 3,780 ± 17 | 175 ± 8 | 1,094 ± 8 | 321 ± 5 |
| SR-2P | 1 | 1 | 5.7 ± 2.1 | 1,741 ± 21 | 21 ± 3 | 279 ± 2 | 67 ± 4 |
* pH of the simulated fluids: vaginal fluid 4.5, semen fluid 7.7, mixture of vaginal and semen fluids 7.5; concentrations after mixing the two components
Physical Appearance and Odor
The placebo gel formulations were checked for physical appearance such as color, consistency, and odor.
pH
The pH of the placebo gels at room temperature was recorded using pH meter 440 (Corning Inc., NY).
Osmolality
The osmolality of the placebo gel formulations was determined using a 3D3 osmometer (Advanced Instruments, Norwood, MA). The measurements were recorded as milliosmoles (mOsm) in 0.2 ml of each placebo gel formulation.
Rheological Characterization
The rheological properties of the gel formulations were characterized by steady shear rheology using a cone-plate geometry viscometer (Brookfield® DV-II programmable viscometer, Middleton, MA). About 2 ml of placebo gel formulation was used and the gap between the geometry and the instrument plate was set at 103 mm as specified by the manufacturer. The viscosity of the ten whole placebo gels and diluted gels in simulated fluids at 37°C was measured at shear rate of 10 s−1, and values were reported as centipoise (cPs).
Dilution Effect of Simulated Fluids on Gel Viscosity
SR-2P gel was diluted with vaginal fluid simulant, and a mixture of semen and vaginal fluid simulants (37°C) at dilution levels that ranged slightly above and below the normal condition so as to mimic vaginal flooding or dryness (slightly modified based on the published literature emphasizing the importance of dilution when studying the flow behavior in the vagina and coitus) (11,12). Briefly, a known volume of gel formulation was placed on the bottom of the scintillation vial. A calculated volume of simulated fluid (vaginal or a mixture of vaginal and seminal fluid) was layered slowly with pipette on top of the previous layer of gel in the vial. Typically, a sequential dilution of SR-2P from 1 to 8 ml of gel with 1 ml of VFS and 4.5 ml of VFS + SFS (1 ml + 3.5 ml) was used in the various experiments. The two components were allowed to equilibrate (under stationary conditions without agitation) with each other for 30 min at 37°C. At the end of equilibration, fluid shear was introduced on a horizontal shaker platform at a speed of 70 rpm (this shear is equivalent to one half the magnitude of in vivo coital shearing which is estimated to be 50 s−1) for a time period of 10 min. After 10 min of shear, the components in the glass vial were kept in contact for an additional 70 min. Viscosity of the gels was measured at time intervals of 30, 35, 40, and 70 min using the viscous gel residue left at the bottom of the vial, after carefully removing the top fluid layer with a Pasteur pipette. Prior to the viscosity measurement, pH of the gel was recorded. The pH of the diluted gels at the end of 70 min and 24 h was also recorded to evaluate the buffering capacity of the gels.
Gel Erosion Assay
In the dilution study, the integrity of the gel was examined visually at the 30, 35, 40, and 70 min time points. Effect of dilution on the gel matrix structure was evaluated qualitatively by (a) magnitude of swelling and (b) retention of gel structure. The effect of swelling and changes in gel structure was quantified as a function of viscosity changes as a function of time in contact with the dilution buffer.
Bioadhesivity Testing of Placebo Gel Formulations
Bioadhesive strength of placebo gel formulations was characterized by a texture analyzer-based method with mini pig vaginal tissues. The bioadhesivity of each formulation was measured using a TA-XT2 Texture Analyzer (Stable Micro Systems, Surrey, UK) in adhesion mode to measure the peak force required to detach the placebo gel formulation from isolated mini pig vaginal tissue. Vaginal tissue (harvested from Gottingen mini pigs) was horizontally attached to the lower end of the texture analysis probe using a rubber band (Fig. 3). At room temperature, samples of each placebo gel formulation (mixed before application) were placed under the analytical probe, and slowly lowered until the mucosal portion of the tissue contacted the surface of the sample. A downward force (0.1 N) was applied to ensure close contact between the tissue and gel formulation. The probe speed was maintained at constant speed of 1.0 mm/s (upwards), and force versus time plots were used to determine the force required to detach vaginal tissue from the gel formulation. The XTRA Dimension software (software provided by the manufacturer) was used for data analysis and force-time plot calculations. A minimum of three analyses was recorded for each formulation.
Fig. 3.

Experimental set up of bioadhesion testing using mini pig vaginal tissues with texture analyzer (Stable Micro Systems, Surrey, UK). Adhesion mode was used to measure peak force required to detach placebo gel formulation from isolated mini pig vaginal tissue
Vaginal Irritation Safety Testing of Placebo Gel Formulations in Mice
All animal work was approved by SRI’s Institutional Animal Care and Use Committee in full compliance with all regulations of the National Institutes of Health Office of Laboratory Animal Welfare. Female 8-week-old CD-1 mice (Harlan, Indianapolis, IN) were conditioned with 3 mg Depo-Provera™ 1 week before and within 1 day prior to study initiation and were randomly assigned to treatment groups (n = 5) by a computerized body weight stratification procedure. Test articles used were as follows: TA 1 (1.0 w/w% Pluronic® F127 + 1.0 w/w% Noveon® AA1; composition of SR-2P); TA 2 (0.5 w/w% Pluronic® F127 + 7.5 w/w% Noveon® AA1), and TA 3 (0.5 w/w% Pluronic® F127 + 4.5 w/w% Noveon® AA1). A 0.050 ml vehicle control (2% carboxymethylcellulose), positive controls (8% N-9, 2% BZK), and test articles (TA 1/SR-2P, TA 2, and TA 3 [components were mixed with two interconnected syringes immediately before dosing; Fig. 2]) were administered intravaginally using a 20 gauge blunt applicator once daily for 12 days. On day 13, animals were euthanized; vaginal tissues were collected and formalin-fixed. Hematoxylin and eosin (H&E)-stained microtome sections were prepared and scored by a board-certified pathologist. Microscopic changes were coded by the most specific topographic and morphologic diagnosis. Systematized Nomenclature of Medicine (SNOMED) and National Toxicology Program (NTP) terminology manuals were used as guidelines. Gradable observations were scored by a four-step grading system: 0, not observed; 1, minimal; 2, mild; 3, moderate; and 4, marked.
RESULTS AND DISCUSSION
Polymer-based gel systems are widely used in formulation development of oral, dental, dermatological, vaginal, and ophthalmic applications (13,14). The gel-based formulations are easy to apply, easy to manufacture on a large scale, and have enhanced patient compliance. Furthermore, these aqueous-based systems display reduced irritancy, which is essential for application to vaginal membranes (13,14). It is understood from the published literature that development of viable semisolid drug delivery platforms for vaginal application requires a fundamental understanding of their rheological, textural, and mucoadhesive properties within the vaginal cavity (15). Furthermore understanding of the factors affecting the dilution of gels with vaginal and semen fluids after the intravaginal administration and subsequent leakage of the gel from the site of application are critical for the development of vaginal gels (16). Mucoadhesive polymers based on acrylic acid and celluloses have been reported to destabilize during dilution resulting in subsequent loss of gel adherence to the vaginal cavity (13,14,16). Mucoadhesive property exhibited by these acrylic acid cellulosic-based polymers alone is not sufficient to resist dilution and coital stress effects (13,14). Research and development efforts are underway for the development of optimum vaginal gel formulations that exhibit non-Newtonian characteristics and possess shear thinning properties, so that they are easy to apply. In addition, these optimum gels should be resistant to in vivo vaginal dilution and maintain structural integrity in spite of coital stress. In addition to the abovementioned gel characteristics, bioadhesivity of the formulation is critical for the prolonged retention and optimal exposure of the therapeutics in the vaginal cavity (13,14). The present study aims to identify placebo gels possessing all the abovementioned characteristics as suitable depot for anti-HIV/HSV agents, for use as microbicides.
Characterization and Optimization of Placebo Gels
The current prototype gels are stored in two compartments until use. This is a requirement for this formulation. The two syringes that make up the formulation constitute the “thermoresponsive” pluronic in one compartment and the “pH-responsive” polycarbophil in the other compartment. The uniqueness of this formulation is that during storage, the two solutions are in the solution form; however, on reconstitution and application to a physiologic site, this formulation “gels.” The sol to gel transformation occurs because the pluronic fraction goes from the ambient temperature to 37°C and therefore gels, and the polycarbophil fraction undergoes a pH increase, which causes it to gel as well. Polycarbophil fraction gels on mixing with the more alkaline pluronic as well due to the presence of physiological fluid, both of which increases the pH of the mixture. The end effect is that the product is a thick spreadable “gel.” If premixed in one syringe, the formulation will not have the sticky property of the polycarbophil; therefore, all the studies performed in this manuscript were done using the two-compartment syringe model.
The gels were evaluated for different properties related to their biophysical and physiological functioning in vivo. All the prototype gel compositions were translucent to off-white in color, without any odor. Compositions containing higher concentrations of Pluronic® F-127 were off-white in color.
The pH of the gels were measured, as the microbicide vehicle should exhibit efficacy over a pH ranging from 3.5 to 7.7, because microbicides encounter wide fluctuations in the pH at the site of application (11). For instance, the normal vaginal pH is acidic, ranging from 3.5 to 4.5, and when semen contacts the vagina, the pH rises to above 6.0 because of the buffering capacity of the male ejaculate (pH 7.2–8.0). Hence, an ideal microbicide should maintain efficacy over the physiological fluid pH level variations, i.e., the microbicide should exhibit ample buffering capacity. The pH of the undiluted prototype gels ranged from 5.1 to 6.4 (Table I). Two gel formulations with higher concentrations of Pluronic® F-127 (>1.8% w/w) and Noveon® AA-1 (1% w/w) resulted in 0.2 to 0.8 unit increase in pH as opposed to other gels (Table I).
Placebo gels were characterized for osmolality, which is used as an indicator of mucosal irritation potential (17). The osmolality of various placebo gel formulations are presented in Table I. From the data, it is evident that compositions with very high concentrations of Pluronic® F127 have approximately eightfold higher osmolality as compared to other gels. Osmolality was found to be lowest in the case of prototypes containing 0.9% w/w of Pluronic® F-127 and 0.5 and 1% w/w of Noveon® AA-1 (15987-16-1 and 15987-18-1, respectively). The osmolality of the remaining six prototype gels was fairly constant at 29 ± 4 mOsm. With reference to the above discussion on osmolality, induction of mucosal toxicity was observed with the hyperosmolar formulations by other research groups (17,18), wherein the adverse toxicological behavior is observed with osmolality values greater than 2,000 mOsm/kg H2O, i.e., substantially greater than those measured for formulations described here.
Rheological Characterization of Placebo Gels
The viscosity of the placebo gels was found to be greatly influenced by the concentration of Pluronic® F-127. Among the various prototype placebo gels, highest viscosity of 3,780 ± 17 cPs was observed in the gel containing the highest amount of Pluronic® F-127 (>10% w/w, 15987-15-1; Table I). On the other hand, the lowest viscosity of 513 ± 3 cPs was observed in case of prototype containing equal (% w/w) concentration of Pluronic® F-127 and Noveon® AA-1 (15987-16-3).
Preliminary evaluation of dilution of gels with simulated fluids and shear (50 rpm) at a 1:1 ratio (volume) with simulated vaginal and a 1:5 ratio of a mixture (5 ml) of vaginal (1 ml) and semen fluids (4 ml) was performed on ten prototype gels. As shown in Table I, dilution with both fluid simulants reduced viscosity by many orders of magnitude (10- to 15-fold), more so with compositions that had less than 1% w/w of Noveon® AA-1. It is worthwhile to mention that SR-2P and the prototype gel containing 15% w/w of Pluronic® F-127 and 1% w/w of Noveon® AA-1 (15987-15-1) resisted the dilution effects of vaginal fluid and seminal fluid simulant mixtures (Table I). These two formulations retained gel structure upon dilution with simulated fluids and unlike the other prototypes tested did not undergo complete dissolution. However, formulation of Noveon® AA-1 (15% w/w, 15987-15-1) was extremely viscous and gelled at room temperature, thus viscosity is deterrent to syringe.
Effect of Shear Force on Placebo Gels under Different Conditions of Dilution
The results of the preliminary dilution study demonstrated that only SR-2P was promising for further testing under simulated physiological conditions of dilution and shear as per the previously reported method (10). In addition, an erosion assay was also used to measure the propensity for the gel to be washed off from the surface by vaginal fluid, semen, and mixture of simulants (19,20). It is evident from the results of the vaginal dilution studies that only SR-2P and the formulation with 15% w/w of Pluronic® F-127 and 1% w/w of Noveon® AA-1 resisted erosion under physiological diluted conditions of simulated fluids and shear (Table I). As per published literature, the physiological shear rates during the passive and coital stress ranges from 0.1 to 100 s−1 (19–21). Since SR-2P has resisted shear (50 rpm) and extreme dilution in simulated fluids, it was further characterized at the physiological simulated dilution and intermediate penile stress (70 rpm; Fig. 4). The entire biologically relevant range of shear rates (19–21) was applied to the whole placebo SR-2P gel in the present study (Figs. 4, 5, 6, and 7).
Fig. 4.
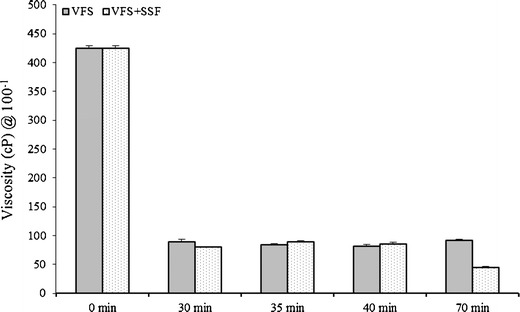
Viscosity of SR-2P gel (3 ml) exposed to VFS (1 ml) or VFS + SFS (4.5 ml) mixture at 37°C under horizontal shear (at 70 rpm) at 0, 30, 35, 40, and 70 min time points. Error bars represent average values with ±SD (n = 3)
Fig. 5.
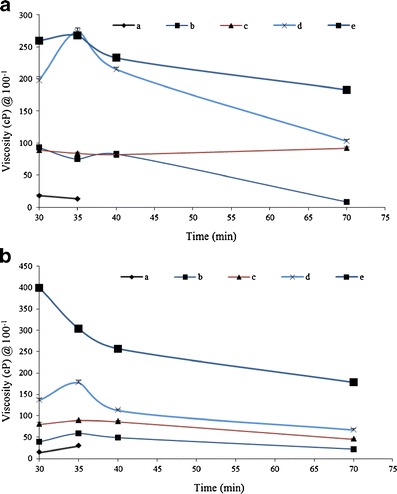
Viscosity of SR-2P gel with increasing volume of 1 ml (a), 2 ml (b), 3 ml (c), 4 ml (d), 8 ml (e) exposed to VFS (1 ml) or VFS + SFS (4.5 ml) mixture at 37°C under horizontal shear (at 70 rpm) at 0, 30, 35, 40, and 70 min time points. a VFS; b VFS + SFS. Error bars represent average values with ±SD (n = 3)
Fig. 6.
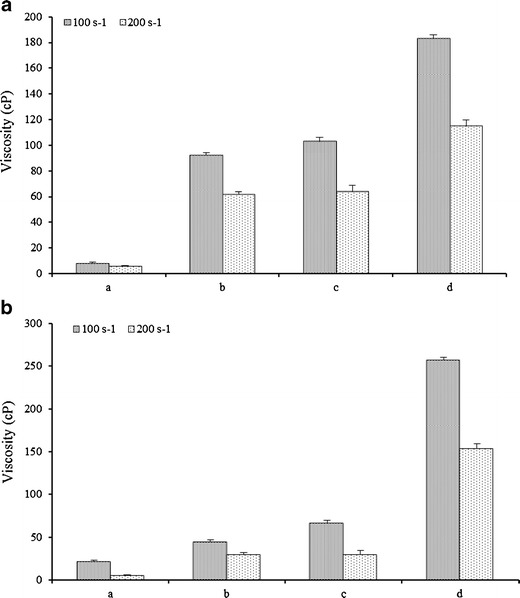
Viscosity of SR-2P gel with increasing volume of 2 ml (a), 3 ml (b), 4 ml (c), and 8 ml (d) exposed to VFS (1 ml) or VFS + SFS (4.5 ml) mixture at 37°C under shear of 70 (100 s−1) and 140 rpm (100 s−1). a VFS; b VFS + SFS. Error bars represent average values with ±SD (n = 3)
Fig. 7.
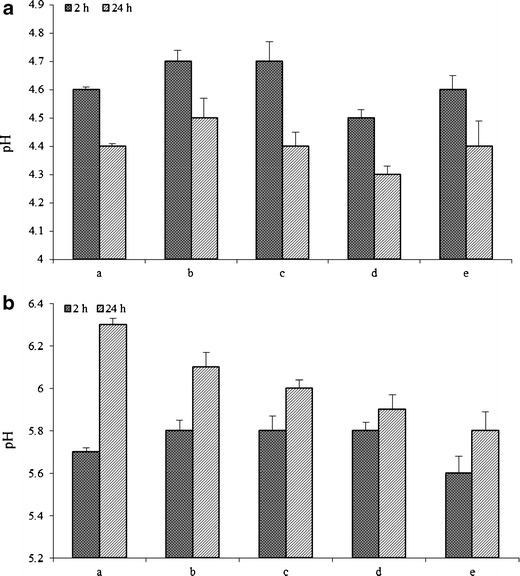
Relative pH buffering capacity of the SR-2P gel with increasing volume of 1 ml (a), 2 ml (b), 3 ml (c), 4 ml (d), and 8 ml (e) exposed to VFS (1 ml) or VFS + SFS (4.5 ml) mixture at 37°C under shear of 70 rpm at 2 and 24 h time points. a VFS; b SFS. Error bars represent average values with ±SD (n = 3)
The most significant challenge for topically applied vaginal gel drug delivery systems is the detrimental effect of dilution by cervicovaginal fluids (and semen during intercourse) on formulation rheology and hence on product retention (19). Current clinical vaginal gels such as those used in contraceptive applications tend to be applied using volumes in the region of 1.5–3 ml (19,21,22). These gel volumes are typically subjected to dilution by approximately 0.75–1 ml of vaginal fluid (11–13,15,21,22). Accordingly, the effects of dilution representative of that encountered in vivo on the viscosity of the gel was examined by sequential dilution of SR-2P from 1 to 8 ml of gel with 1 ml of VFS and 4.5 ml of VFS+SFS (1 + 3.5 ml; Fig. 5). Multiple volumes (1, 2, 3, 4, and 8 ml) of gel of SR-2P (which has 1% w/w of the two polymers) diluted with 1 ml of VFS showed viscosity at 100 cPs up to 70 min (Fig. 5a). It is worthwhile to mention that these gel volumes (1 to 8 ml) also resisted 10 min of simulated coital shear at 70 rpm at shear rate of 100 s−1. At the end of 70 min, viscosity was reduced about four times in the case of 2 ml gel volume, whereas the viscosity of 3-ml gel volume was unchanged (Fig. 5a). In contrast, an increase in viscosity was observed in the case of 4-ml gel volume at 35 min; however, a twofold drop in viscosity was observed at the end of 70 min (Fig. 5a). In the case of the largest volume (8 ml) of SR-2P, a similar trend was observed as was the case with 4 ml, but the drop in viscosity was less, even up to 70 min (Fig. 5a). As evident from the data (Fig. 5b), 1-ml gel volume of SR-2P in VFS + SFS lasted for only 35 min; other higher volumes retained gel structure up to 70 min. In the cases of 2 and 3 ml of SR-2P in VFS + SFS, approximately a twofold drop in viscosity was observed from the initial time point to 70 min (Fig. 5b). In contrast, the 4-ml gel volume of SR-2P exhibited a slight increase in viscosity at 35 min, followed by a drop in viscosity at 40 min and remained plateau up to 70 min (Fig. 5b). An opposite effect was observed in the case of 8-ml gel volume in mixture of VFS and SFS, where a continuous drop in viscosity was observed up to 40 min, and remained constant up to 70 min, retaining threefold of initial viscosity (Fig. 5b).
In addition to studying the effect of dilution on SR-2P at increasing gel volumes in simulated VFS and VFS + SFS mixture, shear effect at 70 and 140 rpm was also studied (Fig. 6). In the case of 1-ml gel volume of SR-2P in VFS, no change in viscosity was observed at 100 and 200 s−1 shear rates (Fig. 6a). Effect of shear rates on the viscosity of SR-2P in increasing gel volumes with VFS + SFS was presented in Fig. 6b. The drop in viscosity was higher in VFS + SFS mixture (Fig. 6b) as opposed to the viscosity drop in simulated VFS alone. Complete solubilization of 1 ml SR-2P gel was observed within 30 min, and in the case of 2 and 3 ml volume, about twofold reduction in viscosity was observed at shear rate of 200 s−1 (Fig. 6b). Similarly at 200 s−1 shear rates, viscosity was decreased by threefold in the case of 4-ml gel volume of SR-2P. In contrast to smaller gel volumes, the lowest drop in viscosity of SR-2P at 8-ml gel was observed at 200 s−1 shear rate (Fig. 6b).
The buffering capacity of SR-2P in VFS and VFS + SFS mixture incubated for up to 24 h at 37°C was presented in Fig. 7. The pH of the SR-2P gels with increasing gel volumes from 1 to 8 ml in 1 ml of simulated VFS was maintained at 4.6 ± 0.2 (Fig. 7a) up to 2-h incubation. However, after 24 h, pH dropped to 4.4 ± 0.3, but within the normal physiological pH of the VFS (Fig. 7a). The drop in the pH between 2 and 24 h was by about 0.2 to 0.3 units across the all gel volumes. The highest drop in pH was in the case of 3-ml SR-2P gel volume, which decreased by 0.3 units, but the drop in pH of the remaining SR-2P gel volumes was 0.2 units from 2 to 24 h incubation (Fig. 7a). In contrast, a raise in the pH of SR-2P gels was observed in the mixture of simulated vaginal fluid (1 ml) and simulated semen (3.5 ml) between 2 and 24 h incubation (Fig. 7b). Moreover, the rise in pH was as high as 0.6-fold in the lowest gel volume of 1 ml and lowest in the case of gel volume of 4 ml. The rise in pH was about 0.3 units in the cases of 2 and 8 ml, and 0.2 units in the case of 3-ml gel volume (Fig. 7b). The rise in the pH of SR-2P could be due to high buffering capacity of simulated SFS (21,22). Another potential explanation is the high ionization of Noveon® AA-1 at the pH of the simulant fluid mixture ∼7.5, which is above its pKa of 6 ± 0.5 (23,24). The two prototype gels with higher concentrations of Pluronic® F-127 (>1.8% w/w) and Noveon® AA-1 (1% w/w) resulted in 0.2 to 0.8 unit increase in pH as opposed to other gels (Table I). The pH ∼6 of the SR-2P in the mixture of the simulated VFS and SFS mixture is near to the ideal conditions of physiological neutral pH of the vagina during the post-intercourse stage (25).
Bioadhesivity of Placebo Gel Formulations
Bioadhesive strength of placebo gel formulations was characterized by a texture analyzer-based method with mini pig vaginal tissues (Fig. 3). The bioadhesivity of each formulation was measured using a TA-XT2 Texture Analyzer (Stable Micro Systems, Surrey, UK) in adhesion mode to measure the peak force required to detach the placebo gel formulation from isolated mini pig vaginal tissue. From the results, it is evident that the SR-2P and placebo gel prototype containing 7.5% w/w Noveon® AA-1 and 0.5% Pluronic® F-127 have shown high peak force of detachment, implying good in vitro bioadhesion (Table II). However, from a practical viewpoint, such a high concentration of Noveon® AA-1 (7.5% w/w) is difficult to fill into an applicator device, as it is extremely viscous (rubbery). Noveon® AA-1-based polymers form gels in water when exposed to pH environment above 4 to 6 (10,23,26). Above their pKa of 6 ± 0.5, the carboxylate groups on the polymer backbone ionize, resulting in repulsion between the anions and further increasing the swelling of the polymer. Cross-linked polymers do not dissolve in water, but form colloidal gel dispersions. The molecular weight of Noveon® AA-1 and related polymers is as high as 3.5 billion that interlink many polymer chains. Due to their chemical nature, these high molecular weight polymers readily swell in water, providing a large adhesive surface for maximum contact with mucin (the glycoprotein predominant in the mucus layer). Generally, carboxylic acid moieties in polyacrylic acid polymers are understood to form noncovalent bonds with the sialic acids in mucin, resulting in adhesion of the polymer to mucin, although the exact mechanism is not yet fully understood (23).
Table II.
Summary of Peak Force of Adhesion of Placebo Gel Formulations Characterized Using Mini Pig Vaginal Tissues
| Formulation ID | Pluronic® F-127 (% w/w) | Noveon® AA-1 (% w/w) | Peak Force of Adhesion (N) |
|---|---|---|---|
| SR-2P | 1 | 1 | 0.09 ± 0.001 |
| 15987-23-1 | 0.5 | 7.5 | 0.13 ± 0.01 |
| 15987-24-1 | 0.5 | 4.5 | 0.06 ± 0.03 |
| 15987-24-2 | 0.5 | 2.25 | 0.02 ± 0.01 |
| 15987-24-3 | 0.5 | 1.25 | 0.01 ± 0.001 |
The bioadhesive capacity of the gel formulation is essential to maximize the residence time and hence the clinical performance. The bioadhesivity of the formulation with vaginal epithelium is influenced by various factors including the nature of the surface of biological substrate, the interfacial layer between the formulation, and biological substrate (23). It was observed during the gel erosion assay that formulations containing a higher percentage of Noveon® AA-1 had a tendency to swell. This may be attributed to the cross-linked nature of the Noveon® AA-1 component of the gels forming an interpenetrating network that allows the gel bolus to imbibe water without undergoing dissolution (24). Consequently, Noveon® AA-1-based formulations would be less susceptible to dissolution and erosion. It has also been reported that the thickening power of pluronics in water increases with hydrophobic molecular weights and ethylene oxide/propylene oxide ratios (20,26). Previously, Hassan and Gallo (27) evaluated the mucoadhesiveness of bioadhesive polymers using the elasticity modulus as an indicator. In this study, the decreased elasticity modulus of the formulations upon dilution with simulated vaginal fluids indicates that the mucoadhesiveness of the formulation may also be reduced by dilution. This effect was attributed to the decrease of elasticity modulus and eventual loss of gelation of the formulation upon dilution with simulated vaginal fluid.
Vaginal Irritation Safety Testing of Placebo Gel Formulations in Mice
For a gel to be successfully used as a vaginal formulation, it needs to be nonirritating to the vaginal tissue, as it otherwise may increase the risk of infection (28). The FDA requires the rabbit vaginal irritation model for preclinical safety testing, as it is the gold-standard model to predict vaginal irritation (29). Rabbits have a simple cuboidal or columnar epithelium that is highly sensitive to mucosal irritants. The absence of a hormonal cycle as a confounding factor simplifies the histopathological analysis in this model. The model is limited, however, since rabbits are markedly different from humans in terms of reproductive organ anatomy and histology, lack of cyclic hormonal and reproductive stages, limited vaginal lactobacilli, neutral vaginal pH, and unresponsiveness to most human genital pathogens. In contrast to rabbits, humans have a stratified squamous vaginal epithelium that is affected by the hormonal cycle (30). Similarly to humans, small rodents such as mice and rats have a stratified squamous epithelium, which has typically been considered less sensitive to vaginal irritants. The height of the stratified squamous epithelium varies during small rodents’ estrous cycle, which is a confounding factor when evaluating the histopathological changes following test article application. To mitigate this problem, small rodents can be conditioned with medroxyprogesterone (Depo-Provera™), which results in thinning of the vaginal epithelium and estrous cycle synchronization. Like humans, mice and rats have a stratified squamous vaginal epithelium, whereas rabbits have a single-layer vaginal epithelium (31). However, significant differences remain, such as the vaginal pH, which is acidic in humans but close to neutral in rabbits, mice, and rats (32). These different animal models should be considered complementary to each other.
We previously developed mouse vaginal irritation models to study the inflammatory response to known vaginal irritants such as nonoxynol-9 (N-9), a previous candidate microbicide that increased HIV infection in clinical trials (33), and benzalkonium chloride (BZK), a contraceptive spermicide that is used outside the US (34). In this present study, we evaluated the vaginal irritation in response to known vaginal irritants N-9, BZK, as well as test articles (TA) 1/SR-2P, TA 2, and TA 3 by histopathology. We observed increased epithelial thinning, erosion, and leukocyte infiltration, as well as decreased mucification after 12 days of treatment with known vaginal irritants (Fig. 8; Table III). In contrast, only minimal changes were observed when treating the animals with SR-2P, suggesting that SR-2P is safe to use as a vaginal microbicide platform gel (Fig. 8; Table III).
Fig. 8.
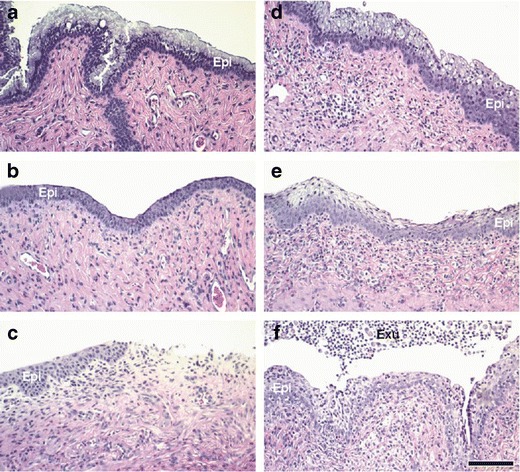
Progesterone-conditioned CD-1 mice were treated intravaginally with a carboxymethylcellulose (CMC) vehicle, b 8.0% N-9, c 2.0% BZK, d TA 1/SR-2P (1% Pluronic® F-127/ 1% Noveon® AA-1), e TA 2 (0.5% Pluronic® F-127/ 7.5% Noveon® AA-1), and f TA 3 (0.5% Pluronic® F-127/ 4.5% Noveon® AA-1) once daily for 12 days. Vaginal tissues were collected 1 day after last dose application, fixed in neutral-buffered formalin, and stained with H&E. After treatment with known vaginal irritants, epithelial thinning (b, c), erosion (c), leukocyte infiltration (b, c), and loss of mucification (b, c) were observed when compared to vehicle-treated animals (a). TA 2 and TA 3 application resulted in leukocyte infiltration and few minimal changes (e, f). In contrast, only minimal changes were observed after SR-2P/TA 1 treatment (d). Epi epithelium, Exu exudate; bar 100 μm
Table III.
Vaginal Histopathology in Progesterone-Conditioned CD-1 Mice
| Histopathology Parameter | Vehicle | N-9 | BZK | TA 1/ SR-2P | TA 2 | TA 3 |
|---|---|---|---|---|---|---|
| Epithelial Thinning | 0 | 1.2 a | 2.2 a | 0.8 | 1.6 | 1 |
| Erosion | 0 | 0.6 | 2.6 | 0 | 0.8 | 0 |
| Exudate | 0.4 | 0.2 | 1.6 | 0 | 0.8 | 1.2 |
| Hemorrhage | 0 | 0 | 0 | 0.6 | 0 | 0.4 |
| Leukocyte Infiltration | 0 | 0.8 | 2.2 | 1.8 | 3 | 3 |
| Keratinization | 0 | 0.2 | 0.2 | 0 | 0 | 0 |
| Mucification | 3.4 | 0.8 | 0.8 | 2.6 | 2 | 1.6 |
aMinimal changes ≥0.5 are highlighted in italics and major changes ≥2 are highlighted with underline
In summary, we have optimized the relative composition of the gel (Fig. 9) to have good buffering capacity and in vitro bioadhesion, and to be nonirritating in vivo. The combination of the two polymers used in this proposed vaginal placebo gel complements each other and helps to withstand dissolution on dilution and application of stress. It is hypothesized that the carboxylic acid moieties in the Noveon polymers retains and swells in the presence of aqueous medium, but the swollen polymer is able to withstand dissolution because of the strength of the pluronic segment that offers additional van der Waals interactions and hydrogen bonding to the polymeric structure. The presence of pluronic segment controls the swelling behavior of the Noveon, as a result of which the bioadhesivity power of the Noveon polymer is not destroyed at the same the pluronic adds mechanical durability to the polymer in the form of “nonswellable” bulk. The interpenetrating network of the Noveon, which discontinuous due to the presence of the pluronic segments, forms a gel, which is difficult to be broken down by dissolution, even on application of force/torque. Future studies will direct towards the development of drug-loaded microbicide gels in SR-2P for vaginal delivery.
Fig. 9.
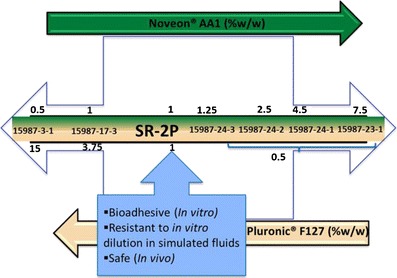
Relative composition of polymers in relation to performance of bioadhesive placebo vaginal gels. Note that the middle horizontal bar defines the molar ratio of the mixture of Noveon and Pluronic polymer
CONCLUSIONS
Novel bioadhesive two-polymer gels were prepared and optimized as a potential microbicide vehicle. The proprietary gel composition SR-2P has shown characteristics of an ideal microbicide candidate. Results have demonstrated that SR-2P retains its gel structure upon dilution at and above the physiologically relevant with simulated fluids. In addition, SR-2P exhibited shear thinning Newtonian behavior under increasing shear rate of 100 and 200 s−1 at 37°C. Moreover, SR-2P maintains the physiological acidic pH of the vagina and the neutral condition of the post-intercourse vaginal environment. SR-2P and a gel prototype with a high amount of Noveon AA-1 have optimal bioadhesive strength with high peak force of adhesion. Our results further demonstrated that the SR-2P platform gel caused no or only minimal signs of irritation when compared to known vaginal irritants like nonoxynol-9 and benzalkonium chloride. Overall, the results presented in this study demonstrated the suitability of SR-2P for use in topical vaginal microbicide applications.
Acknowledgments
We would like to thank SRI International’s Toxicology Technical Services staff for their excellent assistance in performing this study. Additionally, we would like to thank Dr. David Fairchild for his assistance in analyzing the histopathology data.
Research reported in this publication was supported by the National Institute of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R21AI098658.
Disclaimer
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Abbreviations
- BZK
Benzalkonium chloride
- HEC
Hydroxyetheycellulose
- HSV
Herpes simplex virus
- N-9
Nonoxynol-9
- TA
Test article
- SFS
Semen fluid simulant
- VFS
Vaginal fluid simulant
References
- 1.Lisco A, Vanpouille C, Tchesnokov EP, Grivel JC, Biancotto A, Brichacek B, et al. Acyclovir is activated into a HIV-1 reverse transcriptase inhibitor in herpesvirus-infected human tissues. Cell Host Microbe. 2008;4:260–270. doi: 10.1016/j.chom.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(Suppl 1):24A–35A. [PubMed] [Google Scholar]
- 3.Mantell JE, Needham SL, Smit JA, Hoffman S, Cebekhulu Q, Adams-Skinner J, et al. Gender norms in South Africa: implications for HIV and pregnancy prevention among African and Indian women students at a South African tertiary institution. Cult Health Sex. 2009;11:139–157. doi: 10.1080/13691050802521155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myer L, Kuhn L, Stein ZA, Wright TC, Jr, Denny L. Intravaginal practices, bacterial vaginosis, and women's susceptibility to HIV infection: epidemiological evidence and biological mechanisms. Lancet Infect Dis. 2005;12:786–794. doi: 10.1016/S1473-3099(05)70298-X. [DOI] [PubMed] [Google Scholar]
- 5.Global Campaign for Microbicides annual report 2005 to 2006. Available at http://www.global-campaign.org/about_microbicides.htm.
- 6.Abdool KQ, Salim S, Karim A, Janet AF, Anneke CG, Baxter C, et al. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science. 2010;329:1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shankar GN, Burke RL. Bioadhesive delivery system for transmucosal delivery of beneficial agents. U.S. Patent 2009; 7,592,021 B2.
- 8.Milani M, Molteni B, Silvani I. Effect on vaginal pH of a polycarbophil vaginal gel compared with an acidic douche in women with suspected bacterial vaginosis: a randomized, controlled study. Curr Ther Res. 2000;61:781–788. doi: 10.1016/S0011-393X(00)90004-3. [DOI] [Google Scholar]
- 9.Alt C, Harrison T, Zukic M, Mirsalis J, D’Andrea, A. Cytokine measurements in a mouse vaginal irritation model for the safety evaluation of microbicides, presented at the 16th International Inflammation Research Association Conference, 2010, Chantilly, VA.
- 10.Owen DH, Katz DF. A review of the properties of human semen and formulation of a semen simulant. J Androl. 2005;26:459–469. doi: 10.2164/jandrol.04104. [DOI] [PubMed] [Google Scholar]
- 11.Geonnotti AR, Peters JJ, Katz DF. Erosion of microbicide formulation coating layers: effects of contact and shearing with vaginal fluid or semen. J Pharm Sci. 2005;94:1705–1712. doi: 10.1002/jps.20386. [DOI] [PubMed] [Google Scholar]
- 12.Owen DH, Peters JJ, Kieweg SL, Geonnotti AR, Schnaare RL, Katz DF. Biophysical analysis of prototype microbicide gels. J Pharm Sci. 2007;96:661–669. doi: 10.1002/jps.20736. [DOI] [PubMed] [Google Scholar]
- 13.Andrews GP, Donnelly L, Jones DS, Curran RM, Morrow RJ, Woolfson AD, et al. Characterization of the rheological, mucoadhesive, and drug release properties of highly structured gel platforms for intravaginal drug delivery. Biomacromolecules. 2009;10:2427–2435. doi: 10.1021/bm9003332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andrews GP, Jones DS. Rheological characterization of bioadhesive binary polymeric systems designed as platforms for drug delivery implants. Biomacromolecules. 2006;7:899–906. doi: 10.1021/bm050620y. [DOI] [PubMed] [Google Scholar]
- 15.Pillay V, Mashingaidze F, Choonara YE, Du Toit LC, Buchmann E, Maharaj V, et al. Qualitative and quantitative intravaginal targeting: Key to anti-HIV-1 microbicide delivery from test tube to in vivo success. J Pharm Sci. 2012;101:1950–1968. doi: 10.1002/jps.23098. [DOI] [PubMed] [Google Scholar]
- 16.Chopra S, Motwani SK, Iqbal Z, Talegaonkar S, Ahmad FJ, Khar RK. Optimisation of polyherbal gels for vaginal drug delivery by Box-Behnken statistical design. Eur J Pharm Biopharm. 2007;67:120–131. doi: 10.1016/j.ejpb.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 17.Adriaens E, Remon JP. Mucosal irritation potential of personal lubricants relates to product osmolality as detected by the slug mucosal irritation assay. Sex Transm Dis. 2008;35:512–516. doi: 10.1097/OLQ.0b013e3181644669. [DOI] [PubMed] [Google Scholar]
- 18.Lacey CJ, Woodhall S, Qi Z, Sawant S, Cowen M, McCormack S, et al. Unacceptable side effects associated with a hyperosmolar vaginal microbicide in a phase 1 trial. Int J STD AIDS. 2010;21:714–717. doi: 10.1258/ijsa.2010.010215. [DOI] [PubMed] [Google Scholar]
- 19.Owen DH, Peters JJ, Katz DF. Rheological properties of contraceptive gels. Contraception. 2000;62:321–326. doi: 10.1016/S0010-7824(00)00184-0. [DOI] [PubMed] [Google Scholar]
- 20.Chang JY, Oh YK, Choi HG, Kim YB, Kim CK. Rheological evaluation of thermosensitive and mucoadhesive vaginal gels in physiological conditions. Int J Pharm. 2002;241:155–163. doi: 10.1016/S0378-5173(02)00232-6. [DOI] [PubMed] [Google Scholar]
- 21.Gupta KM, Barnes SR, Tangaro RA, Roberts MC, Owen DH, Katz DF, et al. Temperature and pH sensitive hydrogels: an approach towards smart semen-triggered vaginal microbicide vehicles. J Pharm Sci. 2007;96:670–681. doi: 10.1002/jps.20752. [DOI] [PubMed] [Google Scholar]
- 22.Garg S, Anderson RA, Chany CJ, 2nd, Waller DP, Diao XH, Vermani K, et al. Properties of a new acid-buffering bioadhesive vaginal formulation (ACIDFORM) Contraception. 2001;64:67–75. doi: 10.1016/S0010-7824(01)00217-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee JW, Park JH, Robinson JR. Bioadhesive-based dosage forms: the next generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 24.Technical bulletin, number 16 (Bioadhesion), Noveon pharmaceutical polymers, January 2002.
- 25.Tevi-Benissan C, Belec L, Levy M, Schneider-Fauveau V, Si Mohamed A, Hallouin MC, et al. In vivo semen-associated pH neutralization of cervicovaginal secretions. Clin Diagn Lab Immunol. 1997;4:367–374. doi: 10.1128/cdli.4.3.367-374.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Technical bulletin, number 10 (Neutralization procedure), Noveon pharmaceutical polymers, January 2002.
- 27.Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–495. doi: 10.1023/A:1015812615635. [DOI] [PubMed] [Google Scholar]
- 28.Lard-Whiteford SL, Matecka D, O’Rear JJ, Yuen IS, Litterst C, Reichelderfer P, et al. Recommendations for the nonclinical development of topical microbicides for prevention of HIV transmission: an update. J Acquir Immune Defic Syndr. 2004;36:541–552. doi: 10.1097/00126334-200405010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Eckstein P, Jackson MC, Millman N, Sobrero AJ. Comparison of vaginal tolerance tests of spermicidal preparations in rabbits and monkeys. J Reprod Fertil. 1969;20:85–93. doi: 10.1530/jrf.0.0200085. [DOI] [PubMed] [Google Scholar]
- 30.Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel DO. Sex hormone regulation of innate immunity in the female reproductive tract: the role of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol. 2010;63:544–565. doi: 10.1111/j.1600-0897.2010.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Veazey RS, Shattock RJ, Klasse PJ, Moore JP. Animal models for microbicide studies. Curr HIV Res. 2012;10:79–87. doi: 10.2174/157016212799304715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacques M, Olson ME, Crichlow AM, Osborne AD, Costerton JW. The normal microflora of the female rabbit’s genital tract. Can J Vet Res. 1986;50:272–274. [PMC free article] [PubMed] [Google Scholar]
- 33.Van Damme L, Chandeying V, Ramjee G, Rees H, Sirivongrangson P, Laga M, et al. Safety of multiple daily applications of COL-1492, a nonoxynol-9 vaginal gel, among female sex workers. COL-1492 Phase II Study Group. AIDS. 2000;14:85–88. doi: 10.1097/00002030-200001070-00010. [DOI] [PubMed] [Google Scholar]
- 34.Creatsas G, Elsheikh A, Colin P. Safety and tolerability of the new contraceptive sponge Protectaid. Eur J Contracept Reprod Health Care. 2002;2:91–95. doi: 10.1080/ejc.7.2.91.95. [DOI] [PubMed] [Google Scholar]


