Abstract
Objective
To explore the association between the presence of maternal heart disease and maternal, perinatal, and infant outcomes.
Methods
We conducted a population-based retrospective cohort study using Washington State birth certificates linked with hospital discharge records of mothers noted to have maternal congenital heart disease, ischemic heart disease, heart failure or pulmonary hypertension. Women who gave birth between 1987 and 2009 (n=2,171) were compared to a sample of mothers without these conditions (n=21,710). We described characteristics of pregnant women with heart disease over time. Logistic regression estimated the association between reported chronic maternal heart disease and small for gestational age (SGA) birth, as well as perinatal, post-neonatal and maternal death.
Results
The proportion of births to women with reported heart disease increased 224% between the 1987-1994 and 2002-2009 calendar periods. Chronic maternal heart disease was associated with increased risk of SGA birth (62 additional SGA infants per 1,000 births, 95% CI 46-78, p <0.001), perinatal death (14 additional deaths per 1,000 births, 95% CI 8-20, p <0.001), postneonatal death (five additional deaths per 1,000 births, 95% CI 2-9, p<0.001) and maternal death (five additional deaths per 1,000 births, 95% CI 2-9, p<0.001).
Conclusion
The presence of chronic maternal heart disease is associated with elevated risk for poor maternal, perinatal, and postneonatal outcomes.
Introduction
Pregnancy is accompanied by physiologic changes that stress the heart. Normal pregnancy leads to increased blood volume, heart rate and cardiac output(1). These changes appear to support normal fetal growth(2), but can lead to cardiac hypertrophy, dilation and increased cardiac oxygen requirements in the mother(3). Although these changes are well tolerated in healthy women, women with underlying heart disease may suffer adverse consequences from the additional burden(4,5).
Experience in the United Kingdom suggests that heart disease in pregnancy is an increasing public health concern and now represents the leading cause of death in pregnant women(6). The increase in prevalence is thought to be a manifestation of greater longevity in congenital heart disease patients, a trend toward older mothers with more prevalent diabetes and hypertension, and newer guidelines regarding pregnancy risk(6-8). Epidemiologic studies of maternal heart disease in the United States (U.S.) are sparse. A sole U.S. report suggests that the number of mothers with heart disease and the fraction of these mothers who experience complications have increased over time. This report did not address perinatal outcomes and no reports address post-neonatal outcomes(9).
In a population of mothers giving birth in Washington State between 1987 and 2009, we sought to describe pregnant women with heart disease and examine the associations between maternal heart disease and poor outcomes in mothers and children.
Methods
We conducted a population-based retrospective cohort study using hospital discharge records linked with birth certificate data from all nonfederal hospitals in the Comprehensive Hospital Abstract Reporting System (CHARS) in Washington State. Routine linkage of birth records to hospital discharge records, using a combination of sequential probabilistic and deterministic linkages, has been conducted in Washington State for decades with increased accuracy over either modality alone when compared to chart review(10,11). The study was reviewed by the Human Subjects Division at the University of Washington and was determined to be exempt from full IRB review as the authors only had access to de-identified data (Exempt #41371). The primary research question focused on the following types of chronic heart disease: (a) congenital heart disease; (b) heart failure; (c) ischemic heart disease; and (d) pulmonary hypertension. Pulmonary hypertension was included as it is increasingly recognized as a combined disease of the heart and pulmonary vasculature(12). Mothers with isolated valvular heart disease, peripartum cardiomyopathy, infectious heart disease (e.g., endocarditis or myocarditis), diseases of the electrical system or unspecified heart disease who did not also meet our criteria for chronic heart disease were not included, as we believed these could represent isolated, late and/or acute events. We identified mothers with presumed chronic heart disease as those whose discharge records contained ICD-9 codes for specified conditions during the birth or antecedent hospitalizations between 1987 and 2009 (Appendix). Births to mothers with chronic heart disease were stratified by birth year and the distribution of birth years was used to select births to mothers without heart disease. Within each birth year, the selection of births to mothers without heart disease was performed at random. We utilized a sampling ratio of ten to one (births to mothers without chronic heart disease: births to mothers with chronic heart disease) to provide 80% power to detect a 20% increase in the odds of SGA birth, which is comparable to other identified risk factors for SGA birth(13). For women with multiple births in Washington State, the first recorded birth was used.
Isolated valvular heart disease is a form of chronic heart disease, but we chose not to focus on valvular disease because we were concerned that these mothers would be heterogeneous and include many mothers with benign valvular diseases of pregnancy (e.g., mitral valve prolapse), which have no meaningful systemic effects.
The primary outcome was SGA birth, defined as a birth weight of less than the 10th percentile by week of gestational age for all births in Washington State from 1987-2009(14,15). Gestational age and birth weight were collected from the birth certificate and coded centrally using this Washington based standard as SGA. Secondary outcomes, including perinatal and post-neonatal mortality, were collected from linked death certificates. Perinatal mortality included stillbirth (>20 weeks gestation) and neonatal deaths prior to 28 days of life, excluding elective terminations(16). Post-neonatal mortality included death up to one year of age excluding the perinatal period. Combined perinatal and post-neonatal mortality was also assessed. Maternal mortality was estimated from the CHARS database for mothers who died in any nonfederal Washington State Hospital within 90 days of the birth hospitalization.
Clinical characteristics potentially associated with heart disease and maternal, perinatal and post-neonatal outcomes were selected from birth certificate data, including maternal age, race, body mass index (BMI), smoking status, diabetes, and hypertension. Maternal age and BMI were modeled continuously and other covariables were modeled categorically. Three birth cohorts of 92 months each (January 1987 to August 1994, September 1994 to April 2002, May 2002 to December 2009) were created to describe temporal changes over the study period.
Women with and without heart disease were compared descriptively. This included descriptions of maternal heart disease as a proportion of total births in Washington State using published state-wide birth rates(17). Logistic regression estimated the relationship between maternal heart disease, SGA, perinatal, post-neonatal and maternal mortality (unadjusted model). The odds ratio (OR) was felt to be a reasonable proxy for relative risk given the rarity of all outcomes except SGA where the OR may overestimate the relative risk(18). Model 1 was adjusted for cardiac risk factors including maternal age, race, BMI, smoking status, diabetes and hypertension. For the outcomes of perinatal and post-neonatal mortality, model 2 was further adjusted for birth weight and gestational age in addition to model 1. Twenty datasets with multiple imputation for missing variables from chained equations were used in adjusted models(19). Unadjusted rates of SGA, maternal, perinatal and post-neonatal death per 1,000 births and attributable risk (incidence in the exposed cohort – incidence in the unexposed cohort) were reported. Statistical significance was defined as p<0.05. Analyses were performed using STATA 11.0 (StataCorp, College Station, TX, USA).
Results
There were 1,871,770 births between 1987 and 2009 in Washington State. Among these, 2,171 births were to women met our definition of chronic heart disease and 21,710 births, frequency matched by year, were sampled from women without known chronic heart disease. Of mothers with heart disease, 1,262 had congenital heart disease (58.2%), 532 had heart failure (24.5%), 186 had ischemic heart disease (8.6%), 91 had pulmonary hypertension (4.2%) and 100 women had more than one condition (4.6%).
SGA status was available for 2,106 of 2,171 women with heart disease (97%) and 21,349 of 21,710 women without heart disease (98%). Perinatal and neonatal mortality was completely ascertained. Age, race, smoking status, birth weight, gestational age, diabetes and hypertension were missing in less than 10% of mothers; however, BMI was missing in 30.2% of women.
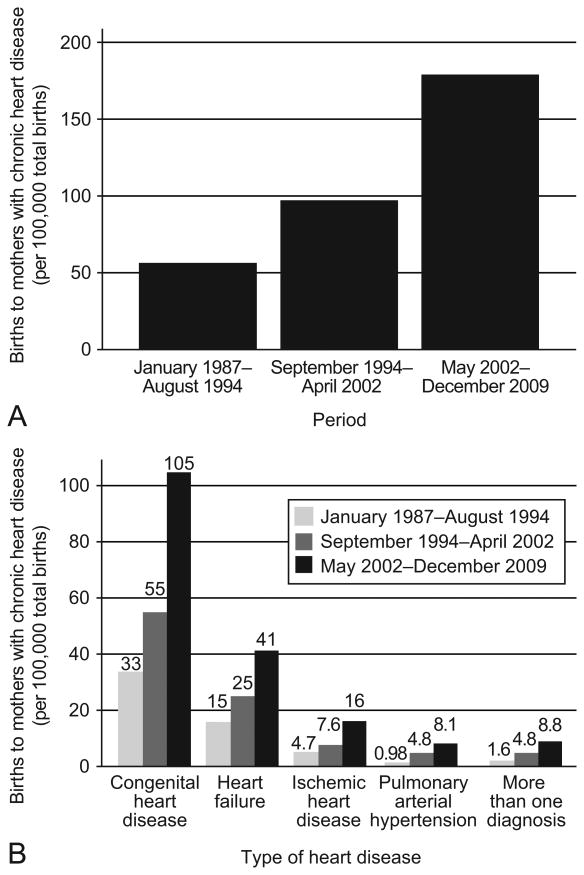
Annual births in Washington increased from 70,409 in 1987 to 89,242 in 2009(17). The proportion of births to mothers with chronic heart disease increased 224% over the study period (56 births increased to 179 per 100,000 births)(Figure 1). The proportion of births to mothers with congenital heart disease increased 218% (33 births increased to 105 per 100,000 births), heart failure increased 173% (15 births increased to 41 per 100,000 births), ischemic heart disease increased 240% (4.7 births increased to 16 per 100,000 births) and pulmonary hypertension increased 727% (1.6 births increased to 8.8 per 100,000 births).
Figure 1.
Proportion of births to mothers with heart disease per 100,000 births, by calendar period, Washington State, 1987–2009. (A) Increase in proportion of births to mothers with heart disease (B) Increase in proportion of births to mothers by type of heart disease (congenital heart disease, ischemic heart disease, or pulmonary arterial hypertension).
Table 1 describes the cohorts. Mothers with chronic heart disease tended to have a higher prevalence of smoking, diabetes and hypertension. Hospital charges were higher and hospitalization longer for mothers with chronic heart disease. Characteristics were similar among the four types of chronic heart disease (data not shown) with the exception that mothers with ischemic heart disease tended to be older (30.4 years old) with a higher prevalence of diabetes (12.6%) and smoking (18.7%). Table 2 describes changes in maternal characteristics over time.
Table 1. Characteristics of Mothers With and Without Heart Disease in Washington State, 1987-2009.
| Women With Heart Disease (n=2,171) | Women Without Heart Disease (n=21,710) | |
|---|---|---|
| Demographics | ||
| Age, years | 26.8 (6.8) | 26.6 (6.2) |
| Race, % | ||
| White | 73.2% | 71.9% |
| Black | 6.7% | 4.1% |
| Asian | 7.6% | 8.4% |
| Other | 12.5% | 15.6% |
| Married | 58.6% | 65.1% |
| Education, years | 12.8 (2.7) | 13.0 (3.0) |
| Income* | $42,850 | $44,003 |
| Comorbidities | ||
| Body mass index, kg/m2 | 25.7 (6.4) | 25.4 (5.9) |
| Smoking | 13.7% | 10.9% |
| Established or gestational diabetes | 6.9% | 4.8% |
| Chronic hypertension | 4.3% | 1.3% |
| Obstetric history | ||
| Number of prior pregnancies | 1.3 (1.9) | 1.1 (1.5) |
| Number of prenatal visits | 11.5 (6.9) | 11.1 (6.0) |
| Birth hospitalization | ||
| Cesarean delivery | 37.1% | 25.0% |
| Length of stay, days | 4.0 (7.6) | 2.3 (1.9) |
| Hospital charges (USD) | $23,622 | $11,558 |
USD, U.S. Dollars.
Data are mean (standard deviation), mean, or percentage unless otherwise specified.
Median income from census tract of residence.
Table 2. Characteristics of Mothers With and Without Heart Disease, by Calendar Period (1-3)* in Washington State, 1987-2009.
| Women With Heart Disease | Women Without Heart Disease | |||||
|---|---|---|---|---|---|---|
| Calendar Period | Calendar Period | |||||
| One | Two | Three | One | Two | Three | |
| n=345 | n=611 | n=1,215 | n=3,427 | n=6,191 | n=12,089 | |
| Demographics | ||||||
| Age, years | 27.2(6.2) | 27.6(6.8) | 26.3(6.9) | 26.5(5.9) | 26.4(6.3) | 26.8(6.2) |
| Race, % | ||||||
| White | 81.2% | 73.8% | 70.7% | 81.6% | 71.0% | 69.6% |
| Non-white | 18.8% | 26.2% | 29.3% | 18.4% | 29.0% | 30.4% |
| Married | 70.9% | 63.5% | 52.6% | 72.2% | 66.1% | 62.6% |
| Education, years | 13.1(2.8) | 12.8(2.8) | 12.8(2.6) | 12.8(2.8) | 12.9(2.9) | 13.1(3.0) |
| Income† | $34,686 | $43,371 | $44,838 | $34,712 | $43,628 | $46,693 |
| Comorbidity | ||||||
| Body mass index, kg/m2 | 23.8(6.5) | 25.5(6.2) | 25.9(6.5) | 24.0(4.9) | 24.4(5.2) | 25.9(6.1) |
| Smoking | 11.6% | 15.0% | 13.4% | 17.7% | 12.6% | 8.7% |
| Established or gestational diabetes | 5.9% | 6.9% | 7.1% | 2.6% | 3.5% | 5.9% |
| Chronic hypertension | 1.6% | 5.2% | 4.6% | 1.1% | 1.2% | 1.3% |
| Obstetric history | ||||||
| Number of prior pregnancies | 1.4(1.9) | 1.3(1.8) | 1.3(2.0) | 1.2(1.6) | 1.0(1.5) | 1.0(1.6) |
| Number of prenatal visits | 13.4(12.8) | 11.7(4.4) | 10.8(4.8) | 12.7(11.4) | 11.2(3.9) | 10.6(4.0) |
| Birth hospitalization | ||||||
| Cesarean delivery | 31.0% | 37.2% | 38.7% | 20.0% | 19.7% | 29.2% |
| Length of stay, days | 4.6(9.4) | 4.3(10.0) | 3.7(5.4) | 2.4(2.1) | 2.1(1.6) | 2.4(1.9) |
| Hospital charges | $9,034 | $32,547 | $31,470 | $4,281 | $16,127 | $16,033 |
| Offspring outcomes | ||||||
| Perinatal and postneonatal death (n per 1,000 births) | 26 | 30 | 29 | 12 | 9 | 9 |
| Small for gestational age | 18.9% | 17.0% | 15.6% | 11.0% | 10.5% | 10.1% |
Data are mean (standard deviation), mean, or percentage unless otherwise specified.
Calendar period: One, December 1987–August 1994; Two, September 1994–April 2002; Three, May 2002–December 2009.
Median income from census tract of residence.
Compared to children of mothers without heart disease, children born to mothers with chronic heart disease had lower mean birth weight (3148g versus 3378g) and shorter mean gestation (38 6/7 versus 39 1/7 weeks). Premature delivery (less than 32 weeks) among women with heart disease occurred in 39 per 1,000 births (n=81) compared to 13 per 1,000 births to mothers without (n=277). SGA birth was significantly more likely in children born to mothers with heart disease compared to children born to mothers without heart disease (62 additional SGA births per 1,000 births, 95% CI 46-78, p-value <0.001)(Table 3). This association was little affected by adjustment for common cardiac risk factors (model 1) and was present within subtypes of chronic non-valvular heart disease.
Table 3. Association Between Maternal Heart Disease* and Poor Outcomes in Washington State, 1987-2009.
| Outcome | With Heart Disease | Without Heart Disease | Attributable Risk, n per 1,000 births | Unadjusted Odds Ratio | Model 1† Odds Ratio | Model 2‡ Odds Ratio |
|---|---|---|---|---|---|---|
| n (per 1,000 births) | n (per 1,000 births) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |
| Maternal Death§ | 12 (6) | 2 (0) | 5 (2-9)‖ | 60 (14-269)‖ | 55 (12-249)‖ | |
| Small for gestational age by | 348 (165) | 2,204 (103) | 62 (46-78)‖ | 1.7 (1.5-1.9)‖ | 1.7 (1.5-1.9)‖ | |
| Pulmonary hypertension | 29 (201) | 2.0(1.3-3.1)‖ | 1.8(1.2-2.8)‖ | |||
| Heart failure | 109 (205) | 2.0(1.6-2.5)‖ | 1.9(1.5-2.4)‖ | |||
| Ischemic heart disease | 47 (216) | 2.1(1.5-3.0)‖ | 2.0(1.4-2.9)‖ | |||
| Congenital heart disease | 201 (154) | 1.5(1.3-1.8)‖ | 1.5(1.3-1.7)‖ | |||
| Perinatal and postneonatal death by | 62 (29) | 205 (9) | 19 (12-26)‖ | 3.1 (2.3-4.1)‖ | 2.8 (2.1-3.8)‖ | 1.7 (1.2-2.5)‖ |
| Pulmonary hypertension | 5 (35) | 3.8 (1.5-9.3)‖ | 3.2(1.3-7.9)‖ | 0.9(0.3-2.9) | ||
| Heart failure | 21 (40) | 4.3 (2.7-6.8)‖ | 3.7(2.3-6.0)‖ | 1.5(0.9-2.7) | ||
| Ischemic heart disease | 6 (28) | 3.0 (1.3-6.8)‖ | 2.4(1.0-5.7)‖ | 1.1(0.4-3.1) | ||
| Congenital heart disease | 34 (26) | 2.8 (1.9-4.1)‖ | 2.7(1.9-3.9)‖ | 2.5(1.6-3.8)‖ | ||
| Perinatal death¶ by | 48 (22) | 176 (8) | 14 (8-20)‖ | 2.8 (2.0-3.8)‖ | 2.5 (1.8-3.5)‖ | 1.3 (0.9-2.0) |
| Pulmonary hypertension | 4 (28) | 3.5(1.3-9.5)‖ | 2.8(1.0-7.9)‖ | 0.6(0.2-2.4) | ||
| Heart failure | 14 (26) | 3.3(1.9-5.7)‖ | 2.7(1.5-4.9)‖ | 0.9(0.4-1.8) | ||
| Ischemic heart disease | 5 (23) | 2.9(1.2-7.1)‖ | 2.2(0.9-5.6) | 0.8(0.2-2.7) | ||
| Congenital heart disease | 26 (20) | 2.5(1.6-3.8)l | 2.4(1.6-3.7)‖ | 2.2(1.3-3.6)‖ | ||
| Postneonatal Death# by | 14 (6) | 29 (1) | 5 (2-9)* | 4.9 (2.6-9.2)* | 4.6 (2.4-8.9)* | 4.0 (2.1-7.8)* |
| Pulmonary hypertension | 1 (7) | 5.2(0.7-38.6) | 5.0(0.7-38.2) | 4.1(0.5-32.3) | ||
| Heart failure | 7(13) | 10.0(4.3-22.9)* | 11.2(4.7-26.3)* | 9.4(3.9-22.9)* | ||
| Ischemic heart disease | 1 (5) | 3.4(0.5-25.4) | 4.3(0.6-33.6) | 3.6(0.4-29.4) | ||
| Congenital heart disease | 8 (6) | 4.6(2.1-10.1)* | 4.0(1.8-8.8)* | 3.7(1.7 - 8.2)* |
CI, confidence interval.
For the subtype analysis, individuals with a combination of heart diseases were included in each component category (pulmonary hypertension, 144 women; heart failure, 532 women; ischemic heart disease, 218; congenital heart disease, 1302).;
Adjusted for education, age, race, body mass index, smoking status, diabetes, and hypertension.
Adjusted for Model 1 covariates plus gestational age and birth weight (model 2 not scientifically appropriate for small for gestational age or maternal death).
Maternal death included deaths between delivery and 90 days postpartum.
P ≤ 0.05
Perinatal death included fetal death after 20 weeks and neonatal death up to 28 days.
Postneonatal death included deaths between 28 and 365 days of life.
Perinatal and post-neonatal deaths were elevated among children of mothers with chronic heart disease (Table 3). Perinatal deaths occurred in 22.1 per 1,000 births (n=48 deaths) to mothers with chronic heart disease compared to 8.1 per 1,000 births (n=176 deaths) to mothers without (14 additional deaths per 1,000 births, 95% CI 8-20, p<0.001). Of these, 17 per 1,000 births (n=37) and 5 per 1,000 births (n=103) were stillbirth in women with and without heart disease respectively. Post-neonatal deaths occurred in 6.4 per 1,000 births (n=14 deaths) to mothers with chronic heart disease compared to 1.3 per 1,000 births (n=29 deaths) to mothers without (5 additional deaths per 1,000 births, 95% CI 2-9, p<0.001). The associations between chronic heart disease, perinatal and post-neonatal mortality remained after adjustment for cardiac risk factors (model 1). With additional adjustment for gestational age and birth weight, chronic heart disease was not associated with perinatal mortality (OR 1.3, 95% CI 0.9-2.0, p=0.16), but remained independently associated with post-neonatal mortality (OR 4.0, 95% CI 2.1-7.8, p<0.001). Risk estimates were similar among types of chronic heart disease. The cause specific post-neonatal death rate to mothers with chronic heart disease appeared higher for sudden infant death syndrome, infection and congenital heart disease. There were no accidental infant deaths among mothers with chronic heart disease (Table 4).
Table 4. Postneonatal Mortality in Washington State, 1987-2009.
| Postneonatal Deaths per 1,000 Births (% of Deaths) | ||
|---|---|---|
|
| ||
| Decedents Among Children of Mothers With Heart Disease (n=14) | Decedents Among Children of Mothers Without Heart Disease (n=29) | |
| All postneonatal deaths | ||
| Cause of death | ||
| Congenital anomaly | 2.3 (36%) | 0.5 (38%) |
| Infection | 1.4 (21%) | 0.05 (3%) |
| Sudden infant death syndrome | 1.8 (29%) | 0.4 (28%) |
| Accidental death | 0 (0%) | 0.2 (17%) |
| Miscellaneous | 0.9 (14%) | 0.2 (14%) |
| Birth characteristics of the deceased | ||
| Mean birth weight, grams (SD) | 2803 (1040) | 3090 (896) |
| Mean gestational age, days (SD) | 263 (26) | 275 (25) |
| Cause of death | Post-neonatal deaths per 1,000 births (% of deaths) | |
| Congenital anomaly | 2.3 (42%) | 0.5 (36%) |
| Infection | 0.5 (8.3%) | 0.05 (4%) |
| Sudden infant death syndrome | 1.8 (33%) | 0.4 (29%) |
| Accidental death | 0 (0%) | 0.2 (18%) |
| Miscellaneous | 0.9 (17%) | 0.2 (14%) |
| Birth characteristics of the deceased | ||
| Mean birth weight, grams (SD) | 3151 (584) | 3181 (770) |
| Mean gestational age, weeks/days (SD) | 38 3/7 (2 1/7) | 38 6/7 (2 6/7) |
Data are n(%) unless otherwise specified.
SD, standard deviation.
Two infants to mothers with heart disease excluded (birth weight 491 grams and 936 grams) and one infant to a mother without cardiac disease excluded (birth weight 637 grams).
Two exploratory analyses of the association between chronic heart disease and post-neonatal mortality were performed. Exclusion of extremely low birth weight infants (ELBW)(<1,000g) did not impact the relationship (unadjusted OR 4.3, 95% CI 2.2-9.5; model 1 OR 4.3, 95% CI 2.2-8.5,all p<0.001). Similarly, analysis restricted to full term births (>37 weeks gestation) did not alter the relationship between maternal heart disease and post-neonatal death (unadjusted OR 4.4, 95% CI 2.0-9.6; model 1 OR 4.3, 95% CI 2.0-9.2, all p<0.001).
Twelve mothers of 2,171 mothers with chronic heart disease died within 90 days of a birth hospitalization (5.5 per 1,000 mothers). One of these women had congenital heart disease and pulmonary hypertension, two had pulmonary hypertension, seven had heart failure and two had ischemic heart disease. Two mothers without chronic heart disease died within 90 days of delivery (0.1 deaths per 1,000 mothers). Maternal deaths in Washington State over the study period averaged 0.1 deaths per 1,000 mothers(17).
Discussion
In this population-based retrospective cohort study in Washington State, the proportion of mothers with chronic heart disease dramatically increased over the 22 years of study. Maternal deaths were more likely in women with chronic heart disease as were SGA birth, perinatal and post-neonatal deaths.
The increase in the proportion of pregnant women with chronic heart disease is similar to one report in the United States (9) and several studies performed in the United Kingdom(6,20). Our study confirms increased maternal heart disease alongside population-wide increases in maternal diabetes, smoking, BMI and hypertension(6-8). The higher frequency of Caesarean section, perinatal death, post-neonatal death and SGA birth among mothers with heart disease was stable over time and mothers with chronic heart disease had longer hospital lengths of stay with higher charges. This reassures us that we accurately describe a similar group of ill mothers across the three birth cohorts with a truly increased prevalence of chronic heart disease rather than an artifact of changes in coding practice.
We included mothers with pulmonary hypertension because of the decreased cardiac output and right ventricular dysfunction that accompanies elevations in pulmonary arterial pressure. Despite firm recommendations for contraception, births to women with pulmonary hypertension saw the greatest increase. This may be related to misclassification given the lack of normative values specific to gravid women where increased cardiac output from pregnancy may exaggerate pulmonary arterial pressures. Similarly, echocardiography was increasingly used during the study period and lacks precision to appropriately identify modest increases in pulmonary pressure without confirmation by right heart catheterization(21). These two factors may have compounded to increase the detection of mild cases with marginal impact in pregnancy. Conversely, ICD-9 codes for pulmonary hypertension may be a reliable, in which case further exploration into this marked rise is warranted(22).
The relationship of maternal heart disease with SGA birth, neonatal and fetal mortality has been described(23,24). We confirm these associations using a U.S. cohort. Furthermore, the magnitude of the association between perinatal death and maternal heart disease is similar to that seen in the United Kingdom(25).
The association between chronic heart disease and post-neonatal mortality has not been previously explored. The most common causes of post-neonatal death are sudden infant death syndrome, congenital abnormalities, accidents/assaults, infections and chronic respiratory disease from birth(26). We see increased post-neonatal death in every category but accidents. As the greatest difference was seen for infectious deaths, we speculated that these deaths might represent delayed death following prolonged hospitalization of sickly neonates. However, elevated risk was present even following restriction of the analysis to full-term pregnancies.
We lack a compelling explanation for the increase in post-neonatal deaths to mothers with chronic heart disease. This may reflect residual confounding from a factor such as smoking or suggest an unrecognized pathway linking subclinical fetal stress to childhood disease susceptibility(27). Alternatively, this association may implicate social factors such as low rates of breastfeeding in mothers with chronic heart disease or avoidance of medical care by mothers following a traumatic birth hospitalization. Further study is needed to reinforce the validity of this finding and then explore potential mechanisms.
Adjustment for diabetes, hypertension and smoking, which contribute to SGA birth (13,28) had little effect on associations with chronic heart disease. Heart disease may confer additional risk beyond a higher density of cardiac risk factors. In a subset of mothers with chronic heart disease this might include inflammation and derangements in hemodynamic and coagulation parameters(29).
Maternal mortality among mothers with chronic heart disease was approximately three times greater than that seen in the previous US study (553 versus 162 deaths per 100,000 women)(9). The previous study only accounts for maternal deaths during birth hospitalizations. When we apply this criterion we find similar maternal mortality (138 deaths per 100,000 women). This suggests that failure to account for maternal death following transfer or in the post-partum period may underestimate the burden of disease. Mortality among mothers with pulmonary hypertension (3%) is less than previously reported (17-50%)(7). When mothers with valvular disease were excluded, we saw death in three women of 43 with pulmonary hypertension (7.0%), which is similar to that seen in a recent case series(30). By comparison, we saw similar mortality among mothers with congenital heart disease (0.07%) to a recent report (0.09%)(8). Our data lacked disease severity metrics, which may allocate risk and these numbers should be used cautiously in the clinical setting(23,24).
This study has several limitations. Unmeasured or residual confounding is possible. Misclassification is present, which would be expected to bias results toward the null.(10,11) Similarly missing data might limit the strength of adjustment, particularly for obesity. Differential misclassification, in which adverse events lead to ICD-9 coding of chronic heart disease, could lead to overestimation the negative ramifications of chronic heart disease. Our approach to try to limit this bias was focus on specific chronic heart diseases, excluding the use of ICD-9 codes for “unspecified heart disease” and diseases with both acute and chronic underpinnings such as sudden cardiac death. This may have led to missed cases and an underestimation of risk.
Acknowledgments
The authors thank William O'Brien for database support, the Washington State Department of Health for data access, and Drs. Stephen Hawes and Beth Mueller (Department of Epidemiology, University of Washington) for their initial recommendations and critiques.
Supported by the National Institutes of Health (T32 HL007287-33).
Appendix: International Classification of Diseases-9 Codes for Selected Maternal Heart Disease
1. Pulmonary Hypertension
416.x Chronic Pulmonary Heart disease
2. Congenital Heart Disease
745.x Bulbus cordis and septal closure anomalies
746.x Other congenital cardiac abnl
747.x Other congenital anomalies of the circulatory system
648.5 Congenital cardiovascular disorders complicating pregnancy childbirth
3. Heart Failure (rheumatic, congestive, hypertensive)/cardiomyopathy
398.91 Rheumatic heart failure
402.01 Malignant htn with heart failure
402.11 benign htn with heart failure
402.91 unspec htn with heart failure (excluded ranges are without heart failure)
404.01 malignant htn AND renal disease with heart failure
404.03 above with renal failure as well
404.11 Benign htn AND renal disease with heart failure
404.13 above with renal failure
404.91 unspecified with heart failure
404.93 and renal failure
425.x cardiomyopathy
428.x Heart Failure
429.3 Cardiomegaly
4. Ischemic Heart Disease
410.x Acute myocardial infarction
411 Other acute and Subacute forms of ischemic heart disease
412 Old Myocardial infarction
414 Other forms of chronic ischemic heart disease
V45.81 Aortocoronary by-pass
V45.82 Percutaneous angioplasty
5. Valvular Heart Disease
394.x Disease of the mitral valve
395.x Disease of the aortic valve
396.x Disease of mitral AND aortic valve
397.x Disease of the tricuspid and/or pulmonary valve
424.0 Mitral Valve disorders
424.1 Aortic Valve Disorders
424.2 Tricuspid Valve Disorders
424.3 Pulmonary Valve Disorders
785.2 Undiagnosed cardiac murmurs
V42.2 Heart valve replacement
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
Contributor Information
Peter J Leary, Email: leary.peterj@gmail.com, University of Washington, Division of Pulmonary and Critical Care Medicine.
Sarah ES Leary, Email: sarah.leary@seattlechildrens.org, University of Washington, Fred Hutchinson Cancer Research Center, Seattle Children's Hospital, Department of Pediatrics.
Karen K Stout, Email: stoutk@uw.edu, University of Washington, Division of Cardiology.
Stephen M Schwartz, Email: sschwart@fhcrc.org, Fred Hutchinson Cancer Research Center, Department of Epidemiology.
Thomas R Easterling, Email: easter@uw.edu, University of Washington, Department of Obstetrics and Gynecology.
References
- 1.Clapp JF, Capeless E. Cardiovascular function before, during, and after the first and subsequent pregnancies. AJC. 1997 Dec 1;80(11):1469–73. doi: 10.1016/s0002-9149(97)00738-8. [DOI] [PubMed] [Google Scholar]
- 2.Nisell H, Lunell NO, Linde B. Maternal hemodynamics and impaired fetal growth in pregnancy-induced hypertension. Obstet Gynecol. 1988 Feb;71(2):163–6. [PubMed] [Google Scholar]
- 3.Simmons LA, Gillin AG, Jeremy RW. Structural and functional changes in left ventricle during normotensive and preeclamptic pregnancy. Am J Physiol Heart Circ Physiol. 2002 Oct;283(4):H1627–33. doi: 10.1152/ajpheart.00966.2001. [DOI] [PubMed] [Google Scholar]
- 4.Harris IS. Management of pregnancy in patients with congenital heart disease. Progress in Cardiovascular Diseases. 2011;53(4):305–11. doi: 10.1016/j.pcad.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelson E, Curry R, Gatzoulis MA, Swan L, Lupton M, Steer P, et al. Effect of maternal heart disease on fetal growth. Obstet Gynecol. 2011 Apr;117(4):886–91. doi: 10.1097/AOG.0b013e31820cab69. [DOI] [PubMed] [Google Scholar]
- 6.Gelson E, Gatzoulis MA, Steer P, Johnson MR. Heart disease--why is maternal mortality increasing? BJOG. 2009 Apr;116(5):609–11. doi: 10.1111/j.1471-0528.2008.02082.x. [DOI] [PubMed] [Google Scholar]
- 7.Simpson LL. Maternal cardiac disease: update for the clinician. Obstet Gynecol. 2012 Feb;119(2 Pt 1):345–59. doi: 10.1097/AOG.0b013e318242e260. [DOI] [PubMed] [Google Scholar]
- 8.Karamlou T, Diggs BS, McCrindle BW, Welke KF. A growing problem: maternal death and peripartum complications are higher in women with grown-up congenital heart disease. Ann Thorac Surg. 2011 Dec;92(6):2193–8. 2198–9. doi: 10.1016/j.athoracsur.2011.05.088. discussion. [DOI] [PubMed] [Google Scholar]
- 9.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995-2006. BJOG. 2011 Feb;118(3):345–52. doi: 10.1111/j.1471-0528.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 10.Parrish KM, Holt VL, Connell FA, Williams B, LoGerfo JP. Variations in the accuracy of obstetric procedures and diagnoses on birth records in Washington State, 1989. American Journal of Epidemiology. 1993 Jul 15;138(2):119–27. doi: 10.1093/oxfordjournals.aje.a116834. [DOI] [PubMed] [Google Scholar]
- 11.Lydon-Rochelle MT, Holt VL, Nelson JC, Cárdenas V, Gardella C, Easterling TR, et al. Accuracy of reporting maternal in-hospital diagnoses and intrapartum procedures in Washington State linked birth records. Paediatr Perinat Epidemiol. 2005 Nov;19(6):460–71. doi: 10.1111/j.1365-3016.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 12.Kawut SM, Al-Naamani N, Agerstrand C, Berman Rosenzweig E, Rowan C, Barst RJ, et al. Determinants of Right Ventricular Ejection Fraction in Pulmonary Arterial Hypertension. Chest. 2009 Mar 1;135(3):752–9. doi: 10.1378/chest.08-1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009 Dec;23(6):779–93. doi: 10.1016/j.bpobgyn.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstet Gynecol. 1982 May;59(5):624–32. [PubMed] [Google Scholar]
- 15.Lipsky S, Easterling TR, Holt VL, Critchlow CW. Detecting small for gestational age infants: the development of a population-based reference for Washington state. Am J Perinatol. 2005 Nov;22(8):405–12. doi: 10.1055/s-2005-872595. [DOI] [PubMed] [Google Scholar]
- 16.Barfield WD. Committee on Fetus and Newborn. Standard terminology for fetal, infant, and perinatal deaths. Pediatrics. 2011 Jul;128(1):177–81. doi: 10.1542/peds.2011-1037. [DOI] [PubMed] [Google Scholar]
- 17.Health WSDO. Washington State Vital Statistics and Induced Terminations of Pregnancy, 2006. 2009 Feb 11;:1–103. [Google Scholar]
- 18.Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA: The Journal of the American Medical Association. 1998 Nov 18;280(19):1690–1. doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
- 19.White IR, Royston P, Wood AM. Multiple imputation using chained equations: Issues and guidance for practice. Statist Med. 2011 Feb 20;30(4):377–99. doi: 10.1002/sim.4067. [DOI] [PubMed] [Google Scholar]
- 20.Cantwell R, Clutton-Brock T, Cooper G, Dawson A, Drife J, Garrod D, et al. Saving Mothers' Lives: Reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011 Mar;118(Suppl 1):1–203. doi: 10.1111/j.1471-0528.2010.02847.x. [DOI] [PubMed] [Google Scholar]
- 21.Rich JD, Shah SJ, Swamy RS, Kamp A, Rich S. Inaccuracy of Doppler echocardiographic estimates of pulmonary artery pressures in patients with pulmonary hypertension. Chest. American College of Chest Physicians. 2011;139(5):988–93. doi: 10.1378/chest.10-1269. [DOI] [PubMed] [Google Scholar]
- 22.Link J, Glazer C, Torres F, Chin K. International Classification of Diseases coding changes lead to profound declines in reported idiopathic pulmonary arterial hypertension mortality and hospitalizations: implications for database studies. Chest. 2011 Mar;139(3):497–504. doi: 10.1378/chest.10-0837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, et al. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001 Jul 31;104(5):515–21. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 24.Siu SC, Sermer M, Harrison DA, Grigoriadis E, Liu G, Sorensen S, et al. Risk and predictors for pregnancy-related complications in women with heart disease. Circulation. 1997 Nov 4;96(9):2789–94. doi: 10.1161/01.cir.96.9.2789. [DOI] [PubMed] [Google Scholar]
- 25.Gelson E, Curry R, Gatzoulis MA, Swan L, Lupton M, Steer P, et al. Effect of maternal heart disease on fetal growth. Obstet Gynecol. 2011 Apr;117(4):886–91. doi: 10.1097/AOG.0b013e31820cab69. [DOI] [PubMed] [Google Scholar]
- 26.Heron M. Deaths: leading causes for 2007. Natl Vital Stat Rep. 2011 Aug 26;59(8):1–95. [PubMed] [Google Scholar]
- 27.McDade TW. Life history, maintenance, and the early origins of immune function. Am J Hum Biol. 2005;17(1):81–94. doi: 10.1002/ajhb.20095. [DOI] [PubMed] [Google Scholar]
- 28.Morgan AR, Thompson JMD, Murphy R, Black PN, Lam WJ, Ferguson LR, et al. Obesity and diabetes genes are associated with being born small for gestational age: results from the Auckland Birthweight Collaborative study. BMC Med Genet. 2010;11:125. doi: 10.1186/1471-2350-11-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Balci A, Sollie KM, Mulder BJM, de Laat MWM, Roos-Hesselink JW, van Dijk APJ, et al. Associations between cardiovascular parameters and uteroplacental Doppler (blood) flow patterns during pregnancy in women with congenital heart disease: Rationale and design of the Zwangerschap bij Aangeboren Hartafwijking (ZAHARA) II study. Am Heart J. 2011 Feb;161(2):269–275.e1. doi: 10.1016/j.ahj.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 30.Curry R, Fletcher C, Gelson E, Gatzoulis M, Woolnough M, Richards N, et al. Pulmonary hypertension and pregnancy-a review of 12 pregnancies in nine women. BJOG. 2012 Mar 6; doi: 10.1111/j.1471-0528.2012.03295.x. [DOI] [PubMed] [Google Scholar]