Abstract
Perivascular adipose tissue (PVAT) directly abuts the lamina adventitia of conduit arteries and actively communicates with the vessel wall to regulate vascular function and inflammation. Mounting evidence suggests that the biological activities of PVAT are governed by perivascular (PV) adipocytes, a unique class of adipocyte with distinct molecular and phenotypic characteristics. Perivascular adipocytes surrounding human coronary arteries (pericoronary PV adipocytes) exhibit a reduced state of adipogenic differentiation and a heightened pro-inflammatory state, secreting up to 50-fold higher levels of the pro-inflammatory cytokine MCP-1 as compared with adipocytes from other regional depots. Thus, PV adipocytes may contribute to upregulated inflammation of PVAT observed in atherosclerotic human blood vessels. On the other hand, PV adipocytes also secrete anti-inflammatory molecules such as adiponectin, and elimination of PVAT in rodent models has been shown to augment vascular disease, suggesting that some amount of PVAT is required to maintain vascular homeostasis. Evidence in animal models and in humans suggests that inflammation of PVAT may be modulated by environmental factors, such as high fat diet and tobacco smoke, which are relevant to atherosclerosis. These findings suggest that the inflammatory phenotype of PVAT is diverse depending on species, anatomic location, and environmental factors, and that these differences are fundamentally important in determining a pathogenic versus protective role of PVAT in vascular disease. Further research into the mechanisms that regulate the inflammatory balance of PV adipocytes may yield new insight into, and treatment strategies for, cardiovascular disease.
Introduction
The traditional model of the pathogenesis of atherosclerosis has focused on intimal disease, following the paradigm of endothelial injury leading to inflammation, monocyte recruitment and foam cell formation.1 This view focuses on the luminal aspect of the vessels with the inflammation initiating inside the vessel wall and radiating outward (“inside-out” model), with adventitia and PVAT occupying a relatively passive role. However, this paradigm has come under intense scrutiny as the extent of adventitial inflammation in atherosclerosis has been better appreciated. Indeed, in apolipoprotein E deficient mice, accumulation of T cells and B cells in the lamina adventitia far exceeds that in the intima (up to 80-fold higher),2 and intense clustering of macrophages and lymphocytes was demonstrated at the border between the lamina adventitia and PVAT in atherosclerotic human aorta,3 suggesting that PVAT could play an active role in promoting vascular inflammation. This view is further supported by observations that balloon or wire injury rapidly induces inflammation and perturbs adipokine gene expression profiles in PVAT in pigs and mice.4, 5 These and other studies have led to a gradual shift towards an “outside-in” model of inflammation in vascular disease and placed PVAT prominently in the spotlight of vascular biology.
The purpose of this review is to discuss current concepts regarding the inflammatory state of PV adipocytes with the recognition that the majority of current knowledge regarding adipocyte origin and function stem from studies of visceral and/or subcutaneous adipose tissues, with limited data on the PV depot. Emerging evidence suggests that PVAT is a unique adipose depot containing a distinct class of adipocytes reflecting the precursor cells (preadipocytes) from which they are derived. A more fundamental understanding of PV adipocytes is required to elucidate the mechanisms whereby PVAT interacts with metabolic factors, chemokines, inflammatory cells and the blood vessel wall to mediate and/or modulate cardiovascular disease.
Adipocytes and inflammation
Adipocytes are traditionally viewed as energy-storage cells that play a key role in energy balance, thermogenesis and glucose homeostasis. However, adipocytes also produce a number of soluble factors (“adipocytokines”) that regulate inflammation, and they possess machinery including toll-like receptors that facilitate inflammatory interactions. Adipokines such as resistin6 and leptin7 exert predominantly pro-inflammatory effects, while adiponectin is classically anti-inflammatory.8 Thus, adipocytes can also be viewed as integral components of the immune system.9, 10 Evolutionarily, adipose tissue was derived from the primitive fat body of insects, which simultaneously serves both the animal’s metabolic and immune functions11. While higher animals have evolved separate systems to serve these functions (i.e., liver and adipose tissues for metabolism and hematopoietic system for immunity), adipose tissue has retained its basic immune functions.12 The contribution of adipocytes to inflammation of adipose tissues in the pathogenesis of obesity-related disease is well established.13, 14 Adipose inflammation, in turn, serves as a major driver of systemic inflammation that links obesity to cardiovascular disease. Although pro-inflammatory adipocytokines are expressed by PVAT, the notion that PVAT may locally contribute to cardiovascular disease is a more recent and less well-founded hypothesis.
Features of PVAT
PVAT is a conglomerate of various cell types including adipocytes, preadipocytes, and mesenchymal stem cells, embedded in a matrix that is invested with microvessels15, 16 and which may contain a similar system of sympathetic innervation seen in richly innervated brown adipose tissue (BAT) and white adipose tissue (WAT),16 although evidence regarding PVAT innervation is scarce. In humans, PVAT directly abuts the adventitia of most large conduit arteries in the body, with the notable exception of the cerebral vasculature, and some microvascular beds such as the mesentary. The absence of a separating fascia layer promotes paracrine communications between PVAT and the associated vasculature (Figure 1). As such, adipocytes from PVAT can infiltrate into the adventitia3, 17 to facilitate their effects on local inflammation and vascular tone. Indeed, PVAT has been shown to modulate vascular contractility via paracrine release of various factors including adiponectin.18–22 Furthermore, PVAT is anatomically co-localized with atherosclerotic lesions in humans, correlating with plaque burden and vascular calcifications.23, 24 Similar to visceral and subcutaneous adipose tissues, PVAT expands during obesity,25–27 with hypertrophy of both brown and white periaortic adipocytes noted in rodent models of high fat feeding.28, 29
Figure 1.
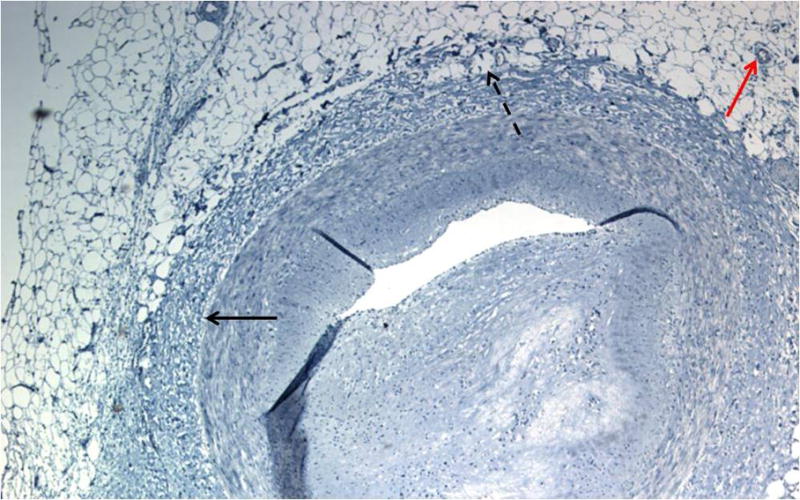
Histological appearance of human perivascular adipocytes surrounding an atherosclerotic coronary artery. Artery was fixed in formalin, sectioned, and counterstained with hematoxylin. Red arrow points to a blood vessel, solid black arrow denotes location of external elastic lamina, and dashed arrow points to PV adipocytes invading the lamina adventitia lacking a separating fascia. Note that these perivascular adipocytes exhibit an appearance more consistent with unilocular white adipocytes rather than multilocular brown adipocytes.
Mounting evidence suggests that the phenotype and function of PVAT varies depending on species and anatomic location, displaying features of traditional WAT versus energy-combusting BAT, or an intermediate between the two.17, 28–32 Most of the published data pertaining to brown adipose phenotype of PVAT were derived from rodent studies; the extent to which this reflects the phenotype of human PVAT is less clear. Human PV adipocytes surrounding coronary arteries exhibit a histological appearance and gene expression pattern more consistent with white rather than brown adipocytes17, 30 (Figure 1). However, as compared to subcutaneous and perirenal adipocytes derived from the same subjects, these PV adipocytes are smaller in size, less efficient at storing lipid, and express lower levels of adipocyte-specific genes, suggesting a reduced state of adipogenic differentiation.17 Global gene expression analyses indicate that these features of PV adipocytes map to early developmental differences, consistent with their origination from a distinct precursor cell,30 as has been proposed for adipose-derived stem cells surrounding perivascular regions of subcutaneous and visceral adipose tissues of mice33. In contrast, in vitro differentiated PV adipocytes derived from human radial arteries are larger in size and exhibit a high capacity to form lipid droplets, suggesting that the origin and/or differentiation state of human PV adipocytes is variable depending on the vascular bed.34
Developmental origins of adipocytes
The majority of research in the origin of adipocytes comes from work on subcutaneous and visceral adipocytes isolated from the stromal vascular fraction (SVF) of homogenized adipose tissues. The emerging consensus is that a majority of the mature adipocytes within each adipose tissue depot derive from precursor cells with distinct embryologic lineages.35 Indeed, in vitro differentiation of preadipocytes typically yields mature adipocytes that recapitulate the phenotype of their in vivo counterparts, thus accounting for the basic differences between individual adipocyte depots. However, heterogeneous, multipotent subpopulations of adipose- stromal cells reside within individual fat depots and can give rise to adipocytes with different phenotypes. Lineage analysis studies suggest that the source of adipose progenitor cells in visceral WAT depots is heterogeneous with the lateral plate mesoderm serving as a major contributor, and Wilms’ tumour gene 1 (Wt1)-positive progenitors giving rise predominately to epididymal as opposed to retroperitoneal fat. In contrast, Wt1 is not expressed in subcutaneous adipose tissue or BAT; BAT appears to originate mainly in the paraxial mesoderm.36, 37 Adipose stromal cells isolated from subcutaneous adipose tissues of mice also were suggested to be heterogeneous, with a subpopulation shown to have a neural crest origin, termed neural crest derived adipose stromal cells.38 These cells were demonstrated to have high adipogenic differentiation potential but markedly attenuated osteogenic and chondrogenic potentials compared to their non-neural crest adipose stromal cell counterparts.38 Studies involving PV adipocytes are relatively scarce, and, to the best of our knowledge, point toward an origin distinct from other adipocytes. Inferential evidence suggests that these cells may arise from vascular smooth muscle cell (VSMC) progenitors.39, 40 Moreover, in a murine model with VSMC-specific PPAR-γ deletion, the animals had normal WAT and BAT depots, but lacked PVAT.41 Although far from definitive proof, the above data suggest a common embryological origin for PV adipocytes and VSMC.
Nature vs. nurture: adipocyte plasticity
Multiple studies support the idea that the surrounding micro-environment is a critical factor in cell lineage determination of adipose stromal cells.30, 35 In addition, mature adipocytes have been suggested to possess the ability to de-differentiate into lipid-free fibroblast-like cells, which under proper induction culture in vivo or in vitro, demonstrated adipogenic, osteogenic, and chondrogenic potentials similar to adipose stromal cells isolated from SVF.42 Thus, the micro-environment may also regulate the differentiation state and phenotype of mature adipocytes. The function of brown adipocytes is generally linked to thermogenesis and temperature homeostasis, whereas white adipocytes are viewed to be important in energy storage and systemic endocrine functioning. However, this dichotomy has recently been challenged with the discovery of brite (brown-in-white) adipocytes detected in white adipose depots, which may contribute to temperature homeostasis and whole-body metabolism in humans.43–45 A bidirectional inter-conversion between brite adipocytes and white adipocytes was demonstrated in inguinal fat depots in mice.32 Cold exposure has been shown to stimulate brown adipocyte hyperplasia and increase metabolic activity in rodent models. In humans, short term cold exposure similarly increased 2-[18F]fluoro-2-deoxyglucose (FDG) uptake in BAT; positron emission tomography images raise the possibility of FDG uptake into fat surrounding the heart and great vessels.46, 47 Whether cold exposure directly or indirectly affects the function or phenotype of PV adipocytes remains to be determined.
Other dietary cues may also regulate the function of PV adipocytes. Chatterjee et al showed that feeding mice a high fat diet for just two weeks produced marked reduction in the expression of adipocyte-related genes (including the anti-inflammatory adipokine adiponectin) in thoracic PVAT, suggesting a reduction in the state of adipogenic differentiation.17 Moreover, expression of the pro-inflammatory cytokine MIP-1α was markedly upregulated in the PVAT, whereas expression in subcutaneous and visceral depots was minimally affected. These changes occurred prior to significant recruitment of inflammatory cells to the PVAT depot, suggesting that the PV adipocytes themselves responded adversely to the high fat diet. Likewise, high fat diet disrupted the balance between oxidant production and anti-oxidant defense mechanisms in murine PVAT;48 the oxidant stress resulting from this imbalance could contribute to amplification of inflammation in the PVAT depot.49 Consistent with this notion, mice deficient in adipose triglyceride lipase, a key lipolytic enzyme that regulates lipid accumulation in adipose tissues, exhibited marked expansion of the thoracic aorta PVAT depot along with amplification of oxidative stress and inflammation50. Interestingly, PV adipocytes (perimesenteric) from smokers, who also exhibit increased oxidative stress, expressed higher levels of MCP-1 mRNA, and enhanced activity of the P2X7R-inflammasome complex, as compared with adipocytes isolated from non-smokers.51 Taken together, these data suggest that adipocytes exhibit considerable plasticity in response to environmental cues, and responsiveness of PV adipocytes to noxious stimuli contained in high fat diet and/or tobacco smoke may promote a pro-inflammatory state conducive to the development of atherosclerosis.
Regulation of inflammation by perivascular adipocytes
The inflammatory state of human PVAT has been the subject of intense research in the last decade. Many studies have shown a heightened state of inflammation in PVAT surrounding atherosclerotic blood vessels, both in terms of inflammatory cell infiltration3, 52, 53 and pro-inflammatory gene expression.3, 53 However, PVAT is comprised of many cell types, so the specific contribution of PV adipocytes to the global inflammatory state of the tissue is impossible to infer from these studies. Only a few publications have investigated the inflammatory potential of PV adipocytes, either freshly isolated or in vitro differentiated. Therefore, the information presented here contains the most current knowledge regarding this topic and is inherently limited in scope. Nevertheless, these data suggest that human PV adipocytes surrounding conduit arteries possess intrinsic pro-inflammatory characteristics. Our laboratory demonstrated that human pericoronary PV adipocytes expressed high levels of MCP-1, IL-8, and IL-617 which play a critical role in inflammatory cell recruitment to adipose tissues3 and are also important in the pathobiology of vascular disease.54 In particular, release of MCP-1 protein from in vitro differentiated PV adipocytes was nearly 50-fold greater than that from adipocytes derived from other regional depots of the same subjects.17 Human periradial adipocytes likewise expressed higher levels of pro-inflammatory MCP-1 and pro-angiogenic mediators as compared with visceral or subcutaneous adipocytes.34 In a wire-induced injury model in mice, Jabs et al showed sequential expression of MCP-1 and other cytokines/cytokine receptors in PV tissue starting on the adventitial aspect of the vessel and progressing toward the intima.55 MCP-1 gene deletion in animal models led to substantial reductions in arterial wall macrophage infiltration and lipid deposition.56, 57 Conditioned media from PV adipocytes elicited chemotaxis of granulocytes, monocytes, and activated T cells, and stimulated VSMC proliferation, which supports a pathological role for cytokines and growth factors produced by PV adipocytes.3, 30, 58
Conversely, pericoronary PV adipocytes secreted markedly less adiponectin (an anti-inflammatory adipokine) as compared with their subcutaneous and perirenal counterparts, which is further consistent with a pro-inflammatory phenotype. It is important to point out that the PVAT was obtained from previously healthy organ donors without a pre-existing history of heart disease, suggesting that the pro-inflammatory phenotype was a fundamental property of the cells and not the product of an atherosclerotic milieu.17 Extending these studies, we performed genome-wide analyses comparing human subcutaneous and PV adipocytes and showed that PV adipocytes had a strikingly higher expression of genes involved in pro-inflammatory pathways including cell adhesion molecules and complement system factors such as complement 7, complement H, SERPINE2, SERPINE1, and cytosolic PLA2.30 In the latter study, the most differentially upregulated gene expressed by PV adipocytes was tumor necrosis factor receptor superfamily member 11b (osteoprotegerin). Osteoprotegerin is a secreted protein that is best known as a decoy ligand for RANK that regulates osteogenesis, but it also exerts both pro-inflammatory and anti-inflammatory effects, and its levels are elevated in humans with atherosclerosis and in mice fed a high fat diet.59 As compared with subcutaneous adipocytes, PV adipocytes secreted markedly higher levels of osteoprotegerin protein, raising the possibility that PVAT contributes importantly to systemic osteoprotegerin levels in obesity and atherosclerosis. The functional importance of pro-inflammatory mediator secretion by PV adipocytes was demonstrated using a transendothelial monocyte migration assay. As compared to conditioned medium from subcutaneous adipocytes, medium from PV adipocytes induced a two-fold increase in monocytic cell migration across an endothelial monolayer, supporting a pro-inflammatory phenotype of human pericoronary PV adipocytes.30
On the other hand, our microarray data also showed increased expression of certain anti-inflammatory genes by the PV adipocytes, including tumor necrosis factor α-induced protein 6 (TAIP6 or TSG6) and suppressor of cytokine signaling 2 (SOCS2), which suggests that PV adipocytes can also exert anti-inflammatory effects.30 A protective effect of PVAT is likewise inferred by the observation that removal of PVAT from the femoral artery enhanced neointima formation after wire injury in high fat fed mice, suggesting that the presence of some amount of PVAT is protective and needed to maintain vascular homeostasis.60 Interestingly, in the latter study, replacement of PVAT with subcutaneous fat from mice fed a chow diet reduced neointima formation, whereas transplanted subcutaneous fat from high fat fed mice was without effect, which further suggests a link between high fat diet and regulation of vascular disease by PVAT. In contrast to these findings, transplanting visceral fat to the murine carotid artery (which is normally devoid of PVAT) enhanced atherosclerosis, mediated in part via P-selectin glycoprotein ligand-1.61 It must be pointed out that none of the aforementioned studies transplanted authentic PVAT, which differs phenotypically from subcutaneous and other visceral fat depots, so the results of these studies may not be directly applicable to endogenous PVAT. With regard to the effects of dietary fat on PVAT, mice fed a longer-term high fat diet demonstrated reduced macrophage infiltration into regions of brown PVAT,29 and atherosclerosis was enhanced in mice devoid of PVAT that were housed under conditions of low temperature, most likely due to loss of the systemic metabolic effects of brown PVAT during adaptive thermogenesis.41 These studies suggest that the inflammatory phenotype of PVAT is diverse depending on species, anatomic location, and environmental conditions, and that these differences are fundamentally important in determining a pathogenic versus protective role of PVAT in vascular disease.
Perspective: balancing pro- versus anti-inflammatory effects of PVAT
The available data, when interpreted in total, suggest that PVAT is capable of both positively and negatively regulating vascular inflammation to modulate vascular disease (Figure 2). Data in human PV adipocytes suggest that MCP-1 is likely to be an important pro-inflammatory mediator that is expressed at high levels and may contribute to vascular disease. PV adipocytes also secrete pro-inflammatory adipokines and express complement system factors and cell adhesion/matrix proteins that may facilitate inflammation. A number of anti-inflammatory molecules likely counterbalance the pro-inflammatory state of human PV adipocytes. Data in rodent models clearly indicate that PVAT secretes anti-inflammatory factors that can favorably influence vascular pathophysiology. A number of questions remain unanswered, such as how PV adipocytes interact with inflammatory cells, and what role the nervous system might play in regulating PVAT inflammation. Further research into the mechanisms that regulate the inflammatory balance of PV adipocytes may yield new insight into, and treatment strategies for, cardiovascular disease.
Figure 2.
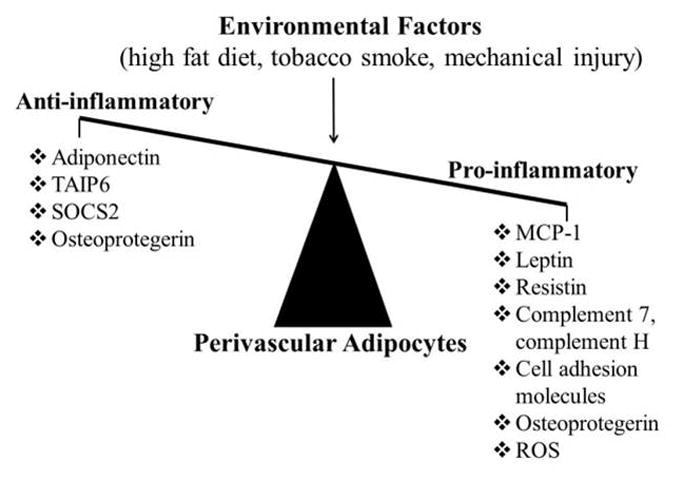
Balancing pro- versus anti-inflammatory effects of PV adipocytes.
Abbreviations: TAIP6, tumor necrosis factor α-induced protein 6; SOCS2, suppressor of cytokine signaling 2; MCP-1, monocyte chemoattractive peptide-1; ROS, reactive oxygen species.
Acknowledgments
This work was supported by NIH grants HL076684 and HL112640 (to N.L.W.), HL086555 (to Y.T.), and DK74932 (to D.Y.H).
Abbreviations
- BAT
brown adipose tissue
- FDG
2-[18F]fluoro-2-deoxyglucose
- PV
perivascular
- PVAT
perivascular adipose tissue
- SOCS2
suppressor of cytokine signaling 2
- SVF
stromal vascular fraction
- VSMC
vascular smooth muscle cell
- WAT
white adipose tissue
- Wlt1
Wilms’ tumour gene 1
References
- 1.Maiellaro K, Taylor WR. The role of the adventitia in vascular inflammation. Cardiovascular research. 2007;75:640–648. doi: 10.1016/j.cardiores.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moos MP, John N, Grabner R, Nossmann S, Gunther B, Vollandt R, Funk CD, Kaiser B, Habenicht AJ. The lamina adventitia is the major site of immune cell accumulation in standard chow-fed apolipoprotein e-deficient mice. Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2386–2391. doi: 10.1161/01.ATV.0000187470.31662.fe. [DOI] [PubMed] [Google Scholar]
- 3.Henrichot E, Juge-Aubry CE, Pernin A, Pache JC, Velebit V, Dayer JM, Meda P, Chizzolini C, Meier CA. Production of chemokines by perivascular adipose tissue: A role in the pathogenesis of atherosclerosis? Arteriosclerosis, thrombosis, and vascular biology. 2005;25:2594–2599. doi: 10.1161/01.ATV.0000188508.40052.35. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto E, Couse T, De Leon H, Vinten-Johansen J, Goodman RB, Scott NA, Wilcox JN. Perivascular inflammation after balloon angioplasty of porcine coronary arteries. Circulation. 2001;104:2228–2235. doi: 10.1161/hc4301.097195. [DOI] [PubMed] [Google Scholar]
- 5.Takaoka M, Suzuki H, Shioda S, Sekikawa K, Saito Y, Nagai R, Sata M. Endovascular injury induces rapid phenotypic changes in perivascular adipose tissue. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1576–1582. doi: 10.1161/ATVBAHA.110.207175. [DOI] [PubMed] [Google Scholar]
- 6.Pang SS, Le YY. Role of resistin in inflammation and inflammation-related diseases. Cellular & molecular immunology. 2006;3:29–34. [PubMed] [Google Scholar]
- 7.Al-Suhaimi EA, Shehzad A. Leptin, resistin and visfatin: The missing link between endocrine metabolic disorders and immunity. European journal of medical research. 2013;18:12. doi: 10.1186/2047-783X-18-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohashi K, Ouchi N, Matsuzawa Y. Anti-inflammatory and anti-atherogenic properties of adiponectin. Biochimie. 2012;94:2137–2142. doi: 10.1016/j.biochi.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circulation research. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 10.Tilg H, Moschen AR. Adipocytokines: Mediators linking adipose tissue, inflammation and immunity. Nature reviews. Immunology. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 11.Azeez OI, Meintjes R, Chamunorwa JP. Fat body, fat pad and adipose tissues in invertebrates and vertebrates: The nexus. Lipids in health and disease. 2014;13:71. doi: 10.1186/1476-511X-13-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 13.Ghigliotti G, Barisione C, Garibaldi S, Fabbi P, Brunelli C, Spallarossa P, Altieri P, Rosa G, Spinella G, Palombo D, Arsenescu R, Arsenescu V. Adipose tissue immune response: Novel triggers and consequences for chronic inflammatory conditions. Inflammation. 2014 doi: 10.1007/s10753-014-9914-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huh JY, Park YJ, Ham M, Kim JB. Crosstalk between adipocytes and immune cells in adipose tissue inflammation and metabolic dysregulation in obesity. Molecules and cells. 2014 doi: 10.14348/molcells.2014.0074. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eringa EC, Bakker W, van Hinsbergh VW. Paracrine regulation of vascular tone, inflammation and insulin sensitivity by perivascular adipose tissue. Vascular pharmacology. 2012;56:204–209. doi: 10.1016/j.vph.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 16.Bulloch JM, Daly CJ. Autonomic nerves and perivascular fat: Interactive mechanisms. Pharmacology & therapeutics. 2014;143:61–73. doi: 10.1016/j.pharmthera.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Chatterjee TK, Stoll LL, Denning GM, Harrelson A, Blomkalns AL, Idelman G, Rothenberg FG, Neltner B, Romig-Martin SA, Dickson EW, Rudich S, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes: Influence of high-fat feeding. Circulation research. 2009;104:541–549. doi: 10.1161/CIRCRESAHA.108.182998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lohn M, Dubrovska G, Lauterbach B, Luft FC, Gollasch M, Sharma AM. Periadventitial fat releases a vascular relaxing factor. FASEB journal: official publication of the Federation of American Societies for Experimental Biology. 2002;16:1057–1063. doi: 10.1096/fj.02-0024com. [DOI] [PubMed] [Google Scholar]
- 19.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, Alloosh M, Sturek M, Tune JD. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: Influence of obesity. Circulation. 2013;128:9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Payne GA, Borbouse L, Kumar S, Neeb Z, Alloosh M, Sturek M, Tune JD. Epicardial perivascular adipose-derived leptin exacerbates coronary endothelial dysfunction in metabolic syndrome via a protein kinase c-beta pathway. Arteriosclerosis, thrombosis, and vascular biology. 2010;30:1711–1717. doi: 10.1161/ATVBAHA.110.210070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Withers SB, Agabiti-Rosei C, Livingstone DM, Little MC, Aslam R, Malik RA, Heagerty AM. Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arteriosclerosis, thrombosis, and vascular biology. 2011;31:908–913. doi: 10.1161/ATVBAHA.110.221705. [DOI] [PubMed] [Google Scholar]
- 22.Lynch FM, Withers SB, Yao Z, Werner ME, Edwards G, Weston AH, Heagerty AM. Perivascular adipose tissue-derived adiponectin activates bk(ca) channels to induce anticontractile responses. American journal of physiology. Heart and circulatory physiology. 2013;304:H786–795. doi: 10.1152/ajpheart.00697.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lehman SJ, Massaro JM, Schlett CL, O’Donnell CJ, Hoffmann U, Fox CS. Peri-aortic fat, cardiovascular disease risk factors, and aortic calcification: The framingham heart study. Atherosclerosis. 2010;210:656–661. doi: 10.1016/j.atherosclerosis.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahabadi AA, Reinsch N, Lehmann N, Altenbernd J, Kalsch H, Seibel RM, Erbel R, Mohlenkamp S. Association of pericoronary fat volume with atherosclerotic plaque burden in the underlying coronary artery: A segment analysis. Atherosclerosis. 2010;211:195–199. doi: 10.1016/j.atherosclerosis.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Ding J, Hsu FC, Harris TB, Liu Y, Kritchevsky SB, Szklo M, Ouyang P, Espeland MA, Lohman KK, Criqui MH, Allison M, Bluemke DA, Carr JJ. The association of pericardial fat with incident coronary heart disease: The multi-ethnic study of atherosclerosis (mesa) The American journal of clinical nutrition. 2009;90:499–504. doi: 10.3945/ajcn.2008.27358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greif M, Becker A, von Ziegler F, Lebherz C, Lehrke M, Broedl UC, Tittus J, Parhofer K, Becker C, Reiser M, Knez A, Leber AW. Pericardial adipose tissue determined by dual source ct is a risk factor for coronary atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 27.Kim HM, Kim KJ, Lee HJ, Yu HT, Moon JH, Kang ES, Cha BS, Lee HC, Lee BW, Kim YJ. Epicardial adipose tissue thickness is an indicator for coronary artery stenosis in asymptomatic type 2 diabetic patients: Its assessment by cardiac magnetic resonance. Cardiovascular diabetology. 2012;11:83. doi: 10.1186/1475-2840-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Police SB, Thatcher SE, Charnigo R, Daugherty A, Cassis LA. Obesity promotes inflammation in periaortic adipose tissue and angiotensin ii-induced abdominal aortic aneurysm formation. Arteriosclerosis, thrombosis, and vascular biology. 2009;29:1458–1464. doi: 10.1161/ATVBAHA.109.192658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgibbons TP, Kogan S, Aouadi M, Hendricks GM, Straubhaar J, Czech MP. Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. American journal of physiology. Heart and circulatory physiology. 2011;301:H1425–1437. doi: 10.1152/ajpheart.00376.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chatterjee TK, Aronow BJ, Tong WS, Manka D, Tang Y, Bogdanov VY, Unruh D, Blomkalns AL, Piegore MG, Jr, Weintraub DS, Rudich SM, Kuhel DG, Hui DY, Weintraub NL. Human coronary artery perivascular adipocytes overexpress genes responsible for regulating vascular morphology, inflammation, and hemostasis. Physiological genomics. 2013;45:697–709. doi: 10.1152/physiolgenomics.00042.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galvez-Prieto B, Bolbrinker J, Stucchi P, de Las Heras AI, Merino B, Arribas S, Ruiz-Gayo M, Huber M, Wehland M, Kreutz R, Fernandez-Alfonso MS. Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. The Journal of endocrinology. 2008;197:55–64. doi: 10.1677/JOE-07-0284. [DOI] [PubMed] [Google Scholar]
- 32.Rosenwald M, Wolfrum C. The origin and definition of brite versus white and classical brown adipocytes. Adipocyte. 2014;3:4–9. doi: 10.4161/adip.26232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tokunaga M, Inoue M, Jiang Y, Barnes RH, 2nd, Buchner DA, Chun TH. Fat depot-specific gene signature and ecm remodeling of sca1 adipose-derived stem cells. Matrix biology: journal of the International Society for Matrix Biology. 2014 doi: 10.1016/j.matbio.2014.03.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rittig K, Dolderer JH, Balletshofer B, Machann J, Schick F, Meile T, Kuper M, Stock UA, Staiger H, Machicao F, Schaller HE, Konigsrainer A, Haring HU, Siegel-Axel DI. The secretion pattern of perivascular fat cells is different from that of subcutaneous and visceral fat cells. Diabetologia. 2012;55:1514–1525. doi: 10.1007/s00125-012-2481-9. [DOI] [PubMed] [Google Scholar]
- 35.Tchkonia T, Lenburg M, Thomou T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. American journal of physiology. Endocrinology and metabolism. 2007;292:E298–307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 36.Chau Y-Y, Bandiera R, Serrels A, Martínez-Estrada OM, Qing W, Lee M, Slight J, Thornburn A, Berry R, McHaffie S, Stimson RH, Walker BR, Chapuli RM, Schedl A, Hastie N. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16:367–375. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chau YY, Brownstein D, Mjoseng H, et al. Acute multiple organ failure in adult mice deleted for the developmental regulator wt1. PLoS genetics. 2011;7:e1002404. doi: 10.1371/journal.pgen.1002404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sowa Y, Imura T, Numajiri T, Takeda K, Mabuchi Y, Matsuzaki Y, Nishino K. Adipose stromal cells contain phenotypically distinct adipogenic progenitors derived from neural crest. PloS one. 2013;8:e84206. doi: 10.1371/journal.pone.0084206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cai X, Lin Y, Hauschka PV, Grottkau BE. Adipose stem cells originate from perivascular cells. Biology of the cell / under the auspices of the European Cell Biology Organization. 2011;103:435–447. doi: 10.1042/BC20110033. [DOI] [PubMed] [Google Scholar]
- 40.Zhao X, Gong P, Lin Y, Wang J, Yang X, Cai X. Characterization of alpha-smooth muscle actin positive cells during multilineage differentiation of dental pulp stem cells. Cell proliferation. 2012;45:259–265. doi: 10.1111/j.1365-2184.2012.00818.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chang L, Villacorta L, Li R, Hamblin M, Xu W, Dou C, Zhang J, Wu J, Zeng R, Chen YE. Loss of perivascular adipose tissue on peroxisome proliferator-activated receptor-gamma deletion in smooth muscle cells impairs intravascular thermoregulation and enhances atherosclerosis. Circulation. 2012;126:1067–1078. doi: 10.1161/CIRCULATIONAHA.112.104489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen JF, Sugawara A, Yamashita J, Ogura H, Sato S. Dedifferentiated fat cells: An alternative source of adult multipotent cells from the adipose tissues. International journal of oral science. 2011;3:117–124. doi: 10.4248/IJOS11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee P, Werner CD, Kebebew E, Celi FS. Functional thermogenic beige adipogenesis is inducible in human neck fat. International journal of obesity (2005) 2014;38:170–176. doi: 10.1038/ijo.2013.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: Is beige the new brown? Genes & development. 2013;27:234–250. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye L, Wu J, Cohen P, Kazak L, Khandekar MJ, Jedrychowski MP, Zeng X, Gygi SP, Spiegelman BM. Fat cells directly sense temperature to activate thermogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:12480–12485. doi: 10.1073/pnas.1310261110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito M, Okamatsu-Ogura Y, Matsushita M, Watanabe K, Yoneshiro T, Nio-Kobayashi J, Iwanaga T, Miyagawa M, Kameya T, Nakada K, Kawai Y, Tsujisaki M. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van Marken Lichtenbelt WD, Vanhommerig JW, Smulders NM, Drossaerts JM, Kemerink GJ, Bouvy ND, Schrauwen P, Teule GJ. Cold-activated brown adipose tissue in healthy men. The New England journal of medicine. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 48.Gil-Ortega M, Condezo-Hoyos L, Garcia-Prieto CF, Arribas SM, Gonzalez MC, Aranguez I, Ruiz-Gayo M, Somoza B, Fernandez-Alfonso MS. Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PloS one. 2014;9:e95312. doi: 10.1371/journal.pone.0095312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hajjar DP, Gotto AM., Jr Biological relevance of inflammation and oxidative stress in the pathogenesis of arterial diseases. The American journal of pathology. 2013;182:1474–1481. doi: 10.1016/j.ajpath.2013.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrammel A, Mussbacher M, Wolkart G, Stessel H, Pail K, Winkler S, Schweiger M, Haemmerle G, Al Zoughbi W, Hofler G, Lametschwandtner A, Zechner R, Mayer B. Endothelial dysfunction in adipose triglyceride lipase deficiency. Biochimica et biophysica acta. 2014;1841:906–917. doi: 10.1016/j.bbalip.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rossi C, Santini E, Chiarugi M, Salvati A, Comassi M, Vitolo E, Madec S, Solini A. The complex p2×7 receptor/inflammasome in perivascular fat tissue of heavy smokers. European journal of clinical investigation. 2014;44:295–302. doi: 10.1111/eci.12232. [DOI] [PubMed] [Google Scholar]
- 52.Verhagen SN, Buijsrogge MP, Vink A, van Herwerden LA, van der Graaf Y, Visseren FL. Secretion of adipocytokines by perivascular adipose tissue near stenotic and non-stenotic coronary artery segments in patients undergoing cabg. Atherosclerosis. 2014;233:242–247. doi: 10.1016/j.atherosclerosis.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Verhagen SN, Visseren FL. Perivascular adipose tissue as a cause of atherosclerosis. Atherosclerosis. 2011;214:3–10. doi: 10.1016/j.atherosclerosis.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 54.Lee HY, Despres JP, Koh KK. Perivascular adipose tissue in the pathogenesis of cardiovascular disease. Atherosclerosis. 2013;230:177–184. doi: 10.1016/j.atherosclerosis.2013.07.037. [DOI] [PubMed] [Google Scholar]
- 55.Jabs A, Okamoto E, Vinten-Johansen J, Bauriedel G, Wilcox JN. Sequential patterns of chemokine- and chemokine receptor-synthesis following vessel wall injury in porcine coronary arteries. Atherosclerosis. 2007;192:75–84. doi: 10.1016/j.atherosclerosis.2006.05.050. [DOI] [PubMed] [Google Scholar]
- 56.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (mcp-1): An overview. Journal of interferon & cytokine research: the official journal of the International Society for Interferon and Cytokine Research. 2009;29:313–326. doi: 10.1089/jir.2008.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schober A. Chemokines in vascular dysfunction and remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2008;28:1950–1959. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 58.Miao CY, Li ZY. The role of perivascular adipose tissue in vascular smooth muscle cell growth. British journal of pharmacology. 2012;165:643–658. doi: 10.1111/j.1476-5381.2011.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Van Campenhout A, Golledge J. Osteoprotegerin, vascular calcification and atherosclerosis. Atherosclerosis. 2009;204:321–329. doi: 10.1016/j.atherosclerosis.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Takaoka M, Nagata D, Kihara S, Shimomura I, Kimura Y, Tabata Y, Saito Y, Nagai R, Sata M. Periadventitial adipose tissue plays a critical role in vascular remodeling. Circulation research. 2009;105:906–911. doi: 10.1161/CIRCRESAHA.109.199653. [DOI] [PubMed] [Google Scholar]
- 61.Ohman MK, Luo W, Wang H, Guo C, Abdallah W, Russo HM, Eitzman DT. Perivascular visceral adipose tissue induces atherosclerosis in apolipoprotein e deficient mice. Atherosclerosis. 2011;219:33–39. doi: 10.1016/j.atherosclerosis.2011.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


