Abstract
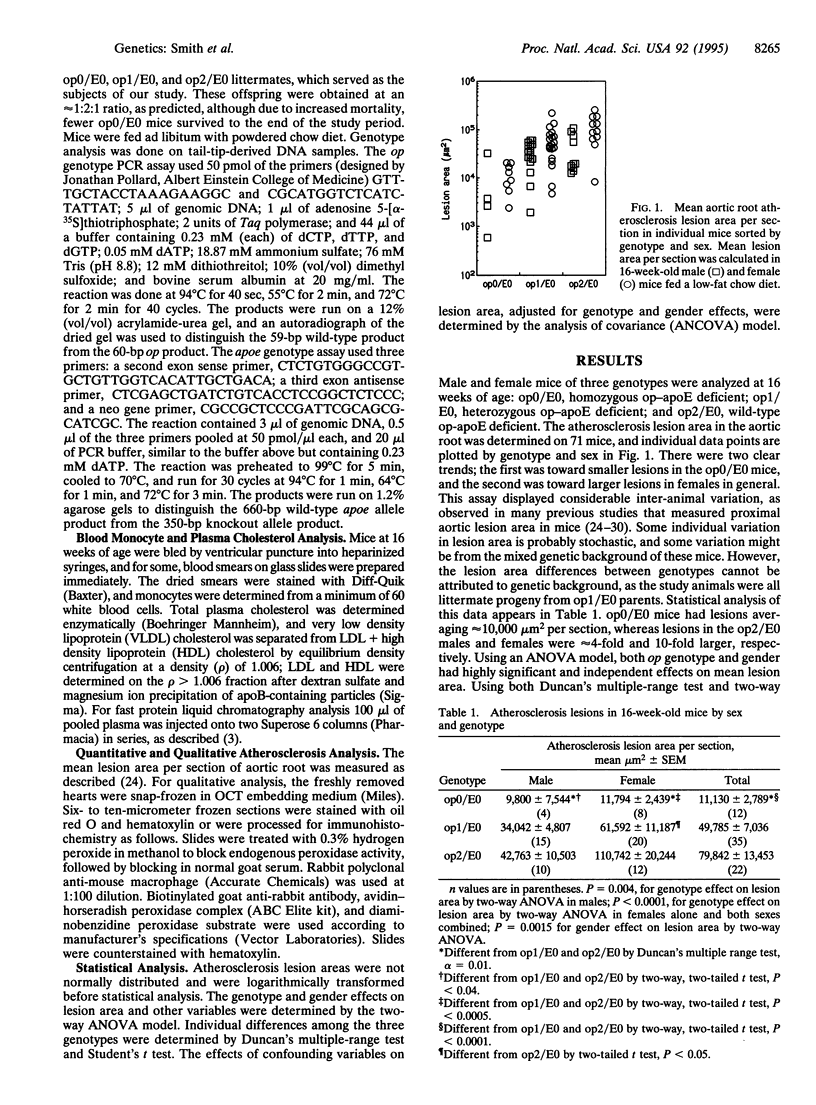
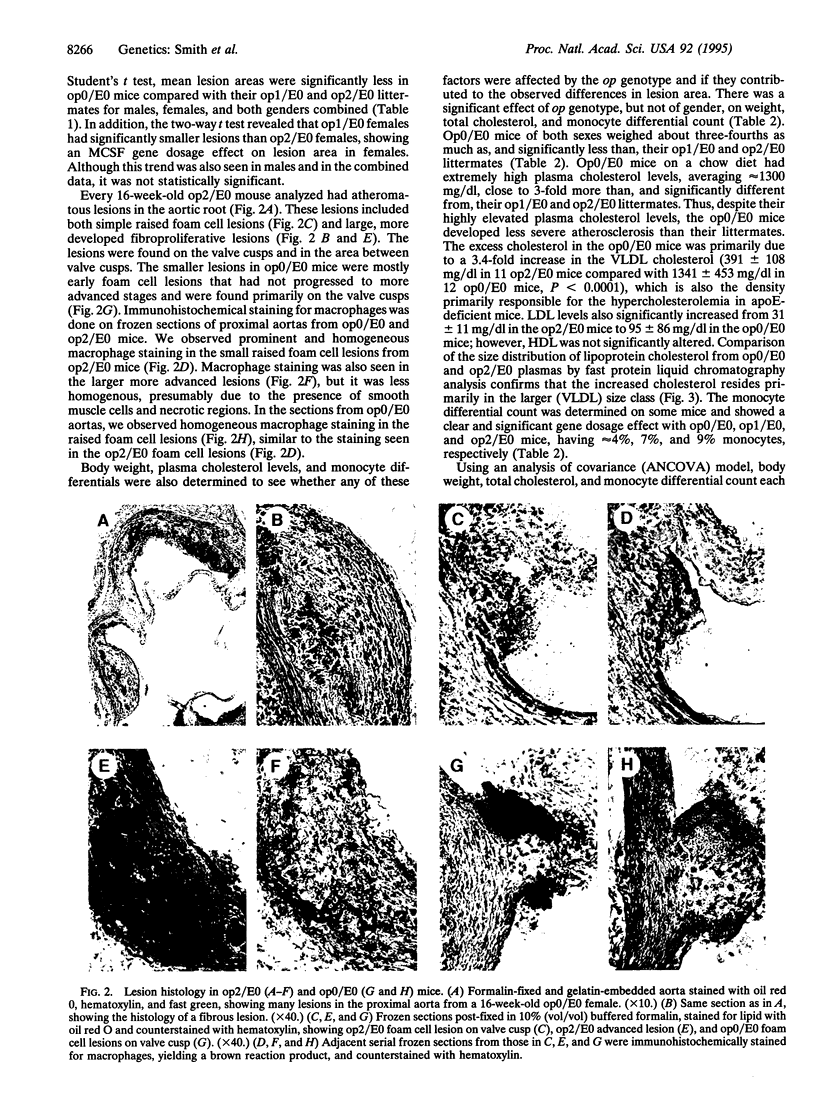
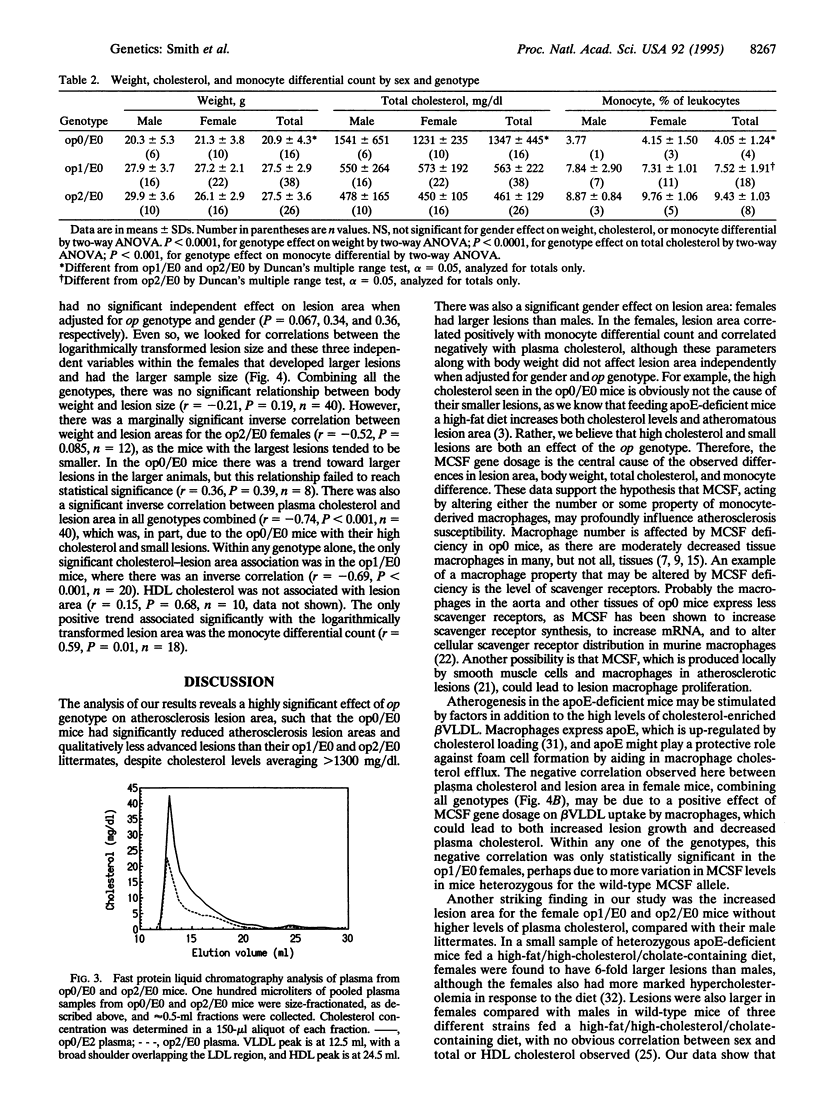
To develop a murine model system to test the role of monocyte-derived macrophage in atherosclerosis, the osteopetrotic (op) mutation in the macrophage colony-stimulating factor gene was bred onto the apolipoprotein E (apoE)-deficient background. The doubly mutant (op/apoE-deficient) mice fed a low-fat chow diet had significantly smaller proximal aortic lesions at an earlier stage of progression than their apoE-deficient control littermates. These lesions in the doubly mutant mice were composed of macrophage foam cells. The op/apoE-deficient mice also had decreased body weights, decreased blood monocyte differentials, and increased mean cholesterol levels of approximately 1300 mg/dl. Statistical analysis determined that atherosclerosis lesion area was significantly affected by the op genotype and gender. The confounding variables of body weight, plasma cholesterol, and monocyte differential, which were all affected by op genotype, had no significant additional effect on lesion area once they were adjusted for the effects of op genotype and gender. Unexpectedly, there was a significant inverse correlation between plasma cholesterol and lesion area, implying that each may be the result of a common effect of macrophage colony-stimulating factor levels. The data support the hypothesis that macrophage colony-stimulating factor and its effects on macrophage development and function play a key role in atherogenesis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basu S. K., Ho Y. K., Brown M. S., Bilheimer D. W., Anderson R. G., Goldstein J. L. Biochemical and genetic studies of the apoprotein E secreted by mouse macrophages and human monocytes. J Biol Chem. 1982 Aug 25;257(16):9788–9795. [PubMed] [Google Scholar]
- Begg S. K., Radley J. M., Pollard J. W., Chisholm O. T., Stanley E. R., Bertoncello I. Delayed hematopoietic development in osteopetrotic (op/op) mice. J Exp Med. 1993 Jan 1;177(1):237–242. doi: 10.1084/jem.177.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchini M. G., Dominguez M. G., Mocci S., Wetterwald A., Felix R., Fleisch H., Chisholm O., Hofstetter W., Pollard J. W., Stanley E. R. Role of colony stimulating factor-1 in the establishment and regulation of tissue macrophages during postnatal development of the mouse. Development. 1994 Jun;120(6):1357–1372. doi: 10.1242/dev.120.6.1357. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Fazio S., Sanan D. A., Lee Y. L., Ji Z. S., Mahley R. W., Rall S. C., Jr Susceptibility to diet-induced atherosclerosis in transgenic mice expressing a dysfunctional human apolipoprotein E(Arg 112,Cys142). Arterioscler Thromb. 1994 Nov;14(11):1873–1879. doi: 10.1161/01.atv.14.11.1873. [DOI] [PubMed] [Google Scholar]
- Fyfe A. I., Qiao J. H., Lusis A. J. Immune-deficient mice develop typical atherosclerotic fatty streaks when fed an atherogenic diet. J Clin Invest. 1994 Dec;94(6):2516–2520. doi: 10.1172/JCI117622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue I., Inaba T., Motoyoshi K., Harada K., Shimano H., Kawamura M., Gotoda T., Oka T., Shiomi M., Watanabe Y. Macrophage colony stimulating factor prevents the progression of atherosclerosis in Watanabe heritable hyperlipidemic rabbits. Atherosclerosis. 1992 Apr;93(3):245–254. doi: 10.1016/0021-9150(92)90261-e. [DOI] [PubMed] [Google Scholar]
- Ishibashi S., Inaba T., Shimano H., Harada K., Inoue I., Mokuno H., Mori N., Gotoda T., Takaku F., Yamada N. Monocyte colony-stimulating factor enhances uptake and degradation of acetylated low density lipoproteins and cholesterol esterification in human monocyte-derived macrophages. J Biol Chem. 1990 Aug 25;265(24):14109–14117. [PubMed] [Google Scholar]
- Kodama H., Yamasaki A., Nose M., Niida S., Ohgame Y., Abe M., Kumegawa M., Suda T. Congenital osteoclast deficiency in osteopetrotic (op/op) mice is cured by injections of macrophage colony-stimulating factor. J Exp Med. 1991 Jan 1;173(1):269–272. doi: 10.1084/jem.173.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn R. M., Wade D. P., Hammer R. E., Chiesa G., Verstuyft J. G., Rubin E. M. Atherogenesis in transgenic mice expressing human apolipoprotein(a) Nature. 1992 Dec 17;360(6405):670–672. doi: 10.1038/360670a0. [DOI] [PubMed] [Google Scholar]
- Li H., Cybulsky M. I., Gimbrone M. A., Jr, Libby P. An atherogenic diet rapidly induces VCAM-1, a cytokine-regulatable mononuclear leukocyte adhesion molecule, in rabbit aortic endothelium. Arterioscler Thromb. 1993 Feb;13(2):197–204. doi: 10.1161/01.atv.13.2.197. [DOI] [PubMed] [Google Scholar]
- Linton M. F., Atkinson J. B., Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995 Feb 17;267(5200):1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- Liu A. C., Lawn R. M., Verstuyft J. G., Rubin E. M. Human apolipoprotein A-I prevents atherosclerosis associated with apolipoprotein[a] in transgenic mice. J Lipid Res. 1994 Dec;35(12):2263–2267. [PubMed] [Google Scholar]
- Motoyoshi K., Takaku F. Serum cholesterol-lowering activity of human monocytic colony-stimulating factor. Lancet. 1989 Aug 5;2(8658):326–327. doi: 10.1016/s0140-6736(89)90505-9. [DOI] [PubMed] [Google Scholar]
- Naito M., Hayashi S., Yoshida H., Nishikawa S., Shultz L. D., Takahashi K. Abnormal differentiation of tissue macrophage populations in 'osteopetrosis' (op) mice defective in the production of macrophage colony-stimulating factor. Am J Pathol. 1991 Sep;139(3):657–667. [PMC free article] [PubMed] [Google Scholar]
- Nakashima Y., Plump A. S., Raines E. W., Breslow J. L., Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994 Jan;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Palinski W., Ord V. A., Plump A. S., Breslow J. L., Steinberg D., Witztum J. L. ApoE-deficient mice are a model of lipoprotein oxidation in atherogenesis. Demonstration of oxidation-specific epitopes in lesions and high titers of autoantibodies to malondialdehyde-lysine in serum. Arterioscler Thromb. 1994 Apr;14(4):605–616. doi: 10.1161/01.atv.14.4.605. [DOI] [PubMed] [Google Scholar]
- Plump A. S., Scott C. J., Breslow J. L. Human apolipoprotein A-I gene expression increases high density lipoprotein and suppresses atherosclerosis in the apolipoprotein E-deficient mouse. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9607–9611. doi: 10.1073/pnas.91.20.9607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plump A. S., Smith J. D., Hayek T., Aalto-Setälä K., Walsh A., Verstuyft J. G., Rubin E. M., Breslow J. L. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992 Oct 16;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Pollard J. W., Hennighausen L. Colony stimulating factor 1 is required for mammary gland development during pregnancy. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9312–9316. doi: 10.1073/pnas.91.20.9312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard J. W., Hunt J. S., Wiktor-Jedrzejczak W., Stanley E. R. A pregnancy defect in the osteopetrotic (op/op) mouse demonstrates the requirement for CSF-1 in female fertility. Dev Biol. 1991 Nov;148(1):273–283. doi: 10.1016/0012-1606(91)90336-2. [DOI] [PubMed] [Google Scholar]
- Qiao J. H., Xie P. Z., Fishbein M. C., Kreuzer J., Drake T. A., Demer L. L., Lusis A. J. Pathology of atheromatous lesions in inbred and genetically engineered mice. Genetic determination of arterial calcification. Arterioscler Thromb. 1994 Sep;14(9):1480–1497. doi: 10.1161/01.atv.14.9.1480. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schaub R. G., Bree M. P., Hayes L. L., Rudd M. A., Rabbani L., Loscalzo J., Clinton S. K. Recombinant human macrophage colony-stimulating factor reduces plasma cholesterol and carrageenan granuloma foam cell formation in Watanabe heritable hyperlipidemic rabbits. Arterioscler Thromb. 1994 Jan;14(1):70–76. doi: 10.1161/01.atv.14.1.70. [DOI] [PubMed] [Google Scholar]
- Stoudemire J. B., Garnick M. B. Effects of recombinant human macrophage colony-stimulating factor on plasma cholesterol levels. Blood. 1991 Feb 15;77(4):750–755. [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W. W., Ahmed A., Szczylik C., Skelly R. R. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982 Nov 1;156(5):1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Bartocci A., Ferrante A. W., Jr, Ahmed-Ansari A., Sell K. W., Pollard J. W., Stanley E. R. Total absence of colony-stimulating factor 1 in the macrophage-deficient osteopetrotic (op/op) mouse. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4828–4832. doi: 10.1073/pnas.87.12.4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiktor-Jedrzejczak W., Urbanowska E., Aukerman S. L., Pollard J. W., Stanley E. R., Ralph P., Ansari A. A., Sell K. W., Szperl M. Correction by CSF-1 of defects in the osteopetrotic op/op mouse suggests local, developmental, and humoral requirements for this growth factor. Exp Hematol. 1991 Nov;19(10):1049–1054. [PubMed] [Google Scholar]
- Witmer-Pack M. D., Hughes D. A., Schuler G., Lawson L., McWilliam A., Inaba K., Steinman R. M., Gordon S. Identification of macrophages and dendritic cells in the osteopetrotic (op/op) mouse. J Cell Sci. 1993 Apr;104(Pt 4):1021–1029. doi: 10.1242/jcs.104.4.1021. [DOI] [PubMed] [Google Scholar]
- Yoshida H., Hayashi S., Kunisada T., Ogawa M., Nishikawa S., Okamura H., Sudo T., Shultz L. D., Nishikawa S. The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature. 1990 May 31;345(6274):442–444. doi: 10.1038/345442a0. [DOI] [PubMed] [Google Scholar]
- de Villiers W. J., Fraser I. P., Hughes D. A., Doyle A. G., Gordon S. Macrophage-colony-stimulating factor selectively enhances macrophage scavenger receptor expression and function. J Exp Med. 1994 Aug 1;180(2):705–709. doi: 10.1084/jem.180.2.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree J. H., van den Broek W. J., Dahlmans V. E., Groot P. H., Vidgeon-Hart M., Frants R. R., Wieringa B., Havekes L. M., Hofker M. H. Diet-induced hypercholesterolemia and atherosclerosis in heterozygous apolipoprotein E-deficient mice. Atherosclerosis. 1994 Nov;111(1):25–37. doi: 10.1016/0021-9150(94)90188-0. [DOI] [PubMed] [Google Scholar]




