Abstract
In this study a simple protocol was developed for purifying phycocyanin (PC) from Spirulina platensis (CCC540) by using ammonium sulphate precipitation, followed by a single step chromatography by using DEAE-Cellulose-11 and acetate buffer. Precipitation with 65 % ammonium sulphate resulted in 80 % recovery of phycocyanin with purity of 1.5 (A620/A280). Thro1ugh chromatography an 80 % recovery of phycocyanin with a purity of 4.5 (A620/A280) was achieved. In SDS_PAGE analysis, the purified PC showed the presence of two subunit α (16 kD) and β (17 kD).
Electronic supplementary material
The online version of this article (doi:10.1007/s40502-014-0094-7) contains supplementary material, which is available to authorized users.
Keywords: Spirulina, DEAE-Cellulose-11, Phycocyanin
Introduction
Cyanobacteria, also known as blue green algae (BGA) are a class of gram negative bacteria, which are considered to be the oldest form of life on the earth. They possess a wide range of coloured components, including carotenoids, chlorophyll and phycobiliproteins. Phycobiliproteins (PBPs) are large water soluble supramolecular protein aggregates involved in light harvesting in these organisms and may comprise as much as 40–60 % of the total soluble protein in these cells (Bogorad 1975). These can be divided broadly into three classes based on their spectral properties: Phycoeryhrin (λmax-565 nm), phycocyanin (λmax-620 nm) and allophycocyanin (λmax-650 nm) (Glazer and Bryant 1975). These are composed of two different kinds of polypeptide of which one is low molecular weight α unit (MW: 12–19 kD) and other is large β unit (MW: 14–21 kD), and are generally present in equimolar amounts (Bernard et al. 1992).
The PBPs, mainly phycocyanin have been widely used as nutritional ingredients, natural dyes, florescent markers (Glazer and Stryer 1984), pharmaceuticals such as antioxidants (Romay and Gonzalez 2000) and anti-inflammatory reagents (Qureshi et al. 1996). Phycocyanin is used as colorant in food (chewing gums, dairy products, gellies etc.) and cosmetics such as lipstick and eye liners in Japan, Thailand and China. It has also shown to have therapeutic value (immuno-modulating activity and anticancer activity) (Lijima and Shimamatsu 1982). Phycocyanin is the most important natural blue pigment used in the food and biotechnology because of their colour, fluorescence and antioxidant properties. Cyanobacteria, as a source of PC are being exploited for a long time. But most studies have focussed on production and purification of PC from Spirulina platensis (Boussiba and Richmond 1979; Hirata et al. 2000; Minkova et al. 2003; Yan et al. 2011).
There are some difficulties in phycocyanin extraction because of multilayered cell walls and large amounts of contaminants (Stewart and Farmer 1984). Several methods have been reported for successful purification of C-PC (Bermejo et al. 2006; Boussiba and Richmond 1979; Chen et al. 2006; Patil et al. 2006; Santiago-Santos et al. 2004; Zhang and Chen 1999), but these methods comprised multiple steps and are time consuming, which may lead to increase in production costs and limit their widespread application.
In the present study we have described a one-step anion exchange chromatography with pH gradient elution for purification of C-Phycocyanin with high purity and recovery from S. Platensis (CCC540). The purity of C-PC was further demonstrated by sodium dodecyl sulfte- polyacrylamide gel electrophoresis (SDS-PAGE) and analysed by HPLC.
Materials and methods
Growth and maintenance of culture
Spirulina platensis (CCC540) was procured from the culture collection of Centre for Conservation and Utilization of Blue Green Algae (CCUBGA), IARI, New Delhi, India. Culture was maintained in chemically defined Z-Medium (Zarrouk 1966) at 28 ± 2 °C under a light intensity of 52–55 µmol photon m−2 s−1 and light/dark cycles of 16:8 h.
Extraction and estimation of C-Phycocyanin
A 500 ml of homogenized log phase (15 days old) culture was centrifuged at 4,000 rpm to obtain pellet. The pellet was suspended in 100 ml of 20 mM acetate buffer containing 50 mM sodium chloride and 0.002 M sodium azide (pH-5.10). C-Phycocyanin was extracted by repeated freezing (−20 °C) and thawing at room temperature until the blue color becomes in acetate buffer (Step I). Cell debris was removed by centrifugation at 5,000 rpm for 10 min and the extract thus obtained was termed as crude extract. Amount of C-PC was measured as described by Bennett and Bogard (1973) and purity was determined by using the formulae: Purity = A620/A280
Purification
The crude extract was subjected to a single step precipitation using 65 % (NH4)2SO4 (Bio Xtra, >99 %; Sigma-Aldrich) and kept overnight at 4 °C. The pellet was recovered by centrifugation at 27,000 rpm for 15 min at 4 °C and dissolved in 10 ml of the same extraction buffer and termed as ammonium sulfate extract (ASE). Ten ml of ASE was dialyzed against the extraction buffer using dialyses membrane (Dialyses membrane-70, MWCO; 12–14 kD) procured from Hi-Media. Dialyses was performed twice against 1,000 ml extraction buffer, first at room temperature and again dialysed against 1,000 ml of extraction buffer at 4 °C overnight. The resultant extract was recovered from the dialyses membrane and filtered through 0.45 µm filter.
DEAE-Cellulose from Sisco Research Laboratory (SRL) was used for anion exchange chromatography. A column (30 × 2 cm) was prepared for purifying the phycocyanin, and equilibrated with 150 ml of acetate buffer (pH-5.10). Dialyzed filtered sample (10 ml) was placed on the column. A linear gradient of acetate buffer with pH ranging from 3.76 to 5.10 was used to developed the column and elutes were collected in 5 ml fractions. Flow rate was kept 20 ml h−1. Absorption spectrum was also determined by scanning the sample in the range of 300–750 nm by using Specord 200 spectrophotometer (Analytikjena, Germany).
SDS-PAGE
A 7.5 % continuous PAGE under non-denaturing conditions was carried out to reconfirm the purity of phycocyanin. The bands were visualized by Coomassie blue staining. Molecular weight of the purified phycocyanin was determined by running Novex Sharp pre stained protein marker along with the sample.
High performance liquid chromatography
Phycobiliprotein subunit separation by HPLC was performed using a reversed phase Discovery BIO Widepore C5 (Supelco, Sigma Aldrich) column (250 × 4.6 mm i.d.) packed with 5 µm porous silica particles (300 angstrom pore diameter). This column was operated at a flow rate of 1 ml min−1 for optimum separation efficiency. All solutions were filtered through 0.5 µm membrane filter and degassed by bubbling with helium before use. Optimization of chromatographic separations was performed using a Alliance system (Waters) with 2695 separation module with auto-sampler consisting of a Waters 2998 Photo Diode-Array detector (PDA) and Waters 2475 Multi λ fluorescence detector. Excitation and emission wavelength for fluorescence detector was set at 580 and 640 nm. The Discovery BIO Widepore C5 column was pre-equilibrated with 20 % (v/v) aqueous acetonitrile (ACN) solution containing 0.1 % (v/v) trifluoroacetic acid (TFA). Twenty µl sample (200 µg ml−1) was injected and elution was performed using a linear gradient from 20 to 100 % (v/v) aqueous ACN (containing 0.1 % TFA) in 45 min. Both PDA and fluorescence detector were connected in series for the detection of biliprotein subunits.
Results and discussion
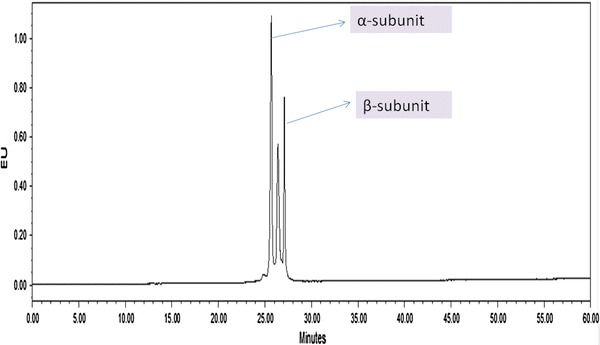
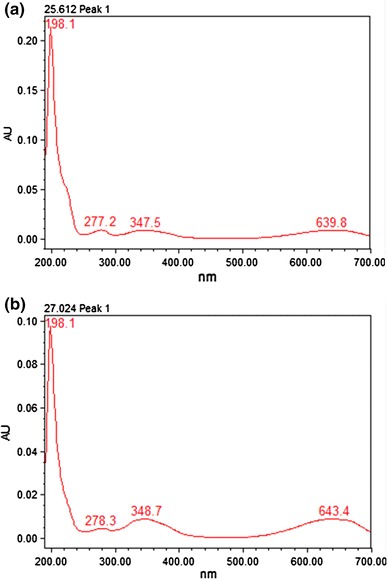
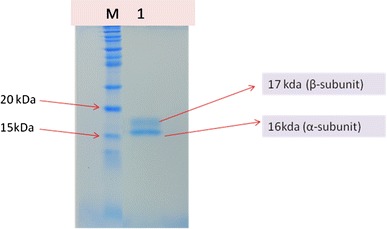
Spirulina is used as a high quality protein mainly for phycocyanin (Eriksen 2008), which is a important cyanobacterial accessory pigment having a number of industrial applications. Although a number of reports are available for the extraction and purification of phycocyanin from cyanobacterial strains (Schoenleber et al. 1984; Swanson et al. 1991). Extraction and purification of phycocyanin, was completed in four major steps: crude extract preparation (Step I), ammonium sulfate precipitation (Step II), dialyses (Step III) and anion exchange chromatography (Step IV) (Fig. 1). After ammonium sulphate precipitation the purity of phycocyanin was 1.5 and after dialyses purity were 2.93. But the final purity (A620/A280) after anion exchange chromatography was 4.58. So from crude extract purification to anion exchange chromatography, purity increased nearly six times. During the chromatographic separation, PC with maximum purity was eluted as a bright blue colored solution at pH 3.76 (Table 1). The absorption spectra of the purified PC showed a prominent peak at 620 nm (Fig. 2). Purity was also reconfirmed by the presence of single bands of α-subunit (16 kDa) and β-subunit (17 kDa) during native gel electrophoresis (Fig 5). In order to further characterize the PC purified from BGA, reverse phase HPLC was performed using C5 column (Fig. 3). PDA detector set at 620 and 226 nm revealed two major peaks at 25.612 and 27.024 min. When absorption spectra of these two chromatogram peaks were critically analyzed, it was found that A620:A280 for the first peak RT = 25.612 min (Fig. 4a) was approximately 1, which is due to the presence of one phycocyanobilin (PCB) chromophore, thus indicating that this peak corresponds to α subunit of PC, while the A620:A280 for the second peak RT = 27.024 min (Fig. 4b) was approximately 2, which is due to the presence of two PCB chromophores and therefore this peak corresponds to β subunit of PC.
Fig. 1.
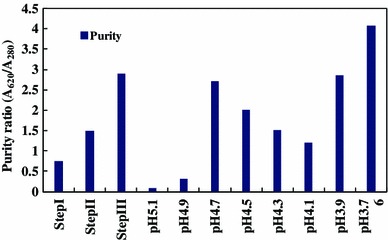
Purity of phycocyanin attained at different steps with a linear gradient of pH from 3.76 to 5.1
Table 1.
Purity and recovery ratio of phycocyanin at each purification steps
| Step | Volume (ml) | PC (µg ml−1) | Purity (A620/A280) | Recovery (%) |
|---|---|---|---|---|
| Crude extract | 200 | 77.4 | 0.75 | 100 |
| Ammonium sulphate pptn | 10 | 123.8 | 1.5 | 80 |
| Dialysis | 10 | 601.4 | 2.93 | 39 |
| DEAE column chromatography | 5 | 413 | 4.58 | 14 |
Fig. 2.
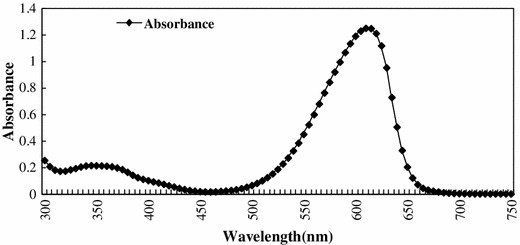
Absorbtion spectrum of purified Phycocyanin
Fig. 5.
SDS-PAGE of purified phycocyanin (M molecular marker, lane 1 PC)
Fig. 3.
Revere phase-HPLC profile of phycocyanin from Spirulina strain using PDA Detector (620 nm) showing α and β subunit peaks
Fig. 4.
a Absorbtion spectra of α-subunit with RT 25.612. b Absorpsion spectra of β-subunit with RT 27.024
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
The authors are thankful to Director IARI, New Delhi for allowing the present study at the institute and to NAIP, ICAR for financial support.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- Bennett, A. (1972). Metabolic and structural investigations of Fremeyella diplosiphon phycobiliproteins. Ph.D. thesis, Harvard University, Cambridge.
- Bennett A, Bogorad L. Comparative chromatic adaptation in a filamentous blue-green alga. Journal of Cell Biology. 1973;58:419–435. doi: 10.1083/jcb.58.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard C, Thomas JC, Mazel D, Mousseau A, Castets AM, Tandeau de Marsac N, Dubacq JP. Characterization of genes encoding phycoerythrin in the red alga Rhodella violacea: evidence for a splitting of the rpeB gene by an intron. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:9564–9568. doi: 10.1073/pnas.89.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogorad L. Phycobiliproteins and complementary chromatic adaptation. Annual Review of Plant Physiology. 1975;26:369–401. doi: 10.1146/annurev.pp.26.060175.002101. [DOI] [Google Scholar]
- Boussiba, S., & Richmond, A. E. (1979). Isolation and characterization of phycocyanins from the blue-green alga Spirulina platensis. Archives of Microbiology, 120, 155–159.
- Bryant DA. Phycoerythrocyanin and phycoerythrin: Properties and occurrence in cyanobacteria. Journal General Microbiology. 1982;128:835–844. [Google Scholar]
- Eriksen NT. Production of phycocyanin—a pigment with applications in biology, biotechnology, foods and medicine. Applied Microbiology and Biotechnology. 2008;80:1–14. doi: 10.1007/s00253-008-1542-y. [DOI] [PubMed] [Google Scholar]
- Glazer AN. Light harvesting by phycobilisomes. Annual Review of Biophysics and Biophysical Chemistry. 1985;14:47–77. doi: 10.1146/annurev.bb.14.060185.000403. [DOI] [PubMed] [Google Scholar]
- Glazer AN, Bryant DA. Allo-phycocyanin B (λmax 671, 618 mm): a new cyanobacterial phycobiliprotein. Archives of Microbiology. 1975;104:15–22. doi: 10.1007/BF00447294. [DOI] [PubMed] [Google Scholar]
- Glazer AN, Cohen-Bazire G. Subunit structure of the phycobiliproteins of blue-green algae. Proceedings of the National Academy of Sciences. 1971;68:1398–1401. doi: 10.1073/pnas.68.7.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A, Bhaya D, Apt K, Kohoe D. Light-harvesting complexes in oxygenic photosynthesis: diversity, control and evolution. Annual Review of Genetics. 1995;29:231–288. doi: 10.1146/annurev.ge.29.120195.001311. [DOI] [PubMed] [Google Scholar]
- Guan X, Qin S, Zhao F, Zhang X, Tang X. Phycobilisomes linker family in cyanobacterial genomes: divergence and evolution. International Journal of Biological Sciences. 2007;3:434–455. doi: 10.7150/ijbs.3.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata, T., Tanaka, M., Ooike, M., Tsunomura, T., & Sakaguchi, M. (2000). Antioxidant activities of phycocyanobilin prepared from Spirulina platensis. Journal of Applied Phycology, 12, 435–439.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MacColl R. Cyanobacterial phycobilisomes. Journal of Structural Biology. 1998;124:311–334. doi: 10.1006/jsbi.1998.4062. [DOI] [PubMed] [Google Scholar]
- MacColl R, Eisele LE, Williams EC, Bowser SS. The discovery of a novel R-phycoerythrin from and Antartic Red Alga. Journal of Biological Chemistry. 1996;271:17157–17160. doi: 10.1074/jbc.271.29.17157. [DOI] [PubMed] [Google Scholar]
- Mieras GA, Wall RA. Sub-units of the algal biliprotein phycoerythrin. Biochemical Journal. 1968;107:127. doi: 10.1042/bj1070127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkova, K. M., Tchernov, A. A., Tchorbadjieva, M. I., Fournadjieva, S. T., Antova, R. E., & Busheva, M. C. (2003). Purification of C-phycocyanin from Spirulina (Arthrospira) fusiformis. Journal of Biotechnology, 102, 55–59. [DOI] [PubMed]
- Oi VT, Glazer AN, Stryer L. Fluorescent phycobiliprotein conjugates for analyses of cells and molecules. Journal of Cell Biology. 1982;93:981–986. doi: 10.1083/jcb.93.3.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong LJ, Glazer AN. Phycoerythrins of marine unicellular cyanobacteria. I. Bilin types and locations and energy transfer pathways in Synechococcus sp. Phycoerythrins. Journal of Biological Chemistry. 1991;266:9515–9527. [PubMed] [Google Scholar]
- Parmar A, Singh NK, Kaushal A, Sonawala S, Madamwar D. Purification, characterization and comparison of phycoerythrins from three different marine cyanobacterial cultures. Bioresource Technology. 2011;102:1795–1802. doi: 10.1016/j.biortech.2010.09.025. [DOI] [PubMed] [Google Scholar]
- Qureshi, M. A., Garlich, J. D., & Kidd, M. T. (1996). Dietary Spirulina platensis enhances humoral and cell-mediated immune functions in chickens. Immunopharmacology and Immunotoxicology, 18, 465–476. [DOI] [PubMed]
- Ranjitha K, Kaushik BD. Purification of phycobiliproteins from Nostoc muscorum. Journal of Scientific and Industrial Research. 2005;64:372–375. [Google Scholar]
- Reis A, Mendes A, Lobo-Fernandes H, Empis JA, Novais JM. Production, extraction and purification of phycobiliproteins from Nostoc sp. Bioresource Technology. 1998;66:181–187. doi: 10.1016/S0960-8524(98)00064-9. [DOI] [Google Scholar]
- Rossano R, Ungaro N, D’Ambrosio A, Liuzzi GM, Riccio P. Extracting and purifying R-phycoerythrin from Mediterranean red algae Corallina elongate Ellis and Solander. Journal of Biotechnology. 2003;101:289–293. doi: 10.1016/S0168-1656(03)00002-6. [DOI] [PubMed] [Google Scholar]
- Schoenleber RW, Lundell DJ, Glazer AN, Rapoport H. Bilin attachment sites in the α and β subunits in B-Phycoerythrin. Structural studies on the singly linked phycoerythrobilins. Journal of Biological Chemistry. 1984;259:5485–5489. [PubMed] [Google Scholar]
- Stanier RY, Kunisawa R, Mandal M, Cohen-Bazire G. Purification and properties of unicellular blue green algae (order Chroococcales) Bacteriological Reviews. 1971;35:171–205. doi: 10.1128/br.35.2.171-205.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson RV, Ong LJ, Wilbanks SM, Glazer AN. Phycoerythrins of marine unicellular cyanobacteria. II. Characterization of phycobiliproteins with unusually high phycourobilin content. Journal of Biological Chemistry. 1991;266:9528–9534. [PubMed] [Google Scholar]
- Tchernov AA, Minkova KM, Houbavenska NB, Kovacheva NG. Purification of phycobiliproteins from Nostoc sp. by aminohexyl-sepharose chromatography. Journal of Biotechnology. 1999;69:69–73. doi: 10.1016/S0168-1656(98)00206-5. [DOI] [Google Scholar]
- Tripathi SN, Kapoor S, Shrivastave A. Extraction and purification of an unusual phycoerythrin in a terrestrial desiccation tolerant cyanobacterium Lyngbya arboricola. Journal of Applied Phycology. 2007;19:441–447. doi: 10.1007/s10811-006-9151-6. [DOI] [Google Scholar]
- Wilbanks SM, Glazer AN. Rod structure of a phycoerythrin II containing phycobilisome. I. Organization and sequence of the gene cluster encoding the major phycobiliprotein rod components in the genome of marine Synechococcus sp. WH8020. Journal of Biological Chemistry. 1993;268:1226–1235. [PubMed] [Google Scholar]
- Yan, S., Zhu, L., Su, H., Zhang, X., Chen, X., Zhou, B., & Zhang, Y. (2011). Single-step chromatography for simultaneous purification of C-phycocyanin and allophycocyanin with high purity and recovery from Spirulina (Arthrospira) platensis. Journal of Applied Phycology, 23, 1–6.
- Zarrouk, C. (1966). Contribution a I’ etude d’ une cyanobacterie: Influence de divers facteurs physiques et chimiques et la photosynthese de Spirulina maxima (Setchell et Gardener) Geitler Ph.D.thesis, University of paris, France.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


