Summary
Integrin α5β1 is essential for vascular development but it remains unclear precisely where and how it functions. Here, we report that deletion of the gene encoding the integrin-α5 subunit [Itga5] using the Pdgfrb-Cre transgenic mouse line, leads to oedema, haemorrhage and increased levels of embryonic lethality. Unexpectedly, these defects were not caused by loss of α5 from Pdgfrb-Cre expressing mural cells (pericytes and vascular smooth muscle cells), which wrap around the endothelium and stabilise blood vessels, nor by defects in the heart or great vessels, but were due to abnormal development of the lymphatic vasculature. Reminiscent of the pathologies seen in the human lymphatic malformation, fetal cystic hygroma, α5 mutants display defects both in the separation of their blood and lymphatic vasculature and in the formation of the lymphovenous valves. As a consequence, α5-deficient mice develop dilated, blood-filled lymphatic vessels and lymphatic capillaries that are ectopically covered with smooth muscle cells. Analysis of the expression of Pdgfrb during lymphatic development suggests that these defects probably arise from loss of α5β1 integrin in subsets of specialised Prox1+ Pdgfr + venous endothelial cells that are essential for the separation of the jugular lymph sac from the cardinal vein and formation of the lymphovenous valve leaflets.
Keywords: Integrin, mural cells, lymphatic vessels, PDGFRβ
Introduction
The integrin family of extracellular matrix receptors are key regulators of both blood and lymphatic vessel development (Astrof and Hynes, 2009; Avraamides et al., 2008; Chen et al., 2012; Hynes, 2007; Tammela and Alitalo, 2010). Multiple integrins, including the fibronectin receptors (α5β1, αvβ3, αvβ5, αvβ6, α4β1), have been implicated to one degree or another in angiogenesis and a number of integrin inhibitors have entered clinical trials to test their ability to prevent tumour angiogenesis (Goodman and Picard, 2012). Integrin α5β1 binds the Arg-Gly-Asp (RGD) motif in fibronectin and is the predominant fibronectin-binding integrin. α5β1 is poorly expressed on quiescent endothelium, but is upregulated during developmental and tumour angiogenesis (Kim et al., 2000; Xie et al., 2011). Genetic ablation of α5, or its major ligand, fibronectin, results in embryonic lethality at ~E10.5 due to severe vascular defects (Francis et al., 2002; George et al., 1997; George et al., 1993; Yang et al., 1993). Furthermore, inhibition of α5β1 leads to decreases in angiogenesis and tumour growth in both chicken and mouse models (Kim et al., 2000; Muether et al., 2007; Umeda et al., 2006).
Some studies have also implicated α5β1 integrin in development of the lymphatic vasculature (Chen et al., 2012). The main function of the lymphatic system is to maintain tissue fluid balance (Földi et al., 2005). Blind-ended lymphatic capillaries collect excess interstitial fluid extravasated from the blood and drain it back via collecting lymphatic vessels, lymph nodes and finally the lymphatic duct to the venous circulation (Tammela and Alitalo, 2010). Within collecting lymphatic vessels, intra-luminal valves (herein referred to as lymphatic valves) ensure unidirectional lymphatic flow (Bazigou and Makinen, 2013), while at the junctions of the lymphatic ducts with the venous circulation, lymphovenous valves allow fluid to drain into the veins and prevent blood from entering into the lymphatic circulation. In vitro, α5β1 is required for activation of vascular endothelial growth factor receptor-3 (VEGFR-3) (Zhang et al., 2005), which plays an essential role in lymphangiogenesis (Alitalo, 2011). Proliferation and growth of lymphatic endothelial cells (ECs) has also been blocked by small-molecule antagonists of α5β1 in corneal and airway inflammation models in vivo (Dietrich et al., 2007; Okazaki et al., 2009).
The role of endothelial α5β1 during development however remains unclear due to compensation by other integrins and the influence of strain-dependent genetic modifiers (van der Flier et al., 2010). Ablation of endothelial α5β1 fails to produce the major defects seen with global knockouts of α5 (van der Flier et al., 2010). On a 129S4 background, Tie2-Cre-mediated deletion of Itga5 leads to late embryonic lethality apparently due to patent ductus arteriosus (van der Flier et al., 2010). Ablation of both α5 and αv integrin subunits in endothelial cells produced remodelling defects in the major vessels but these were still much less severe than observed in the global α5 knockout mice. These results suggest that α5β1 integrin functions in other cell types to contribute to the observed vascular defects in α5-null embryos.
Much less information exists about the roles of integrins on mural cells, which wrap around both blood and collecting lymphatic vessels. The physiological importance of mural cells in blood vessel development can be seen in mice that lack platelet-derived-growth-factor-B (PDGF-B), or its receptor, PDGF receptor-β (PDGFRβ). PDGF-B is secreted from ECs and promotes the proliferation and migration of PDGFRβ-positive mural cell precursors to the vessel wall (Hirschi et al., 1999). Loss of either PDGF-B or PDGFRβ results in blood vessels that lack, or are incompletely covered by, mural cells (Leveen et al., 1994; Soriano, 1994). As a consequence, ECs hyperproliferate, form abnormal junctions and give rise to dilated, leaky vessels (Hellstrom et al., 1999; Lindahl et al., 1997).
Recently, two papers have shown that integrin-β1 is essential for mural cell function in vivo. In both studies, mural-cell-specific deletion of β1 led to the formation of aneurysms and defects in the assembly of ECM proteins within the vessel wall (Abraham et al., 2008; Turlo et al., 2012). In mice where Itgb1 was deleted using the Pdgfrb-Cre transgene, mural cells also appeared round, poorly spread, and only loosely attached to the subendothelial basement membrane (Abraham et al., 2008). Electron microscopic studies have shown that dense fibronectin-rich plaques form at the pericyte-endothelial interface suggesting an important role for fibronectin-binding integrins (Courtoy and Boyles, 1983). Mural cells express several β1 heterodimers that bind to fibronectin, including α5β1 (Davenpeck et al., 2001), which is upregulated during differentiation of mesenchymal stem cells to pericytes (Kale et al., 2005). Integrin α5β1 has also been shown to promote proliferation, migration, and switching of vascular smooth muscle cells (vSMCs) from a contractile to synthetic phenotype (Barillari et al., 2001; Davenpeck et al., 2001; Hedin and Thyberg, 1987), and to regulate signalling through PDGFRβ (Veevers-Lowe et al., 2011). To date, however, no one has investigated the role of α5β1 on either pericytes or vSMCs in vivo.
To address the function of α5β1 on mural cells in vivo, we used a conditional gene targeting approach to disrupt expression of Itga5 selectively in both pericytes and vSMCs. We found, again unexpectedly, that deletion of α5β1 integrin from mural cells failed to produce major defects in blood vessel development. We did however observe defects in the lymphatics. Accordingly, we report here on the development of both blood vessels and lymphatics in mice lacking α5β1 integrin in cell types that express Pdgfr -Cre–these include pericytes, vSMCs and, as we report here, a subset of endothelial cells that appear to be involved in separations between the two vascular systems.
Materials and Methods
Mouse lines
All mouse strains used were on a 129S4:C57BL/6 mixed genetic background. Itga5 floxed mice (van der Flier et al., 2010), Itgav floxed mice (Lacy-Hulbert et al., 2007), transgenic Pdgfrb-Cre (Foo et al., 2006), Tie2-Cre (Kisanuki et al., 2001), mTmG (Muzumdar et al., 2007), VE-Cadherin-CreERT2 (Benedito et al., 2009) and Pf4-Cre (Tiedt et al., 2007) mouse lines have all been described previously. Genotyping was performed on DNA isolated from tail snips either in-house or by Transnetyx. For experiments involving the VE-Cadherin-CreERT2 line, pregnant females were given intraperitoneal injections of 2 mg Tamoxifen (dissolved in peanut oil) at E8.5, E9.5, and E10.5.
Histology and immunofluorescence staining
Freshly isolated embryos were embedded in Tissue-Tek OCT and sectioned (20μm) on a Cryostat; or fixed in 4% paraformaldehyde (PFA) in PBS at 4°C overnight or in zinc fixative (BD) at room temperature (RT) for 48 hours, followed by embedding and sectioning (5 μm) in paraffin wax. Selected paraffin sections were stained with hematoxylin and eosin (H&E) using standard protocols. For immunofluorescence stainings, deparaffinized tissue sections were subjected to heat-induced epitope retrieval (2×5 min 800 W microwave) in 10 mM Tris, 1 mM EDTA, 0.05% Tween 20, pH 9.0, blocked in PBS containing 0.5% Tween and 2% goat or donkey serum (PBS-Tx), and incubated overnight at 4°C with primary antibodies diluted in 1:1 PBS:PBS-Tx. After washes in PBS-T (PBS 0.1% Tween), tissues were incubated either at RT for 2 hours, or overnight at 4°C, with fluorophore-conjugated secondary antibodies diluted in 1:1 PBS-Tx. Samples were then washed in PBS-T, mounted onto coverslips in Fluoromount (SouthernBiotech) and imaged using Zeiss LSM 510 or Nikon A1R scanning laser confocal microscopes. Images were processed using Volocity (Perkin Elmer) or Nikon elements software. Whole-mount staining of PFA-fixed embryonic back skin followed methods previously described (Foo et al., 2006).
Antibodies
Rat anti-mouse PECAM-1, MEC13.3 (BD Pharmingen), rabbit anti-mouse PECAM-1 (Abcam), mouse anti-human α-SMA, Clone1A4-Cy3 (Sigma), rabbit anti-Desmin (Abcam), rat anti-mouse Ter-119 (BD Pharmingen), rabbit anti-mouse LYVE-1 (Abcam), hamster-anti mouse Podoplanin (Developmental Studies Hybridoma Bank), goat anti-mouse Integrin-α9 (R&D systems), goat anti-human Prox1 (R&D systems), rabbit anti-Prox1 (Angiobio), rat anti-mouse integrin-α5 (BD Pharmingen), goat anti-GFP (Abcam), rabbit anti-GFP (Cell Signalling), rabbit anti-mouse PDGFRβ 28E1 (Cell Signaling), rabbit anti-αSMA (Abcam). Secondary antibodies were Alexa488, Alexa594, and Alexa647 conjugated antibodies (Invitrogen).
Micro-CT scans
PFA (4%)-fixed embryos were stained with a proprietary contrast agent and scanned with a high-resolution volumetric micro-CT scanner (μCT40 ScanCo Medical, Zurich, CH) using the following parameters: 6 μm isometric voxel resolution at 200 ms exposure time, 2000 views and 5 frames per view (Numira Biosciences (Salt Lake City, UT). The micro-CT generated DICOM files were analyzed using OsiriX and Volocity software.
Results
Loss of integrin-α5 from PDGFRβ-positive cells results in oedema
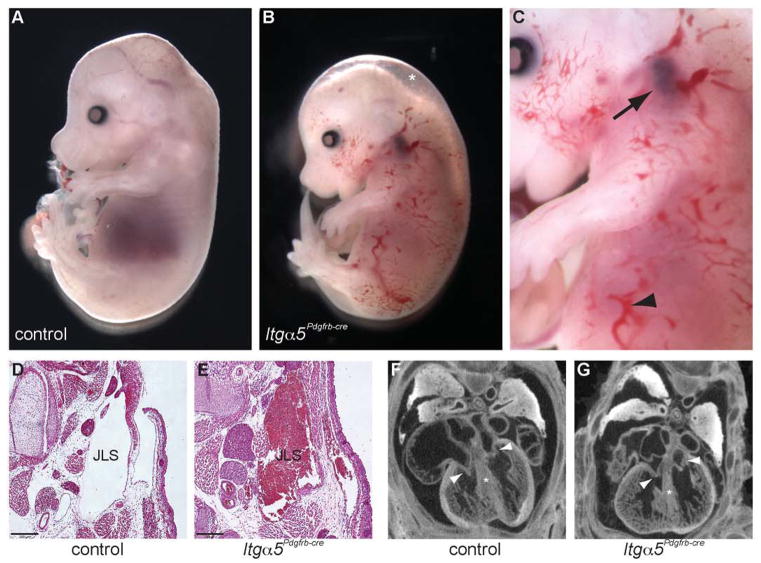
During development of the vascular system, α5β1 is expressed on both endothelial and mural cells within the blood vessel wall (Fig. S1A). To study the role of α5β1 on pericytes and vSMCs, female homozygous Itga5-floxed mice (van der Flier et al., 2010) were crossed to male Pdgfrb-Cre-positive (Foo et al., 2006) heterozygous Itga5-floxed mice. Isolation of the resulting mutant embryos, herein referred to as Itga5Pdgfrb-cre mice, revealed efficient loss of α5 protein within the vSMC layers of the aorta by embryonic day 13.5 (E13.5) (Fig. S1B), and an increased incidence of intrauterine death from E13.5 (Fig. S2A). By E15.5, ~80% of Itga5Pdgfrb-cre mice displayed oedema and, in 35% of mutants, widespread accumulation of blood within the skin (Fig 1A–C, S2B). Itga5Pdgfrb-cre mice that survived to E17.5 (~65%) however appeared to have resolved these defects and survived to birth (Fig. S2A, S2B). Interestingly, Itga5Pdgfrb-cre mice also developed blood-filled jugular lymphatic sacs (Fig. 1C–E). However in the majority of the mice, this was observed only on the left side of the embryos suggesting a left-side predilection (Fig. S2B). To rule out cardiac dysfunction as the cause of these defects, we completed micro-CT scans through the thoracic region of control and Itga5Pdgfrb-cre embryos at E15.5. However, no obvious defects in either the development of the heart or remodelling of the outflow tract were detected in the absence of α5β1 (Fig. 1F, G, Movie S1, S2).
Figure 1. Phenotype of Itga5Pdgfrb-cre mutant mice.
Freshly isolated E15.5 control (A) and Itga5Pdgfrb-cre embryos (B, enlarged in C). Itga5Pdgfrb-cre mutants display oedema (asterisk, B), accumulation of blood within the skin (arrowhead in C), and blood-filled jugular lymph sacs (arrow, C). H&E sections showing control (D) and blood-filled jugular lymph sac (JLS) in E15.5 Itga5Pdgfrb-cre mutants (E). Micro-CT section of E15.5 control (F) and Itga5Pdgfrb-cre hearts showing development of the tricuspid and mitral valves (arrowheads) and the absence of ventricular septal defects (*) in mutant embryos (G). See also Movies 1 and 2 in Supplementary Material. Scale bar: 20 μm.
Normal blood vessel development in Itga5Pdgfrb-cre mice
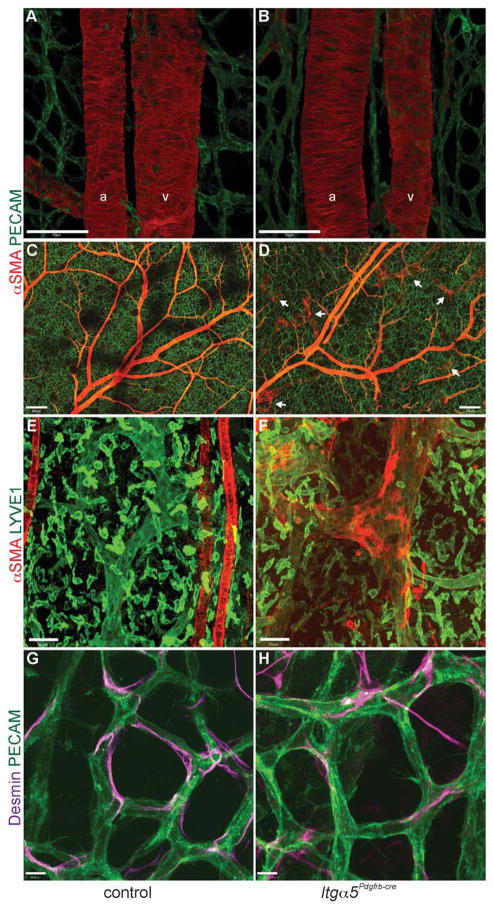
Previous studies have shown that defective mural cell coverage leads to haemorrhage, oedema and embryonic lethality due to instability of the vessel wall (Hellstrom et al., 1999; Kogata et al., 2009). Analysis of the embryonic dermal vasculature by whole-mount immunostaining surprisingly revealed no obvious defects in vSMC or pericyte morphology (Fig. 2), despite efficient deletion of mural cell α5 protein (Fig. S1B). In contrast to the rounded morphology seen in mice lacking mural cell expression of all β1 integrins (Abraham et al., 2008), Itga5-deficient vSMCs formed organised concentric rings around arteries and continuously covered large veins (Fig. 2A–D). Loss of Itga5 did however cause abnormal recruitment of αSMA-positive cells to lymphatic capillaries of the skin (Fig. 2C–F), which in contrast to the collecting lymphatic vessels, are usually devoid of smooth muscle cells in order to allow efficient absorption of interstitial fluid (Petrova et al., 2004). Desmin-positive pericytes also appeared unaffected by the loss of integrin-α5. Pericytes in Itga5Pdgfrb-cre mice were elongated, in close association with the capillary endothelium (Fig. 2G, H), and in similar numbers to those seen in control mice (Fig. S3).
Figure 2. Normal blood vessel morphology in Itga5Pdgfrb-cre mice.
(A–H) Whole-mount immunofluorescence stainings of control and mutant embryonic skin at E17.5. (A, B) No obvious defects seen in vSMC (red) attachment or morphology around arteries (a) and veins (v) in Itga5Pdgfrb-cre mice. (C, D) Low magnification images showing the extent of vSMC coverage throughout the vasculature in a control (C) and Itg α5Pdgfrb-cre embryo (D). Notice the ectopic vSMC coverage around lymphatic capillaries (white arrows) (D), which are absent in control embryos (C). Higher magnification image of a lymphatic capillary (green) in control skin (E) and ectopic αSMA coverage around a lymphatic capillary in an Itg α5Pdgfrb-cre embryo (F). Desmin-positive pericytes apposed to microvessels in control (G) and Itg α5Pdgfrb-cre mutants (H). Scale bars: 50 μm (A, B,), 200 μm (C, D), 50 μm (E, F), 10 μm (G, H).
Itga5Pdgfrb-cre mice display blood-filled, hyperplastic lymphatic vessels
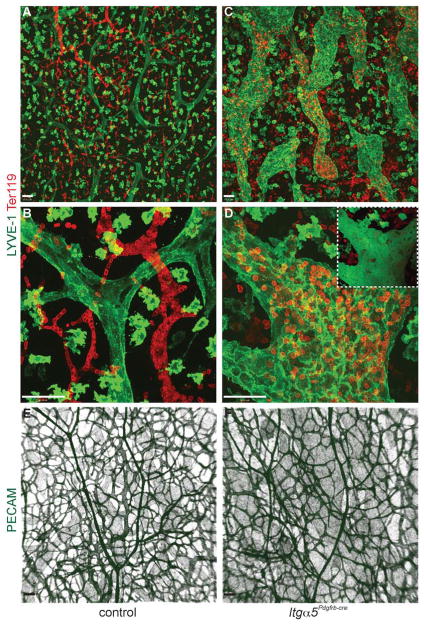
Since the haemorrhage and oedema in Itga5Pdgfrb-cre mice could not be attributed to obvious defects in formation of the vascular wall and ectopic vSMC coverage was seen around lymphatic vessels (Fig. 2C–F), samples of embryonic skin from E15.5 embryos were stained with the pan-endothelial marker PECAM-1, the lymphatic endothelial marker LYVE-1, and the erythroid cell marker Ter-119 (Fig. 3A–F). In control mice, erythrocytes were contained within the blood vasculature (Fig. 3A, B), and blind-ended lymphatic capillaries appeared 2–3 times the size of the largest blood vessels. In contrast, in Itga5Pdgfrb-cre embryos, lymphatic vessels appeared hyperplastic, tortuous and were often filled with blood (Fig. 3C, D). The distribution of LYVE-1 was also different in mutant embryos. Instead of the uniform LYVE-1 expression seen in control mice, endothelial cells in lymphatic capillaries of Itga5Pdgfrb-cre embryos had increased levels of LYVE-1 at their cell junctions (Fig. 3D). Despite these lymphatic defects, blood vessels in Itga5Pdgfrb-cre mutants appeared indistinguishable from those in control mice (Fig. 3E), and even in the most severely affected mutants, no aneurysms or dilation of blood vessels were ever observed (Fig. 3F).
Figure 3. Itg α5Pdgfrb-cre mice display hyperplastic, blood-filled lymphatic vessels.
(A–D) Confocal images showing the lymphatic capillaries (LYVE-1, green; note that LYVE-1 is also expressed on macrophages) and erythrocytes (Ter119, red) in E15.5 control and Itg α5Pdgfrb-cre skin. In contrast to control mice (A and B), lymphatic vessels in Itg α5Pdgfrb-cre mutants are hyperplastic, tortuous and filled with red blood cells (C and D). Inset shows 3D rendering of image in (D) confirming presence of blood within the lymphatic vessel. Blood vessels in Itg α5Pdgfrb-cre mice (strong PECAM stain, green) however remain unaffected by the deletion of integrin-α5 (E, F). Note lymphatic vessels (weak PECAM stain) are also visible in both E and F. Scale bars: 50 μm.
Pdgfrb-Cre is expressed in a population of cells within the jugular lymph sac and lymphovenous valves
Previous results have shown that Pdgfrb is expressed on newly formed lymphatic vessels in the corneas of mice treated with PDGF-B (Cao et al., 2004) and in endothelial cells in vitro (Heldin and Westermark, 1999). To address whether the defects observed in Itga5Pdgfrb-cre mutants could be due to deletion of α5 throughout the lymphatic endothelium we analysed the expression of Pdgfrb, by crossing the Pdgfrb-Cre line with the mTmG reporter mouse (Pdgfrbmtmg), in which Cre-mediated excision results in the expression of membrane-bound GFP (Muzumdar et al., 2007). As expected, Pdgfrb is highly expressed in both pericytes and vSMCs, but is absent from the vascular endothelium and lymphatic capillaries within the skin (Fig. S4A–D).
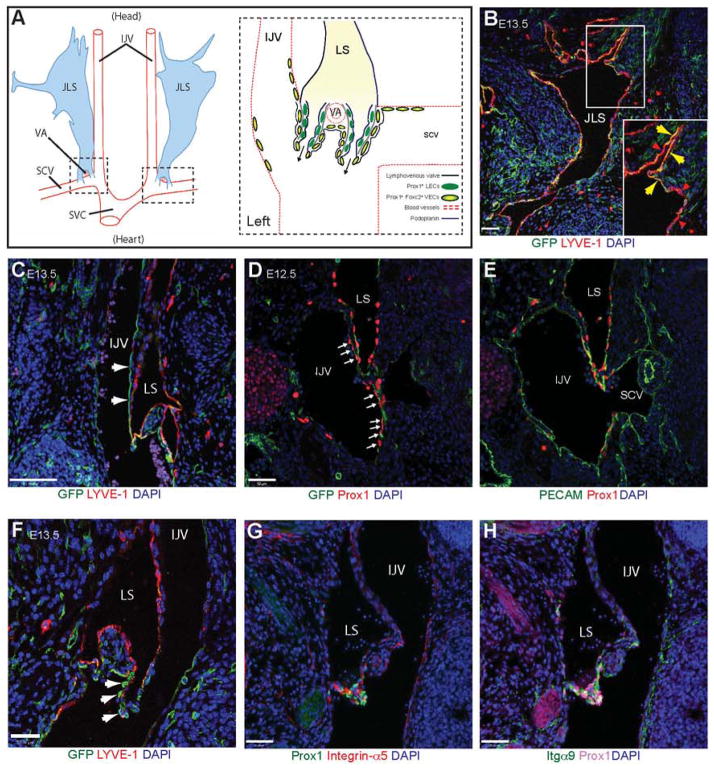
GFP+ cells were however detected in a subset of LYVE-1+ lymphatic ECs in the jugular lymph sacs (JLSs) (Fig. 4A, B), and in a population of Prox1+ cells in the internal jugular (IJV) and subclavian veins (SCV), adjacent to where the lymphatic sac (LS) merges with the venous endothelium to form the two lymphovenous valves and drain fluid back into the blood circulation (Fig. 4C–E). As described by Srinivasan and Oliver (2011), the lymphovenous valves form through the fusion of the LSs (Fig. 4A) with the veins in a region containing a subpopulation of Prox1+ FoxC2+ venous ECs within the IJV and SCV (Fig. 4A). Lymphovenous valves consist of two leaflets, each containing two layers of Prox1+ cells (Fig. 4A, S5A). The inner layer of cells is derived from the LSs and expresses podoplanin (Fig. 4A, S5B), while the outer layer is derived from the specialised Prox1+ FoxC2+ podoplanin− cells found in the walls of the veins (Fig. 4A, S5B) (Srinivasan and Oliver, 2011). Interestingly, analysis of the Pdgfrbmtmg mice revealed that Pdgfrb-Cre is also expressed within these specialised venous ECs in the outer layer of the lymphovenous valve leaflets or their progenitors (Fig. 4F). Furthermore, a subpopulation of PDGFRβ-positive cells was still detectable in the outer layer of the valves at E13.5 (Fig. S5C). These cells did not appear to be pericytes or vSMCs, since they lacked the mural cell markers desmin and SMA (Fig. S5B). In addition to Pdgfrb, the lymphovenous valves also expressed high levels of integrin-α5 (Fig. 4G), along with the lymphatic valve marker (and fibronectin receptor) integrin-α9 (Fig. 4H) (Bazigou et al., 2009). This is in contrast to the valves within the collecting lymphatic vessels, where only low levels of α5 have been reported (Bazigou et al., 2009).
Figure 4. Lymphatic expression of Pdgfrb-Cre.
(A) Schematic representation of the position (modified from Srinivasan and Oliver (2011)) and morphology of the jugular lymphatic sacs (JLS) and the lymphovenous valves in E13.5 mouse embryos. The head is orientated to the top and the heart towards the bottom of the figure. The JLS runs from the neck posteriorly to the level of the thymus, where it is split into two portions by the vertebral artery (VA). The lymphovenous valves (dashed boxes) form at the end of these two lymph sacs (LS) through the fusion of the posterior region of the LSs with the internal jugular (IJV) and subclavian (SCV) veins where they merge and drain into the superior vena cava (SVC). Specialised Prox1+ Foxc2+ podoplanin− venous endothelial cells (VECs) are found in these regions. The lymphovenous valve leaflets consist of two layers of Prox1+ cells. The inner layer is composed of Prox1+ Foxc2− podoplanin− lymphatic endothelial cells (LEC) from the LSs, while the outer layer is derived from the Prox1+ Foxc2+ podoplanin− VECs. (B) Immunofluorescence staining on a coronal section through the JLS of a Pdgfrbmtmg mouse at E13.5 (Pdgfrβ+ cells will be GFP+). Note that the JLS appears to contain both GFP+ (yellow, arrows) and GFP− (red, arrowheads) lymphatic endothelial cells (B, enlarged in inset). (C) GFP+ cells are also present in a population of cells in the IJV and SCV (arrows), adjacent to the JLS where the LS merge with venous circulation to form the lymphovenous valves. (D, E) Sequential transverse sections stained with antibodies against the transcription factor Prox1 and (D) GFP and (E) PECAM showing Pdgfrb-Cre expression in the LS and in a specialised population of venous endothelial cells within the IJV and SCV (arrows) in an E12.5 Pdgfrbmtmg embryo. Note the lack of Pdgfrb-Cre expression in Prox1-negative venous endothelium. (F) Pdgfrb+ cells (arrows) in the outer layer of the lymphovenous valve. (G, H) Immunofluorescence staining on a cryosection through the lymphovenous valve showing high expression of (G) integrin-α5 and (H) integrin-α9 within the Prox1+ valve leaflets. Scale bars: 50 μm.
Separation of the jugular lymph sacs from the cardinal vein is delayed in Itga5Pdgfrb-cre mice
Development of the lymphatic system in mice starts at E9.75 when Prox1 turns on in a subpopulation of LYVE-1+ ECs in the lateral parts of the anterior cardinal vein (CV) (Srinivasan et al., 2007; Wigle and Oliver, 1999). Prox1+ lymphatic progenitor cells then bud, and migrate from the CV in response to VEGF-C from the mesoderm to form the JLSs and the superficial lymphatic vessels, respectively (Francois et al., 2012; Hagerling et al., 2013; Yang et al., 2012). During the budding of the JLSs, nascent lymph sacs become transiently filled with blood until complete separation from the CV occurs at E12.5, and the lymphovenous valves form and allow the lymph sacs to drain into the venous circulation (Francois et al., 2012; van der Putte, 1975). Failure to separate the JLSs from the CV leads to prolonged bleeding into the lymph sacs, tortuous blood-filled cutaneous lymphatic vessels and eventually embryonic death (Abtahian et al., 2003; D’Amico et al., 2009; Uhrin et al., 2010).
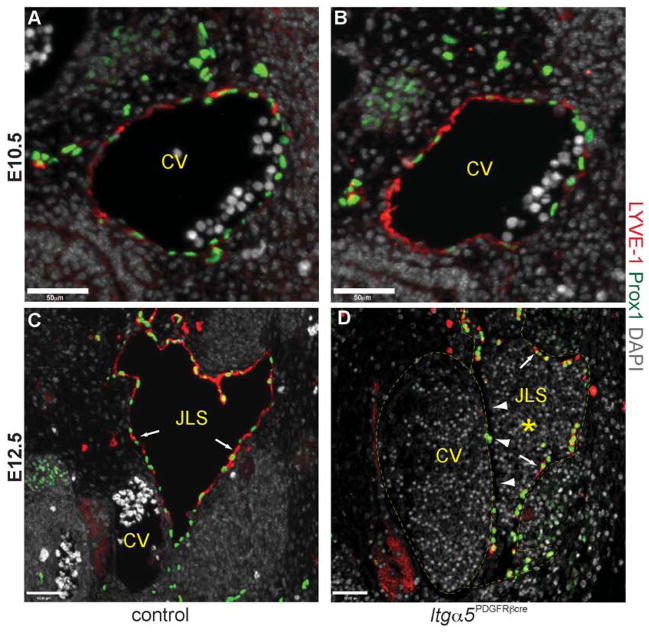
Since Pdgfrb-Cre is expressed within Prox1+ ECs in the venous endothelium and in a subset of cells within the JLSs (Fig. 4), we examined whether the defects observed in Itga5Pdgfrb-cre embryos might also be due to a failure of the JLS to separate from the CV. Consistent with previous reports, at E10.5 the anterior CV of Itga5Pdgfrb-cre embryos expressed LYVE-1 (Wigle et al., 2002) and contained equivalent numbers of Prox1+ progenitor cells when compared with control mice (Fig. 5A, B). By E12.5, LYVE-1 expression was no longer present in the CV, and both control and mutant embryos had clearly visible lymph sacs (Fig. 5C, D). However, in contrast to control embryos (Fig. 5C), JLSs in E12.5 Itga5Pdgfrb-cre embryos were often filled with blood (Fig. 5D, S2) and consisted of both LYVE-1+ lymphatic ECs and LYVE-1− ECs in both haemorrhaging (Fig. 5D) and non-haemorrhaging mice (Fig. S6). The lack of LYVE-1+/Prox1+ lymphatic endothelial cells was particularly noticeable in the region directly adjacent to the CV (Fig. 5D, S6), suggesting that the absence of a5 integrin in these cells compromised the separation of JLS from CV in Itga5Pdgfrb-cre mice, which as a result were susceptible to excessive amounts of blood entering into the lymphatic circulation.
Figure 5. Separation of the jugular lymph sac from the cardinal vein is abnormal in Itg α5Pdgfrb-cre embryos.
Transverse sections through the cardinal vein (CV) showing expression of LYVE-1 (red) and Prox1 (green) in control and Itg α5Pdgfrb-cre embryos. At E10.5, equivalent numbers of Prox1+ lymphatic endothelial progenitor cells are expressed on the dorsal lateral side of the anterior cardinal vein in control (A) and Itg α5Pdgfrb-cre mice (B). Note that LYVE-1 is expressed throughout the CV at this stage.
These cells then bud, and migrate from the CV to form the jugular lymph sacs (JLS) by E12.5. In control embryos the JLSs now consist entirely of Prox1+ LYVE-1+ lymphatic endothelial cells (arrows, C) and contain no red blood cells. In contrast, JLSs in mutant embryos often contained blood (asterisk, D) and appeared to consist of both Prox1+ LYVE-1+ lymphatic endothelial cells (arrows, D), and Prox1+ Lyve1− and Prox1− Lyve-1− blood endothelial cells. This is most apparent directly adjacent to the CV (arrowheads, D). Scale bars: 50 μm.
Previous studies have shown platelets are essential for separation of the JLS from the CV (Bertozzi et al., 2010). Mutant mice with defects in podoplanin-induced platelet activation fail to separate their lymphatic vessels from the blood circulation and, as a result, develop blood-filled lymphatics (Bertozzi et al., 2010). Platelets have been reported to express PDGFRβ in humans (Yang et al., 1997), therefore, Itga5 might have been deleted from platelets in our mutant mice and be the cause of the lymphatic defects observed. However, deletion of Itga5 specifically within platelets, using the Pf4-Cre mouse line (Tiedt et al., 2007) failed to replicate the phenotype seen in Itga5Pdgfrb-cre mice. Itga5Pf4-cre mice developed to term and formed organised lymphatic capillaries within the skin (Fig. S7). Thus, the development of blood-filled lymphatic vessels in Itga5Pdgfrb-cre mice is not due to platelet defects.
Integrin-α5 is required for formation of the lymphovenous valves
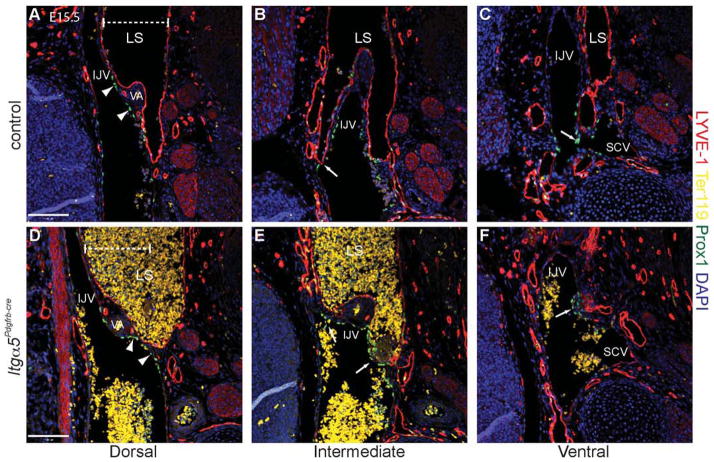
In the human lymphatic malformation, fetal cystic hygroma, failure to connect the LSs to the venous circulation results in large swollen lymphatic vessels, oedema and, unless resolved, death due to inability to drain interstitial fluid back into the venous circulation (Chervenak et al., 1983). To investigate whether the defects in Itga5Pdgfrb-cre mice might also involve a failure to form the lymphovenous valves, we analysed sequential coronal sections through E15.5 control and mutant embryos. At E15.5, the lymphovenous valves should have been fully formed for at least 48 hours (Fig. 4C–E) (Srinivasan and Oliver, 2011). Indeed, in control mice (Fig. 6A–C) the first valve of the left JLS is present just below the vertebral artery, where the LS splits into two portions, and drains into the IJV (arrow, Fig. 6B), while the second valve is positioned more caudally, and drains into the junction of the IJV and SCV (arrow, Fig. 6C). In oedematous Itga5Pdgfrb-cre mice however, no obvious connections between the LSs and the venous circulation were apparent, and despite careful examination of ~30 oedematous embryos, no lymphovenous valves were detected (Fig. 6D–F). Instead, by E15.5, only Prox1+ lymphovenous valve rudiments were found on the walls of both the jugular and subclavian veins (Fig. 6E, F).
Figure 6. Itg α5Pdgfrb-cre mice fail to form lymphovenous valves.
Sequential coronal sections at the junction of the IJV and SCVs stained with LYVE-1 (red), Prox1 (green), and Ter119 (yellow), showing the morphology of the left lymphovenous valves in a control (A–C) and an Itga5Pdgfrb-cre embryo (D–F) at E15.5. In control mice, the first valve leaflets open into the IJV (arrow, B), while the second valve drains into the SCV/IJV junction (arrow, C). Note the Prox1 expression in the venous endothelium adjacent to the LS (arrowheads, A), and the lack of red blood cells (yellow) within the LS in control mice (A–C). In contrast, no valve-like structures are observed in Itga5Pdgfrb-cre mice (D–F) and, at the regions where the LS should connect at either the IJV (E) or the SCV (F), only Prox1+ rudiments are visible (arrows, E and F). Consequently, lymph fluid and sometimes blood (D and E), accumulates and leads to dilation of the lymphatic sac (D). Dashed line indicates the width of the LS in a control embryo (A) for comparison. Note, just as observed in control embryos, Prox1+ VECs are also clearly visible in mutant embryos (arrowheads, D). Scale bars: 50 μm.
As a result, lymph fluid and blood that has entered the lymph sacs during the separation from the CV, is unable to drain into the venous circulation, accumulates in the lymphatic network (Fig. 6D, E), and leads to dilation of the lymph sac (Fig. 6D). Interestingly, lymphovenous valves were found in Itga5Pdgfrb-cre embryos that lacked any obvious signs of oedema (Fig. S8A). Furthermore, in agreement with the predisposition of mutants to develop a blood-filled JLS only on the left side of the embryo, we also observed that in a large proportion of Itga5Pdgfrb-cre embryos analysed, only the left lymphatic sac failed to connect to the venous endothelium, and only in rare cases were both lymphovenous valves absent (Fig. S8B). Lymphovenous valve defects have previously been reported in mice lacking a single copy of the Prox1 transcription factor and as a result they develop oedema and occasionally blood-filled JLSs (Srinivasan and Oliver, 2011). In that study, the authors proposed that the development of the lymphovenous valves is dependent on expression of Prox1 in the population of venous ECs that contribute to formation of the valve leaflets. Loss of Prox1 expression did not seem to be the cause for the valve defects in Itga5Pdgfrb-cre embryos however, since similar numbers of Prox1+ vascular ECs were visible at regions adjacent to the lymphatic sacs, in both control and Itga5Pdgfrb-cre mice (arrowhead, Fig. 6A, D).
Endothelial expression of both α5 and αv integrins are required for proper lymphatic development
Since our results suggest that deletion of α5 in a subset of venous endothelial cells, rather than mural cells (Fig. S5B) or platelets (Fig. S7), is the cause of the lymphatic defects seen in Itga5Pdgfrb-cre embryos, we re-examined embryos in which Itga5 had been deleted using Tie2-Cre, which is expressed in both the lymphatic and blood vasculature. As previously reported by van der Flier et al. (2010), on a C57BL/6 background none of the Itga5 flox/KOTie2-cre embryos analysed developed oedema or blood-filled lymphatics (Fig. S9A, B), and only relatively mild dilation of lymphatics was observed (Fig. S9B). In contrast, on a 129S4 background, ~40% of the Itga5 flox/floxTie2-cre embryos developed oedema (4/9 analysed), and hyperplastic blood-filled lymphatic vessels (1/4 mutants) by E14.5 (Fig S9C, D), confirming that the lymphatic defects in Itga5Pdgfrb-cre embryos are indeed endothelium-specific. The penetrance of this phenotype was enhanced further by the addition of a single integrin-αv KO allele (Fig. S9E), or by deleting both Itga5 and Itgav specifically within the endothelium from E8.5 using the inducible VE-Cadherin-Creert2 mouse (6/6 mutants) (Fig. S9F). The phenotype of endothelium-specific α5, and possibly Itga5Pdgfrb-cre embryos, appears therefore to be dependent on a strain-dependent genetic modifier and on the ability of αv integrins to compensate for the loss of α5β1.
Discussion
We report here two unexpected findings about the role of α5β1 integrin in vascular development. First, ablation of α5 integrin from mural cells fails to cause any notable defects in the development of the blood vasculature. Second, deletion of α5 integrin in Itga5Pdgfrb-cre mice produces lymphatic defects.
Integrin-α5 is dispensable for mural cell functions during vascular development
Despite efficient ablation of α5β1 integrin from mural cells (Fig. S1B), blood vessels in Itga5Pdgfrb-cre embryos lack obvious mural cell defects (Fig. 2). α5-deficient pericytes covered capillaries and extended long cellular extensions (Fig. 2F), while vSMCs formed organised layers around the aorta (Fig. S1B) and aligned in concentric rings around arteries within the skin of mutant mice (Fig. 2B). This was particularly surprising since α5β1 is highly expressed on mural cells (Fig. S1A) (Moiseeva, 2001). We have reported earlier that mice lacking endothelial expression of α5β1 also fail to display the angiogenic defects observed in global α5-KO mice (van der Flier et al., 2010). Thus, α5β1 integrin seems to be largely dispensable in each cell type for formation of the blood vasculature. It is possible that α5β1 integrin in both cell types contributes to assembly of a fibronectin-rich ECM between them and that either cell type can perform this function. Alternatively, other fibronectin-binding integrins could contribute overlapping or compensatory functions that cover for the absence of α5β1 integrin. αv integrins can also assemble fibronectin but global knockouts of all αv integrins or ablation of αvβ3 and αvβ5 also fail to produce general vascular defects (Bader et al., 1998; Hodivala-Dilke et al., 1999; Huang et al., 2000a; Reynolds et al., 2002), demonstrating that, if αv integrins are involved, they are less essential than α5β1 integrin. We have shown that mice lacking endothelial expression of both α5 and αv integrin subunits, both of which are capable of fibronectin assembly in vitro, show no obvious defects in the deposition of fibronectin within their basement membranes in vivo (van der Flier et al., 2010).
Mural cell deletion of Itgb1, the common beta subunit of a dozen different integrins, including α5, as well as other potential minor fibronectin receptors (α4, α8, α9) allows development until late in gestation, although skin vessels do show dilations. Some pups are born but, postnatally, aneurysms form and the mural cells show defects in their adherence to vessels and in the assembly of fibronectin and other ECM proteins around vessels within the skin (Abraham et al., 2008). None of those other alpha subunits, when individually deleted globally, has much if any effect on vascular development and, in the absence of α5β1 on mural cells, αv integrins are presumably still expressed by the mural cells. In light of these results, there are two possible explanations for the absence of detectable blood vessel defects in Itga5Pdgfrb-cre embryos. First, the role of α5 may be adequately replaced (through overlapping functions or by compensation) by other integrins expressed on the mural cells, or by α5 expressed on ECs during angiogenesis. Mural cells express several fibronectin receptors (α5β1, αvβ3, αvβ5, αvβ6, α4β1) (Moiseeva, 2001), and defects in pericyte and vSMCs distribution have been reported in α4-KO mice (Garmy-Susini et al., 2005; Grazioli et al., 2006). To address these possibilities, tissue-specific mutants lacking multiple fibronectin integrins and mice lacking α5 in both endothelial and mural cell compartments will need to be studied. Second, mural cell function and vessel stability may in fact, be more reliant on the collagen and laminin receptors included among the β1 integrins. vSMCs reportedly express high levels of α3β1 (a laminin receptor) and α1β1 (a collagen receptor) in vivo; however to date, only the α7β1 integrin (another laminin receptor) has been shown to have a role in mural cell function during development (Flintoff-Dye et al., 2005).
Selective deletion of α5β1 integrin causes lymphatic defects
Lymphatic malformations occur in 1 in 1750 live births and 1 in 200 spontaneous abortions. The exact genes and cells involved in these malformations remain unclear. Here we report that α5β1, the major fibronectin receptor, is essential for proper lymphatic development. Reminiscent of the lymphatic malformation, fetal cystic hygroma (Chervenak et al., 1983), Itga5Pdgfrb-cre embryos fail to form lymphovenous valves on their left side (Fig. 6D–F). Consequently, α5 mutants fail to drain their left jugular lymph sacs into the venous circulation, accumulate interstitial fluid in their lymphatic circulation and develop swollen hyperplastic lymphatic vessels and oedema (Fig. 1B, 3C). Itga5Pdgfrb-cre mice that manage to connect their JLSs before intrauterine death appear to resolve their oedema and survive to birth (Fig. S2, S8). Nevertheless, even without obvious persistent oedema, these mice still displayed enlarged lymphatic vessels and ectopic smooth muscle cell coverage around their capillary lymphatic vessels (Fig. 2D, E), just as observed in patients with lymphedema distichiasis, and in mice lacking the transcription factor Foxc2 (Petrova et al., 2004). Interestingly, lymphatic hyperplasia and abnormal recruitment of vSMCs to the lymphatic capillaries, without blood vessel defects, are also seen in mice lacking the PDZ-binding domain of ephrin-B2, which like FoxC2 mutants have defective lymphatic valves and abnormal lymphatic circulation (Makinen et al., 2005; Petrova et al., 2004). Intriguingly, deletion of ephrin-B2 using the same Pdgfrb-Cre line used in this study, also leads to ectopic vSMC cell coverage on the dermal lymphatic capillaries, in addition to causing mural cell defects (Foo et al., 2006).
Since the majority of Itga5Pdgfrb-cre embryos developed oedema in the absence of obvious haemorrhaging, and blood-filled lymph sacs were observed before the formation of the lymphovenous valves at E12.5 (Fig. 5D, S2), we believe that the appearance of a blood-filled lymphatic network in α5 mutants requires both a delay in the separation of the JLS from the CV (Fig. 5D) and defective lymphovenous valve formation (Fig. 6). Previous studies have shown that, even in wild-type mice, blood can enter the lymphatic circulation as the nascent lymph sacs bud from the CV (Francois et al., 2012; van der Putte, 1975). Therefore, prolonging this process would result in excess blood entering the lymphatic system. Indeed, numerous reports have shown that failure or delay in separating the JLS from the CV results in blood-filled lymphatic vasculature (Abtahian et al., 2003; D’Amico et al., 2009; Uhrin et al., 2010). In wild-type mice, blood that enters during development is, most likely, drained back into the venous circulation via the lymphovenous valves. However, since no such connection is made in the majority of Itga5Pdgfrb-cre embryos by E15.5, this blood is prevented from leaving the lymphatics and leads to the blood-filled phenotype observed. Interestingly, blood-filled lymphatics have also been reported to occur in Prox1 heterozygous mutants, which also lack lymphovenous valves (Srinivasan and Oliver, 2011). In that study, the authors suggest that blood could enter the lymphatic circulation through the formation of abrupt fusions between the lymph sac and the IJV close to the valves (Srinivasan and Oliver, 2011). However, since <10% of Prox1 heterozygous embryos develop blood-filled lymphatic vessels (Srinivasan and Oliver, 2011) and an abrupt fusion between the LS and the IJV was only seen in one Itga5Pdgfrb-cre embryo (data not shown), it is unlikely that this connection is the primary site where blood enters the lymphatic circulation in our mutant embryos.
Expression of PDGFRβ and integrin α5β1 during lymphatic development
Our analysis of Pdgfrb-Cre expression during embryonic development suggests that the lymphatic defects observed in Itga5Pdgfrb-cre embryos are caused by loss of α5β1 in a specialised population of Prox1+ Pdgfr + venous endothelial cells that contribute to the outer layer of the lymphovenous valve leaflets (Fig. 4C–G). Elegant work from Srinivasan and Oliver (2011) has shown that these cells also express the transcription factor Foxc2, in contrast to the lymphatic ECs that form the inner layer of the valve leaflets which are Prox1+ but Foxc2− (Fig. 4A). Interestingly, Foxc2 is a direct transcriptional activator of PDGFRβ expression and promotes mesenchymal transition (Hollier et al., 2013). This suggests that expression of PDGFRβ within the outer layer of the valves and in the JLS is downstream of Foxc2, and therefore of Prox1 (Harada et al., 2009), and may indicate an important role for PDGF-B signalling in formation of both the JLS and the lymphovenous valves. Consistent with this model, Pdgfb KO mice, in addition to displaying severe blood vasculature defects, also display dilated, tortuous, blood-filled lymphatic vessels, analogous to those seen in Itga5Pdgfrb-cre embryos (Haiko, 2008). Furthermore, mice lacking a single copy of Prox1 lose expression of Prox1 and Foxc2 in the cells within the IJV and SCV that contribute to the outer layer of the valve and fail to develop lymphovenous valves (Srinivasan and Oliver, 2011).
Possible roles of integrin-α5 in lymphatic development
In this study, we have found that α5 is highly expressed in the lymphovenous valves (Fig. 4G), and is required by the specialised population of Prox1+ Pdgfrb+-expressing cells for the proper formation of the JLS and the lymphovenous valves. The importance of α5 within these cells could be attributable to several factors. First, α5 may be mediating growth factor signalling. Integrin-α5 is required for optimal activation of the lymphatic endothelial growth factor receptor, VEGFR-3 (Zhang et al., 2005), and proliferation of lymphatic ECs is blocked by small-molecule antagonists of α5β1 in vitro (Dietrich et al., 2007). α5β1 has also been implicated in the activation of PDGFRβ, in the absence of growth factor stimulation, and anti-α5 blocking antibodies significantly reduce PDGFRβ phosphorylation (Veevers-Lowe et al., 2011). Second, loss of α5 may reduce cell migration or adhesion of cells to fibronectin and fibrillin, the main component of the anchoring filaments securing lymphatic vessels to the interstitial matrix. Finally, α5 may be required for deposition of fibronectin. Integrin-α5 plays a major role in assembly of fibronectin (Wierzbicka-Patynowski and Schwarzbauer, 2003) and loss of α5 in either vascular or lymphatic ECs leads to a reduction in fibronectin fibrillogenesis (Bazigou et al., 2009; van der Flier et al., 2010). Interestingly, assembly of fibronectin containing the extra type III domain (EIIIA) has been shown to be essential for the elongation of the intra-lymphatic valve leaflets (Bazigou et al., 2009). Mice lacking endothelial integrin-α9, fail to organise EIIIA+ fibronectin into fibrils and as a result develop ring-like valve structures that are unable to prevent the backflow of lymph fluid within the body (Bazigou et al., 2009). It is conceivable therefore, that a similar process regulates the development of lymphovenous valve leaflets.
Conclusions
Despite numerous in vitro and in vivo studies, the roles of α5β1 in vascular development remain unclear. Both endothelial and mural cells express α5β1 during development but it is not essential in either cell type alone – maybe it functions similarly and, to some degree redundantly, in both cell types, for example in correct assembly of extracellular matrix. Furthermore, other integrins appear to play more minor, but perhaps overlapping, functions in vascular development. Some such functions have been revealed in experiments where multiple integrins have been ablated (van der Flier et al., 2010) and additional experiments of that sort, although difficult to accomplish, may shed further light on the complex roles of integrins in development of the cardiovascular system. It is also becoming clear that integrins, in particular fibronectin-binding integrins, have prominent roles in controlling the development of the lymphatic system. Mice lacking integrin-α9 develop abnormal lymphatic valves and chylothorax (Bazigou et al., 2009; Huang et al., 2000b), while integrin-α4 is required for tumour lymphangiogenesis (Garmy-Susini et al., 2010). Our present study has shown that integrin α5β1 is essential in a subset of Pdgfrb+ cells for the proper separation of the blood and lymphatic vasculature and the development of the lymphovenous valves. Thus, while the known roles served by integrins in vascular and lymphatic development continue to increase, there undoubtedly remain yet other contributions to be discovered.
Supplementary Material
α5β1 integrin is expressed on mural cells but is dispensable for normal blood vessel development.
α5β1 integrin is also expressed on a subset of Prox1+ Pdgfr + venous endothelial cells.
Deletion of α5β1 integrin from these ECs causes distended, blood-filled lymphatics.
α5β1 integrin is required for separation of jugular lymph sacs from cardinal veins.
α5β1 integrin is required for proper formation of lymphovenous valves.
Acknowledgments
We are grateful to Ralf Adams for the Pdgfrb-Cre mouse line and members of the Hynes laboratory for advice and for critically reading the manuscript. This work was supported by grants from the National Institutes of Health (PO1-HL66105, PI, Monty Krieger), the NIGMS Cell Migration Consortium, (GC11451.126452, PI, A.F. Horwitz), and by the Koch Institute Support (core) Grant P30-CA14051 from the National Cancer Institute. CJT was a postdoctoral associate and ROH is an Investigator of the Howard Hughes Medical Institute, which also supported this research.
Footnotes
Author Contributions
Experiments were conceived, designed and interpreted by CJT, KB-N and ROH.
Experiments were performed by CJT and KB-N; DC provided sections and AF generated Itga5 flox/KOTie2cre mice. The manuscript was written by CJT and ROH.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abraham S, Kogata N, Fassler R, Adams RH. Integrin beta1 subunit controls mural cell adhesion, spreading, and blood vessel wall stability. Circ Res. 2008;102:562–570. doi: 10.1161/CIRCRESAHA.107.167908. [DOI] [PubMed] [Google Scholar]
- Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alitalo K. The lymphatic vasculature in disease. Nat Med. 2011;17:1371–1380. doi: 10.1038/nm.2545. [DOI] [PubMed] [Google Scholar]
- Astrof S, Hynes RO. Fibronectins in vascular morphogenesis. Angiogenesis. 2009;12:165–175. doi: 10.1007/s10456-009-9136-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Rayburn H, Crowley D, Hynes RO. Extensive vasculogenesis, angiogenesis, and organogenesis precede lethality in mice lacking all alpha v integrins. Cell. 1998;95:507–519. doi: 10.1016/s0092-8674(00)81618-9. [DOI] [PubMed] [Google Scholar]
- Barillari G, Albonici L, Incerpi S, Bogetto L, Pistritto G, Volpi A, Ensoli B, Manzari V. Inflammatory cytokines stimulate vascular smooth muscle cells locomotion and growth by enhancing alpha5beta1 integrin expression and function. Atherosclerosis. 2001;154:377–385. doi: 10.1016/s0021-9150(00)00506-2. [DOI] [PubMed] [Google Scholar]
- Bazigou E, Makinen T. Flow control in our vessels: vascular valves make sure there is no way back. Cell Mol Life Sci. 2013;70:1055–1066. doi: 10.1007/s00018-012-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- Bertozzi CC, Hess PR, Kahn ML. Platelets: covert regulators of lymphatic development. Arterioscler Thromb Vasc Biol. 2010;30:2368–2371. doi: 10.1161/ATVBAHA.110.217281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–345. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Chen J, Alexander JS, Orr AW. Integrins and their extracellular matrix ligands in lymphangiogenesis and lymph node metastasis. Int J Cell Biol. 2012;2012:853703. doi: 10.1155/2012/853703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chervenak FA, Isaacson G, Blakemore KJ, Breg WR, Hobbins JC, Berkowitz RL, Tortora M, Mayden K, Mahoney MJ. Fetal cystic hygroma. Cause and natural history. N Engl J Med. 1983;309:822–825. doi: 10.1056/NEJM198310063091403. [DOI] [PubMed] [Google Scholar]
- Courtoy PJ, Boyles J. Fibronectin in the microvasculature: localization in the pericyte-endothelial interstitium. J Ultrastruct Res. 1983;83:258–273. doi: 10.1016/s0022-5320(83)90133-8. [DOI] [PubMed] [Google Scholar]
- D’Amico G, Jones DT, Nye E, Sapienza K, Ramjuan AR, Reynolds LE, Robinson SD, Kostourou V, Martinez D, Aubyn D, Grose R, Thomas GJ, Spencer-Dene B, Zicha D, Davies D, Tybulewicz V, Hodivala-Dilke KM. Regulation of lymphatic-blood vessel separation by endothelial Rac1. Development. 2009;136:4043–4053. doi: 10.1242/dev.035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenpeck KL, Marcinkiewicz C, Wang D, Niculescu R, Shi Y, Martin JL, Zalewski A. Regional differences in integrin expression: role of alpha(5)beta(1) in regulating smooth muscle cell functions. Circ Res. 2001;88:352–358. doi: 10.1161/01.res.88.3.352. [DOI] [PubMed] [Google Scholar]
- Dietrich T, Onderka J, Bock F, Kruse FE, Vossmeyer D, Stragies R, Zahn G, Cursiefen C. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171:361–372. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flintoff-Dye NL, Welser J, Rooney J, Scowen P, Tamowski S, Hatton W, Burkin DJ. Role for the alpha7beta1 integrin in vascular development and integrity. Dev Dyn. 2005;234:11–21. doi: 10.1002/dvdy.20462. [DOI] [PubMed] [Google Scholar]
- Földi M, Strössenreuther RHK ebrary Inc. Foundations of manual lymph drainage. 3. Mosby; St. Louis: 2005. [Google Scholar]
- Foo SS, Turner CJ, Adams S, Compagni A, Aubyn D, Kogata N, Lindblom P, Shani M, Zicha D, Adams RH. Ephrin-B2 controls cell motility and adhesion during blood-vessel-wall assembly. Cell. 2006;124:161–173. doi: 10.1016/j.cell.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Francis SE, Goh KL, Hodivala-Dilke K, Bader BL, Stark M, Davidson D, Hynes RO. Central roles of alpha5beta1 integrin and fibronectin in vascular development in mouse embryos and embryoid bodies. Arterioscler Thromb Vase Biol. 2002;22:927–933. doi: 10.1161/01.atv.0000016045.93313.f2. [DOI] [PubMed] [Google Scholar]
- Francois M, Short K, Seeker GA, Combes A, Schwarz Q, Davidson TL, Smyth I, Hong YK, Harvey NL, Koopman P. Segmental territories along the cardinal veins generate lymph sacs via a ballooning mechanism during embryonic lymphangiogenesis in mice. Dev Biol. 2012;364:89–98. doi: 10.1016/j.ydbio.2011.12.032. [DOI] [PubMed] [Google Scholar]
- Garmy-Susini B, Avraamides CJ, Schmid MC, Foubert P, Ellies LG, Barnes L, Feral C, Papayannopoulou T, Lowy A, Blair SL, Cheresh D, Ginsberg M, Varner JA. Integrin alpha4beta1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–3051. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George EL, Baldwin HS, Hynes RO. Fibronectins are essential for heart and blood vessel morphogenesis but are dispensable for initial specification of precursor cells. Blood. 1997;90:3073–3081. [PubMed] [Google Scholar]
- George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- Goodman SL, Picard M. Integrins as therapeutic targets. Trends Pharmacol Sci. 2012;33:405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Grazioli A, Alves CS, Konstantopoulos K, Yang JT. Defective blood vessel development and pericyte/pvSMC distribution in alpha 4 integrin-deficient mouse embryos. Dev Biol. 2006;293:165–177. doi: 10.1016/j.ydbio.2006.01.026. [DOI] [PubMed] [Google Scholar]
- Hagerling R, Pollmann C, Andreas M, Schmidt C, Nurmi H, Adams RH, Alitalo K, Andresen V, Schulte-Merker S, Kiefer F. A novel multistep mechanism for initial lymphangiogenesis in mouse embryos based on ultramicroscopy. EMBO J. 2013;32:629–644. doi: 10.1038/emboj.2012.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haiko P. VEGF-C/VEGFR-3 and PDGF-B/PDGFR-beta pathways in embryonic lymphangiogenesis. Haartman Institute and Biomedicum Helsinki. University of Helsinki; 2008. [Google Scholar]
- Harada K, Yamazaki T, Iwata C, Yoshimatsu Y, Sase H, Mishima K, Morishita Y, Hirashima M, Oike Y, Suda T, Miura N, Watabe T, Miyazono K. Identification of targets of Prox1 during in vitro vascular differentiation from embryonic stem cells: functional roles of HoxD8 in lymphangiogenesis. J Cell Sci. 2009;122:3923–3930. doi: 10.1242/jcs.052324. [DOI] [PubMed] [Google Scholar]
- Hedin U, Thyberg J. Plasma fibronectin promotes modulation of arterial smooth-muscle cells from contractile to synthetic phenotype. Differentiation. 1987;33:239–246. doi: 10.1111/j.1432-0436.1987.tb01563.x. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Hellstrom M, Kalen M, Lindahl P, Abramsson A, Betsholtz C. Role of PDGF-B and PDGFR-beta in recruitment of vascular smooth muscle cells and pericytes during embryonic blood vessel formation in the mouse. Development. 1999;126:3047–3055. doi: 10.1242/dev.126.14.3047. [DOI] [PubMed] [Google Scholar]
- Hirschi KK, Rohovsky SA, Beck LH, Smith SR, D’Amore PA. Endothelial cells modulate the proliferation of mural cell precursors via platelet-derived growth factor-BB and heterotypic cell contact. Circ Res. 1999;84:298–305. doi: 10.1161/01.res.84.3.298. [DOI] [PubMed] [Google Scholar]
- Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollier BG, Tinnirello AA, Werden SJ, Evans KW, Taube JH, Sarkar TR, Sphyris N, Shariati M, Kumar SV, Battula VL, Herschkowitz JI, Guerra R, Chang JT, Miura N, Rosen JM, Mani SA. FOXC2 expression links epithelial-mesenchymal transition and stem cell properties in breast cancer. Cancer Res. 2013;73:1981–1992. doi: 10.1158/0008-5472.CAN-12-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Griffiths M, Wu J, Farese RV, Jr, Sheppard D. Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol Cell Biol. 2000a;20:755–759. doi: 10.1128/mcb.20.3.755-759.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XZ, Wu JF, Ferrando R, Lee JH, Wang YL, Farese RV, Jr, Sheppard D. Fatal bilateral chylothorax in mice lacking the integrin alpha9beta1. Mol Cell Biol. 2000b;20:5208–5215. doi: 10.1128/mcb.20.14.5208-5215.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Cell-matrix adhesion in vascular development. J Thromb Haemost. 2007;5(Suppl 1):32–40. doi: 10.1111/j.1538-7836.2007.02569.x. [DOI] [PubMed] [Google Scholar]
- Kale S, Hanai J, Chan B, Karihaloo A, Grotendorst G, Cantley L, Sukhatme VP. Microarray analysis of in vitro pericyte differentiation reveals an angiogenic program of gene expression. FASEB. 2005;J19:270–271. doi: 10.1096/fj.04-1604fje. [DOI] [PubMed] [Google Scholar]
- Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–1362. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kogata N, Tribe RM, Fassler R, Way M, Adams RH. Integrin-linked kinase controls vascular wall formation by negatively regulating Rho/ROCK-mediated vascular smooth muscle cell contraction. Genes Dev. 2009;23:2278–2283. doi: 10.1101/gad.535409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy-Hulbert A, Smith AM, Tissire H, Barry M, Crowley D, Branson RT, Roes JT, Savill JS, Hynes RO. Ulcerative colitis and autoimmunity induced by loss of myeloid alphav integrins. Proc Natl Acad Sci U S A. 2007;104:15823–15828. doi: 10.1073/pnas.0707421104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leveen P, Pekny M, Gebre-Medhin S, Swolin B, Larsson E, Betsholtz C. Mice deficient for PDGF B show renal, cardiovascular, and hematological abnormalities. Genes Dev. 1994;8:1875–1887. doi: 10.1101/gad.8.16.1875. [DOI] [PubMed] [Google Scholar]
- Lindahl P, Johansson BR, Leveen P, Betsholtz C. Pericyte loss and microaneurysm formation in PDGF-B-deficient mice. Science. 1997;277:242–245. doi: 10.1126/science.277.5323.242. [DOI] [PubMed] [Google Scholar]
- Makinen T, Adams RH, Bailey J, Lu Q, Ziemiecki A, Alitalo K, Klein R, Wilkinson GA. PDZ interaction site in ephrinB2 is required for the remodeling of lymphatic vasculature. Genes Dev. 2005;19:397–410. doi: 10.1101/gad.330105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseeva EP. Adhesion receptors of vascular smooth muscle cells and their functions. Cardiovasc Res. 2001;52:372–386. doi: 10.1016/s0008-6363(01)00399-6. [DOI] [PubMed] [Google Scholar]
- Muether PS, Dell S, Kociok N, Zahn G, Stragies R, Vossmeyer D, Joussen AM. The role of integrin alpha5beta1 in the regulation of corneal neovascularization. Exp Eye Res. 2007;85:356–365. doi: 10.1016/j.exer.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L. A global double-fluorescent Cre reporter mouse. Genesis. 2007;45:593–605. doi: 10.1002/dvg.20335. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Ni A, Ayeni OA, Baluk P, Yao LC, Vossmeyer D, Zischinsky G, Zahn G, Knolle J, Christner C, McDonald DM. alpha5beta1 Integrin blockade inhibits lymphangiogenesis in airway inflammation. Am J Pathol. 2009;174:2378–2387. doi: 10.2353/ajpath.2009.080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrova TV, Karpanen T, Norrmen C, Mellor R, Tamakoshi T, Finegold D, Ferrell R, Kerjaschki D, Mortimer P, Yla-Herttuala S, Miura N, Alitalo K. Defective valves and abnormal mural cell recruitment underlie lymphatic vascular failure in lymphedema distichiasis. Nat Med. 2004;10:974–981. doi: 10.1038/nm1094. [DOI] [PubMed] [Google Scholar]
- Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- Soriano P. Abnormal kidney development and hematological disorders in PDGF beta-receptor mutant mice. Genes Dev. 1994;8:1888–1896. doi: 10.1101/gad.8.16.1888. [DOI] [PubMed] [Google Scholar]
- Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- Turlo KA, Noel OD, Vora R, LaRussa M, Fassler R, Hall-Glenn F, Iruela-Arispe ML. An essential requirement for beta1 integrin in the assembly of extracellular matrix proteins within the vascular wall. Dev Biol. 2012;365:23–35. doi: 10.1016/j.ydbio.2012.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- Umeda N, Kachi S, Akiyama H, Zahn G, Vossmeyer D, Stragies R, Campochiaro PA. Suppression and regression of choroidal neovascularization by systemic administration of an alpha5beta1 integrin antagonist. Mol Pharmacol. 2006;69:1820–1828. doi: 10.1124/mol.105.020941. [DOI] [PubMed] [Google Scholar]
- van der Flier A, Badu-Nkansah K, Whittaker CA, Crowley D, Bronson RT, Lacy-Hulbert A, Hynes RO. Endothelial alpha5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Putte SC. The development of the lymphatic system in man. Adv Anat Embryol Cell Biol. 1975;51:3–60. [PubMed] [Google Scholar]
- Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through alpha5beta1-integrin-mediated activation of PDGFR-beta and potentiation of growth factor signals. J Cell Sci. 2011;124:1288–1300. doi: 10.1242/jcs.076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzbicka-Patynowski I, Schwarzbauer JE. The ins and outs of fibronectin matrix assembly. J Cell Sci. 2003;116:3269–3276. doi: 10.1242/jcs.00670. [DOI] [PubMed] [Google Scholar]
- Wigle JT, Harvey N, Detmar M, Lagutina I, Grosveld G, Gunn MD, Jackson DG, Oliver G. An essential role for Prox1 in the induction of the lymphatic endothelial cell phenotype. EMBO J. 2002;21:1505–1513. doi: 10.1093/emboj/21.7.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- Xie L, Duncan MB, Pahler J, Sugimoto H, Martino M, Lively J, Mundel T, Soubasakos M, Rubin K, Takeda T, Inoue M, Lawler J, Hynes RO, Hanahan D, Kalluri R. Counterbalancing angiogenic regulatory factors control the rate of cancer progression and survival in a stage-specific manner. Proc Natl Acad Sci U S A. 2011;108:9939–9944. doi: 10.1073/pnas.1105041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JT, Rayburn H, Hynes RO. Embryonic mesodermal defects in alpha 5 integrin-deficient mice. Development. 1993;119:1093–1105. doi: 10.1242/dev.119.4.1093. [DOI] [PubMed] [Google Scholar]
- Yang M, Khachigian LM, Hicks C, Chesterman CN, Chong BH. Identification of PDGF receptors on human megakaryocytes and megakaryocytic cell lines. Thromb Haemost. 1997;78:892–896. [PubMed] [Google Scholar]
- Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–2348. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Groopman JE, Wang JF. Extracellular matrix regulates endothelial functions through interaction of VEGFR-3 and integrin alpha5beta1. J Cell Physiol. 2005;202:205–214. doi: 10.1002/jcp.20106. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.