Abstract
Background
Early adverse life (EAL) events and sex have been identified as vulnerability factors for the development of several stress-sensitive disorders, including irritable bowel syndrome (IBS). We aimed to identify disease and sex-based differences in resting state (RS) connectivity associated with EALs in individuals with IBS.
Method
A history of EALs before age 18 was assessed using the early trauma inventory. RS functional magnetic resonance imaging was used to identify patterns of intrinsic brain oscillations in the form of RS networks in 168 people (58 IBS, 28 females; and 110 healthy controls, 72 females). Partial Least Squares, a multivariate analysis technique was used to identify disease and sex differences and possible correlations between EALs and functional connectivity in six identified RS networks.
Results
Associations between EALs and RS networks were observed. While a history of EALs was associated with altered connectivity in the salience/executive control network to a similar extent in male and female IBS patients (Bootstrap ratio [BSR]=3.28-5.61; p=.046), male IBS patients demonstrated additional EAL-related alterations in the cerebellar network (BSR=3.92-6.79; p=.022).
Conclusion
This cross sectional study identified correlations between RS networks and EALs in individuals with IBS. These results suggest that exposure to EALs before age 18 can shape adult RS in both male and female patients in the salience/executive control network, a brain network that has been implicated in the pathophysiology of central pain amplification.
Keywords: early adverse traumatic life events, irritable bowel syndrome (IBS), resting state networks, salience/executive control network, cerebellar network, sex differences
Introduction
Irritable bowel syndrome (IBS), is the most common functional pain disorder with a prevalence of up to 15% of US adults (1, 2). IBS patients are more likely to report histories of early adverse life events (EALs), such as loss or chronic illness of a parent, discordant relationships between parents or various types of abuse (3-6). A history of EALs is also associated with an increased risk to develop post-infectious IBS (7, 8), psychiatric and somatic co-morbidities (3, 9), and the likelihood of gastrointestinal related complications and outcomes such as lower quality of life, increased visceral pain, and poorer responses to treatment (3, 10). Converging evidence from clinical, epidemiological and neuroimaging studies are consistent with the hypothesis that EALs can modulate brain-gut interactions to increase IBS-related symptoms (11-13).
A large number of functional neuroimaging studies have characterized evoked brain responses during an active task condition in IBS compared to healthy controls (HCs) (14-18). The majority of these studies used controlled rectal distension as the stimulus and showed greater engagement of regions of the salience network (including thalamus [THAL], insula [INS], and anterior cingulate cortex [ACC]) in IBS patients, with some studies showing abnormal responsiveness of brain regions related to emotional arousal (such as amygdala [AMYG], subgenual and pregenual ACC, hippocampus, hypothalamus) and endogenous pain inhibition (such as AMYG, subregions of the ACC, THAL, anterior, middle and posterior INS) (19-22).”
Studies investigating structural (grey and white matter) brain differences between IBS and HCs have demonstrated disease related differences in regions involved in sensory processing, integration, and modulation (THAL, basal ganglia, somatosensory cortex), prefrontal cognitive modulation, and saliency (ACC, INS) (23-26). It remains to be determined if these structural changes reflect the brain's response to chronic nociceptive stimuli from the periphery, or represent preexisting genetic and epigenetic (such as EAL related) brain changes which increase the vulnerability of an individual to develop IBS.
Some evidence suggests that different brain mechanisms may contribute to IBS symptom generation in female and male patients (27, 28). For example, while female patients show evidence for an upregulation of emotional arousal circuits during an aversive rectal stimulus, male patients show greater engagement of prefrontal regions (21, 29). In females, the greater emotional arousal circuit activation was seen primarily during expectation (rather than actual delivery) of the rectal stimulus (2). Recently, marked sex related differences in regional alterations in the power of intrinsic oscillation frequency were reported in IBS, consistent with alterations in the salience network. Similar to the sex differences observed in evoked brain responses, females showed evidence for higher activity in regions of an emotional arousal circuit (30).
EAL-related alterations in the effective connectivity of the emotional arousal circuitry following visceral (2) and emotional (21) stimuli have been reported in IBS patients (2, 21, 31), suggesting that higher EALs are associated with reduced cortical inhibition of emotional arousal circuits (29). Based on this evidence supporting a role of EALs in IBS etiology, we aimed to test the general hypothesis that a history of EALs is related to brain activity in networks that are involved in determining the salience of somatic, visceral or environmental stimuli in IBS. To test this hypothesis, we used resting state fMRI to determine 1) IBS, and 2) EAL related alterations in the connectivity within six pain, emotion and cognition related RS networks.
Methods
Participants
185 participants were recruited through UCLA Digestive Diseases Clinic and advertisements in the Los Angeles community from April 2009 till March 2012. Advertisements were circulated through online social media websites, local newspapers, university and hospital community listservs and mailing lists, and flyers posted in the greater Los Angeles area and on the UCLA campus. 17 participants were dropped from the analyses, due to either abnormalities in scanning data acquisition or because behavioral data was not available (did not complete the early traumatic events questionnaire). 168 subjects consisting of 58 IBS (28 females) and 110 HC (72 females) were used in the analyses. All participants were right-handed. Participants were screened via medical exam for absence of significant medical conditions other than a diagnosis of IBS. A gastroenterologist or nurse practitioner made a diagnosis of IBS based on the ROME symptom criteria (32). IBS is defined as recurrent abdominal pain or discomfort for at least 3 days/month in the last 3 months and is associated with two or more of the following: 1) Improvement with defecation. 2) Onset associated with a change in frequency of stool. 3) Onset associated with a change in form (appearance) of stool. Exclusion criteria in both groups comprised pregnancy, substance abuse, abdominal surgery, tobacco dependence, excessive physical exercise (more than 8 hours/week of continuous exercise such as marathon runners or triathlon athletes), and ongoing psychiatric illness as determined by the Mini International Neuropsychiatric Interview (MINI) (33). Subjects taking medications that interfere with the central nervous system (full dose antidepressants including SSRI, NSRIs, sedatives or anxiolytics, and opioids) were also excluded. However, as most IBS patients take low dose tricyclic antidepressants, subjects on tricyclic antidepressants were allowed into the study provided that the dose of the drug was between 10-50mg, and that the subject had been on a stable dose for at least 3 months. Since female sex hormones such as estrogen are known to effect brain structure and function, in this study we used women who were premenopausal and who were during the follicular phase of their menstrual cycles. Study protocols were performed after approval by the review committee at UCLA's Office of Protection for Research Subjects, and written informed consent was obtained from all subjects. The demographic characteristics of the subjects are shown in Table 1.
Table 1. Study Demographics and Clinical Behavioral Measures.
| Male HC | Female HC | Male IBS | Female IBS | F | Sig | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | |||
| N | 38 | 72 | 30 | 28 | - | - | ||||
| Age (yrs) | 35.95 | 12.97 | 29.39 | 9.93 | 37.28 | 10.75 | 30.65 | 10.71 | 5.53 | .001 |
| ETI | 4.17 | 3.97 | 3.27 | 3.45 | 6.48 | 4.77 | 5.33 | 4.97 | 5.01 | .002 |
| NEO_Neuroticism | 47.03 | 10.40 | 43.60 | 10.14 | 52.72 | 14.78 | 49.06 | 9.52 | 5.26 | .002 |
| HAD Anxiety | 3.26 | 2.60 | 3.04 | 2.44 | 5.69 | 4.45 | 5.03 | 3.67 | 7.12 | .000 |
| HAD Depression | 1.64 | 1.56 | 1.09 | 1.73 | 2.79 | 3.40 | 2.42 | 2.50 | 5.48 | .001 |
Questionnaires: Early Traumatic Inventory (ETI), NEO_Neuroticism; Hospital Anxiety and Depression (Anxiety), Hospital Anxiety and Depression (Depression).
Subject Number (N), Standard Deviation (SD)
Significant = p<0.05*, p<0.005**
Behavioral Measures
Questionnaires were completed before scanning.
The Early Traumatic Inventory (ETI) (34) was used to access histories of childhood traumatic and adverse life events in four domains: general trauma, physical, emotional, and sexual abuse. General traumatic events comprise a range of stressful and traumatic events that can be mostly secondary to chance events. Physical abuse involves physical contact, constraint, or confinement, with intent to hurt or injure. Emotional abuse is verbal communication with the intention of humiliating or degrading the victim. Sexual abuse is unwanted sexual contact performed solely for the gratification of the perpetrator or for the purposes of dominating or degrading the victim. The ETI has been found to have good internal consistency (Cronbach α=.70) (35).
The Neuroticism subscale on the NEO-Personality Inventory (36) was used to access personality traits associated with neuroticism. Internal reliability measures range between .75 to .82 (37). Common clinical mental comorbidities associated with IBS such as anxiety and depression were measured using a self-report 14-item instrument (Hospital Anxiety and Depression Inventory; HAD) (38). Internal consistency measures have ranged between .78 and .90 (39).
fMRI Acquisition
Whole brain functional magnetic resonance imaging (fMRI) data was obtained by aggregating data from multiple studies, which has been documented to be a viable form of using data from multiple sources in order to describe comprehensive functional connectome maps in human studies (40). A high resolution structural image was acquired from each subject for registration purposes with a magnetization-prepared rapid gradient-echo (MP-RAGE) sequence, repetition time (TR) = 2200ms, echo time (TE) = 3.26ms, TA = 9m3s, slice thickness = 1 mm, 176 slices, 256 × 256 voxel matrices, and 13 mm voxel size. The duration of the resting scan varied from 5 - 10 minutes. Noise reducing headphones were used. Subjects rested with eyes closed while functional blood oxygen-level dependent (BOLD) images were acquired. We pooled resting state fMRI data using different scanning protocols and with different TRs (2000-3000ms) for the purposes of comparing patterns of BOLD oscillation intrinsic connectivity patterns in a large sample size. Scanning protocol details follow:
Siemens 3 Tesla Trio using the following parameters: 40-slice whole brain volumes, slice thickness = 4mm, Repetition time (TR) = 2000ms, Echo time (TE) = 28ms, Resting duration (TA) = 10m 6s, flip angle = 77°, FOV = 220. (23 female HCs, 24 male HCs, 20 male IBS subjects, 22 female IBS subjects) (21, 41).
Siemens 3 Tesla Trio using the following parameters: 38-slice whole brain volumes, slice thickness 3mm, TR = 2500ms, TE = 26ms, TA = 5m 8s, flip angle = 90°, FOV = 200. (41 female HCs) (42).
Siemens 3 Tesla Trio using the following parameters: 40-slice whole brain volumes, slice thickness 4mm, TR = 2000ms, TE = 28ms, TA = 8m 6s, flip angle = 77°, FOV = 220. (8 female HCs, 6 female IBS subjects) (43).
Siemens 3 Tesla Allegra using the following parameters: 38-slice whole brain volumes, slice thickness = 3mm, TR = 3000ms, TE = 28ms, TA = 6m 6s, flip angle = 90°, FOV = 200. (14 male HCs, 10 male IBS subjects) (21).
Following the resting scan, some subjects completed additional fMRI scans involving threat and pain related tasks; these data are not included in the current report, and have been reported elsewhere (25, 44).
fMRI Preprocessing
Functional imaging preprocessing and statistical analyses were carried out using SPM8 software (Welcome Department of Cognitive Neurology, London, UK). Images were realigned to the first image and were slice-time and motion corrected. The anatomical image was spatially normalized to the Montreal Neurological Institute (MNI) template, and the resulting parameter file was used to normalize the functional images (voxel size: 2×2×2 mm3) and was spatially smoothed with a 5 mm3 Gaussian kernel. The first two volumes were discarded to allow for stabilization of the magnetic field.
Statistical Analyses
Group independent component analysis (gICA) was performed to quantify resting state networks (RSN) in IBS and HCs and was implemented with GIFT 2.0c (http://www.icatb.sourceforge.com). The minimum description length (MDL) was used to objectively identify the number of independent components (IC) to be extracted (45). The RS data was decomposed into 15 IC maps per subject using infomax algorithm and ICASSO (46, 47). Spatial correlation was used to determine which ICs represented the RSNs of interest based on prior neuroimaging studies associated with IBS disease, sex differences or EALs (19, 21, 30). Specifically, we chose the ICs that correlated highest with canonical RSN templates (48), and where the majority of power spectral distribution was in the lower frequencies (< .08 Hz). RSNs of interest included: 1) Salience/Executive Control (referred to throughout the text as salience network) (associated with cognitive control, executive attention, emotion, perception-somesthesis-pain) 2) Left Frontal Parietal, (associated with cognitive modulation, somatosensory perception), 3) Right Frontal Parietal (associated with cognition, language), 4) Cognitive Control, 5) Cerebellar (associated with action-execution, perception-somesthesis-pain), and 6) Default Mode Network (associated with self-referential activity) (48). Individual subject maps for each IC were extracted. These maps contained z-score values that represented the degree of correlation between the voxel signal and the group averaged time-course of the component (i.e. network functional connectivity). Higher z values indicate greater connectivity strength or influence of that voxel on the network (49). As a final noise reduction step, all individual maps were entered into one-sample t-tests in SPM8 and thresholded at p<0.05 corrected for family-wise error (FWE).
Thresholded individual subject component maps were entered into a partial least squares (PLS) analysis. PLS (http://www.rotman- baycrest.on.ca) is a multivariate statistical technique similar to principle component analysis (PCA), where solutions are restricted to the part of the covariance structure that is attributable to group difference contrasts (non-rotated task PLS) or covariate measures (behavioral PLS) (50). PLS was our analysis of choice as it allows for inferences about networks (51). PLS essentially extracts common information from two data sets (e.g., intrinsic network connectivity maps and EAL scores), which generalize to the population. PLS is considered a random effects model as it employs permutation tests that assess generalizability of the pattern and bootstrapping to identify the stable or most reliable elements of the pattern (For more detail on PLS methodology, see Krishnan et al., 2011) (50).
To examine diagnosis differences in network connectivity, a non-rotated task PLS was performed specifying a main effect contrast: IBS vs. HC. This analysis produced patterns or spatial maps representing differences due to IBS diagnosis (52). To examine the relationship between EALs and RSN connectivity, a behavioral PLS was performed. In this analysis, threshholded individual resting state maps and EAL scores were used to compute group correlation maps (IBS females, IBS males, HC males, HC females). The behavioral PLS selects only the most robust patterns in these across-subject correlations maps, negating the need for specifying contrasts of interest (53). Significance of the patterns was assessed by permutation testing with 1000 permutations, and was applied to determine whether spatial maps could be differentiated from noise (52). Each voxel comprising the spatial map has an associated numerical weight called a “salience” and can be positive or negative, indicating the magnitude and direction in which each voxel correlates with the variable being tested (disease contrast, EAL score). The reliability of voxel saliences for non-rotated task and behavioral PLS analyses were assessed by bootstrap estimation (500 samples). Clusters with a peak voxel with a bootstrap ratio (BSR) exceeding ±2.81 (p<.005) with an extent of at least 60 voxels were considered reliable and reported.
Behavioral Analyses
Behavioral analyses were performed using Statistical Package for the Social Sciences (SPSS) software (version 19). Group differences in behavioral measure scores were evaluated by applying ANOVA and independent sample t-tests. Post-hoc false-discovery rate (FDR) correction tests (54) were conducted following one-way ANOVAs, whereby each significant main effect was identified by specifying 4 individual contrasts: Male IBS vs. Female IBS, Male IBS vs. Male HC, Female IBS vs. Female HC, and Male HC vs. Female HC. Clinical and behavioral variables tested included early adverse life trauma (ETI), neuroticism (NEO-PI), and anxiety and depression (HAD).
Results
Behavioral and Subject Data
Mean age, and EAL ratings are provided in Table 1. One-way ANOVAs indicated that EAL (p=.002), and Neuroticism (p=.002) scores were significantly higher for patients compared to HCs (Table 1). Although within normal clinical ranges, patients also had higher anxiety and depression symptom scores on the HAD compared to HCs, with male IBS having significantly higher scores than male HCs on anxiety (p=.001), and female IBS having significantly higher anxiety (p=.003) and depression (p=.005) symptoms than female HCs. A one-way ANOVA factoring sex, indicated that there were no significant differences on neuroticism (F=.247, p=.620), anxiety (F=1.633, p=.203), or depression (F=3.392, p=.067) between males and females.
IBS-related group differences in resting state network connectivity
Differences were found in the intrinsic connectivity of the salience, the left frontal parietal, and the default mode networks in IBS compared to HCs. IBS displayed greater within network connectivity of certain brain regions while no resting state networks displayed greater within network connectivity in HCs compared to IBS patients (Table 2). In the salience network of IBS patients, greater within-network connectivity was observed for the putamen, INS, ACC, mid cingulate cortex [MCC], and supramarginal gyrus (p=.010). In the left frontal parietal network increased connectivity was identified between numerous regions, including putamen, ACC, THAL, and frontal cortex (p=.009). In default mode network of IBS patients, increased connectivity was observed of parietal regions including the precuneus, inferior parietal gyrus and angular gyrus (p=.005).
Table 2. Regions within the Intrinsic Connectivity Networks Associated with Disease.
| COMPONENT: Salience/Executive Control | ||||||||
|---|---|---|---|---|---|---|---|---|
| Positive correlation in IBS; no correlation in HC | ||||||||
| Region | BA | Hemisphere | X | Y | Z | Cluster k | Bootstrap Ratio | p-value |
| Insula | Left | -40 | 14 | -2 | 86 | 4.69 | <.001 | |
| Left | -32 | 16 | -10 | 117 | 3.59 | <.001 | ||
| 13 | Right | 32 | 18 | -14 | 238 | 3.78 | <.001 | |
| Supramarginal Gyrus |
Right | 58 | -44 | 34 | 148 | 4.06 | <.001 | |
| MCC | Right | 6 | 24 | 34 | 133 | 3.83 | <.001 | |
| ACC | Left | -10 | 42 | 14 | 288 | 3.54 | <.001 | |
| Right | 4 | 40 | 0 | 323 | 3.44 | <.001 | ||
| Putamen | Left | -20 | 10 | 2 | 67 | 3.09 | <.001 | |
| COMPONENT: Left Frontal Parietal | ||||||||
|---|---|---|---|---|---|---|---|---|
| Positive correlation in IBS; no correlation in HC | ||||||||
| Region | BA | Hemisphere | X | Y | Z | Cluster k | Bootstrap Ratio | p-value |
| Putamen | Left | -16 | 14 | -2 | 162 | 4.23 | <.001 | |
| ACC | Left | -6 | 28 | 32 | 99 | 3.04 | .002 | |
| IPG | Left | -24 | -66 | 44 | 95 | 4.10 | <.001 | |
| Left | -40 | -40 | 42 | 388 | 3.75 | <.001 | ||
| SFG | 8 | Left | -24 | 4 | 66 | 62 | 3.96 | <.001 |
| MFG | Right | 30 | 4 | 54 | 63 | 3.92 | <.001 | |
| IFG | Left | -46 | 42 | 8 | 238 | 3.92 | <.001 | |
| ITG | Left | -46 | -64 | -6 | 260 | 3.70 | <.001 | |
| MOG | Left | -42 | -70 | 30 | 189 | 3.07 | <.001 | |
| COMPONENT: Default Mode Network | ||||||||
|---|---|---|---|---|---|---|---|---|
| Positive correlation in IBS; no correlation in HC | ||||||||
| Region | BA | Hemisphere | X | Y | Z | Cluster k | Bootstrap Ratio | p-value |
| Precuneus | Right | 12 | -56 | 24 | 929 | 4.70 | <.001 | |
| Angular Gyrus | Right | 50 | -56 | 28 | 130 | 3.88 | <.001 | |
| IPG | Left | -34 | -60 | 50 | 98 | 3.63 | <.001 | |
| Calcarine | Left | -4 | -58 | 6 | 100 | 3.48 | <.001 | |
BSR=2.81 (p<0.005); (voxel>60)
HC: Healthy Control, IBS: Irritable Bowel Syndrome, BA: Broadmann's Area
All regions represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates
Regions: MCC, mid cingulate cortex; ACC, anterior cingulate cortex; IPG, inferior parietal gyrus; SFG, superior frontal gyrus; MFG, middle frontal gyrus; IFG, inferior frontal gyrus; ITG, inferior temporal gyrus; MOG, middle occipital gyrus
Early adversity related differences in resting state network connectivity
Two of the identified resting state networks, the salience and cerebellar networks, displayed significant correlations between EAL measures and within-network intrinsic connectivity (Table 3). In the salience network, higher levels of EALs in IBS subjects (both males and females), but not in HCs were associated with increased connectivity with the left putamen, and decreased connectivity with the supplementary motor area (SMA), INS, ACC, parietal and frontal regions (p = .046) (Figure 1). In the cerebellar network, (Table 4) a disease and sex specific relationship between intrinsic connectivity and EAL were identified (p=.022). Higher levels of EALs were associated with increased connectivity of THAL, INS, ACC, pINS, cerebellum and middle temporal gyrus (MTG) with the cerebellar network, and decreased connectivity of right caudate with the cerebellar network in a greater extent in IBS males than in HC male subjects (Figure 2), while no correlations were seen in females. In addition, no significant correlations with anxiety, depression, neuroticism or age were identified for the salience and cerebellar networks (p>.05). None of the other resting state networks showed significant correlations with EAL scores.
Table 3. Regions of the Salience/Executive Control Network Associated with Early Adverse Life Events.
| Region | BA | Hemisphere | X | Y | Z | Cluster k | Bootstrap Ratio | p-value |
|---|---|---|---|---|---|---|---|---|
| Positive correlation in IBS; no correlation in HC | ||||||||
| Lentiform Nucleus/Putamen | Left | -20 | 8 | 2 | 75 | 3.32 | <.001 | |
| Negative correlation in IBS; no correlation in HC | ||||||||
| SMA | Left | -4 | 12 | 66 | 72 | 3.28 | .001 | |
| ACC | 32 | Right | 2 | 44 | 2 | 515 | 5.61 | <.001 |
| 32 | Bilateral | 0 | 36 | 34 | 293 | 3.68 | <.001 | |
| Cingulate Gyrus | 24 | Right | 4 | -10 | 44 | 219 | 4.16 | <.001 |
| IFG/Insula | Left | -48 | 12 | 0 | 486 | 5.17 | <.001 | |
| 13 | Right | 28 | 16 | -12 | 196 | 4.06 | <.001 | |
| MFG | Right | 30 | 54 | 18 | 171 | 4.13 | <.001 | |
| 9 | Left | -30 | 38 | 40 | 118 | 3.94 | <.001 | |
| SFG | 6 | Right | 12 | 16 | 64 | 68 | 3.73 | <.001 |
| Parietal | 39 | Left | -46 | -68 | 32 | 123 | 3.45 | <.001 |
BSR=2.81 (p<0.005); (voxel>60)
HC: Healthy Control, IBS: Irritable Bowel Syndrome, BA: Broadmann's Area
All regions represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates
Regions: SMA, supplementary motor area; ACC, anterior cingulate cortex; IFG, inferior frontal gyrus; MFG, middle frontal gyrus; SFG, superior frontal gyrus
Figure 1. Influence of Early Adverse Life Events in the Salience/Executive Control Network.
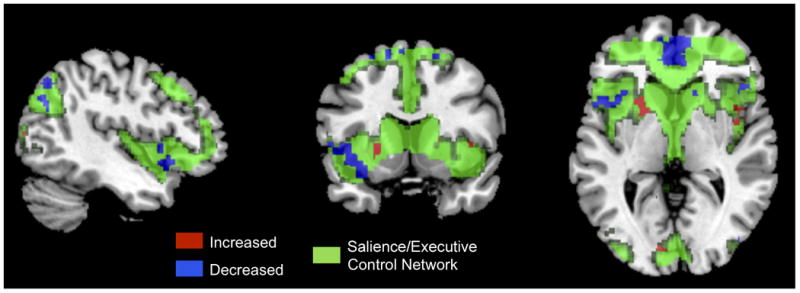
Green: Salience/Executive Control Network
Red: Positive correlations between brain regions and the salience/executive control network with increasing EALs
Blue: Negative correlations between brain regions and the salience/executive control network with increasing EAL
Table 4. Regions of the Cerebellar Network Associated With Early Adverse Life Events.
| Region | BA | Hemisphere | X | Y | Z | Cluster k | Bootstrap Ratio | p-value |
|---|---|---|---|---|---|---|---|---|
| More positive correlation in IBS males than in HC males; no correlation in females | ||||||||
| Thalamus | Left | -12 | -16 | 6 | 667 | 6.79 | <.001 | |
| Insula | Left | -34 | -10 | 0 | 66 | 5.37 | <.001 | |
| ACC | 32 | Bilateral | 0 | 14 | 38 | 126 | 5.55 | <.001 |
| Posterior Insula | Right | 36 | 0 | -10 | 103 | 5.63 | <.001 | |
| Cerebellum | Right | 18 | -60 | -20 | 121 | 3.99 | <.001 | |
| Right | 2 | -58 | -20 | 216 | 3.92 | <.001 | ||
| MTG | 39 | Left | -40 | -72 | 26 | 109 | 4.19 | <.001 |
| More negative correlation in IBS males than in HC males, no correlation in females | ||||||||
| Caudate | Right | 12 | 18 | 6 | 74 | 5.12 | <.001 | |
BSR=2.81 (p<0.005); (voxel>60)
HC: Healthy Control, IBS: Irritable Bowel Syndrome, BA: Broadmann's Area
All regions represented in Montreal Neurological Institute (MNI) space with X, Y, Z coordinates
Regions: ACC, anterior cingulate cortex; MTG, middle temporal gyrus
Figure 2. Influence of Early Adverse Life Events in the Cerebellar Network.
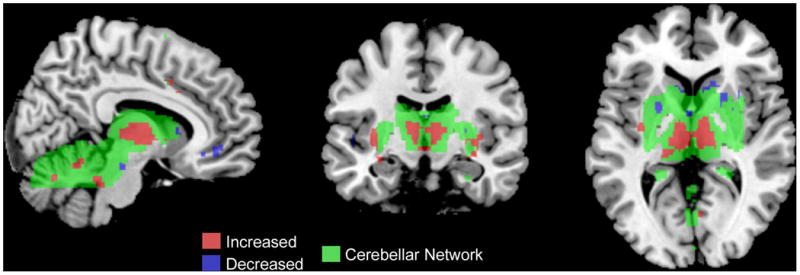
Green: Cerebellar Network
Red: Positive correlations between brain regions and the cerebellar network with increasing EALs
Blue: Negative correlations between brain regions and the cerebellar network with increasing EALs
Discussion
We examined relationships between a history of EALs and resting state networks of regional intrinsic BOLD fluctuations in IBS subjects and in HCs, with a special emphasis on identifying sex related differences in these networks. We used a data-driven multivariate approach in order to investigate the relationship between EAL scores and resting state brain connectivity within six networks. The main findings of the study were: 1. Disease related differences were present in the salience, left frontal parietal, and default mode networks. 2. Both male and female IBS subjects showed significant EAL related correlations with intrinsic connectivity within the salience network. 3. The intrinsic connectivity within the cerebellar network was related to EALs in a sex specific manner, with male IBS subjects showing EAL-related alterations compared to male HCs. Despite the fact that EALs are common environmental factors associated with IBS and other stress sensitive disorders, to our knowledge, this is the first report that demonstrates sex and disease related differences in resting state network connectivity that are related to a history of EALs in a large patient population.
IBS and EAL related alterations in the salience network
IBS patients (males and females) compared to HCs displayed greater within-network connectivity of the putamen, INS, ACC, MCC, and supramarginal gyrus within the salience network. With increasing levels of EALs, increased connectivity of the left putamen with the salience network was observed in IBS patients, while the connectivity of several other regions of the salience network (SMA, INS, ACC, parietal and frontal regions) was reduced. The salience network is thought to play a key role in selecting from multiple internal (including cognitive, emotional, interoceptive) and extrapersonal (including somatosensory) stimuli those with the greatest personal relevance, in order to optimally guide behavior and adjust autonomic nervous system output (55-57). Patients with chronic pain conditions such as fibromyalgia, chronic back pain, migraines and IBS show abnormal activation in core structures of the salience network in response to stimuli, and such alterations in the engagement of the salience network have been implicated in the pathophysiology of these conditions (58, 59).
The putamen-salience network connectivity is increased in the presence of salient nociceptive stimuli (60). This increased connectivity not only guides motor behavior, and motivation towards salient stimuli, but also is associated with reduced mental processing speed (61). In the current study, the putamen-salience connectivity was increased in IBS compared to HCs, and was further enhanced in the presence of EALs, suggesting that a history of EALs is related to increased impairments in the ability to detect, process and modulate sensory information, and decreased cognitive modulation and flexibility functions (58, 59) beyond that seen in IBS alone.
Two additional regions with increased salience network connectivity in IBS subjects, the INS and ACC, also demonstrated a relationship with EALs in IBS. However, unlike with the putamen, increased EALs were associated with reduced INS and ACC connectivity with the salience network. Thus, IBS with lower EALs appeared to have mainly INS/ACC-salience alterations while IBS with higher EALs appeared to have mainly putamen-salience alterations. The INS is the integral hub of the salience network which interfaces with other regions within the salience network (e.g. ACC, MCC, SMA, prefrontal cortex [PFC]), and with regions within other networks such as the default mode network and cognitive control network (62). As a result, the INS-salience network connectivity plays a role in cognitive, attention, affective and homeostatic processes which link the external stimulus driven environment and the internal milieu of the body. The current study demonstrated similar results to those reported in trauma patients (63), in that decreased connectivity between certain limbic and paralimbic regions within the salience network (anterior INS and subgenual ACC) were observed. These results associated with the salience network in IBS patients suggest that experiences of adversity or trauma early in life are associated with intrinsic network alterations, which may lead to an impaired ability to detect, process and modulate sensory information and to generate appropriate autonomic behavioral responses (56).
IBS, EALs and sex related alterations in the cerebellar network
Although no significant IBS-related differences in the connectivity of the cerebellar network were identified, significant sex and IBS-related differences in the relationship between EALs and cerebellar network connectivity were demonstrated. Higher EAL scores were associated with increased connectivity of the THAL, aINS, pINS, ACC, cerebellum, and MTG with the cerebellar network and decreased connectivity of caudate with the cerebellar network in male IBS but not HC males with no relationship demonstrated in female subjects. The cerebellar network is involved in fear perception, motor function and visual-motor learning (64, 65). More recently the cerebellar network has been identified as being central to both physical pain (66) and psychological pain (67), and abnormalities in cerebellar network connectivity have been reported in various disorders, including substance abuse (68, 69), and major depression (70, 71).
A recent meta-analysis has shown that trauma related alterations in the connectivity between the THAL, cerebellum, INS and cingulate regions within the cerebellar network may play an important role in sensorimotor integration (64, 65, 72). Thus, the observation of greater connectivity within the cerebellar network with increasing EALs in IBS males may suggest a role of EALs in altering sensorimotor integration in IBS males. Since the caudate is known to modulate pain intensity through expectancy and attentional mechanisms, the decreased connectivity between the caudate-cerebellar network with increasing EALs in male patients compared to male HCs is consistent with an impaired pain modulation system which is influenced by the interaction of disease status and level of EALs. The reasons and the functional consequences of the sex specific alterations in cerebellar network alterations remain to be determined.
Study Limitations
Limitations of the study include the fact that data was pooled from multiple studies, possibly contributing heterogeneity to the sample. There were also unbalanced sample sizes for disease groups and for sex, and there were significant differences between groups in age. However, we were still able to detect significant differences in the groups with smaller sample sizes (e.g. IBS). Future studies that would potentially match patients and healthy control subjects on various demographic indices such as age, demographics, socioeconomic status (SES), and body mass index (BMI) would require greater samples, and would be possible through the availability of mechanisms such as large data pain repositories. Even though this study used predominantly premenopausal women during the follicular phase of the menstrual cycle, we did not measure female sex hormones and therefore could not address a possible influence of sex hormones on the current findings. Since a psychiatric diagnosis was an exclusion criterion, patients with severe IBS and comorbid pathological levels of anxiety were excluded from the study sample, resulting in a less severe study population. It is true that some of the measures including the ETI are based on subjective recall, and could therefore be influenced by recall and reporting bias. However, previous studies have demonstrated that the ETI has acceptable psychometric properties and it has been validated against more extensive interview measures of EALs. Lastly, some of the observed differences could be influenced by the intake of centrally acting medications, in particular tricyclic antidepressants, a factor that could not be addressed in the current study.
Conclusions and Clinical Implications
These results are consistent with previous reports that have shown long lasting effects of EALs on brain responses in patients with chronic pain disorders. The results indicate that EALS are correlated with resting state network alterations, which play a role in anxiety and altered sensorimotor integration. The fact that certain resting state network patterns were not seen in HCs with a history of EALs, implies that it is not the experience of EAL per se, but that the interaction of EALs with other factors involved in IBS pathophysiology must be involved. Interestingly, some of the observed changes were only seen in male patients, highlighting the importance in the need to consider sex differences in these interactions. The functional and behavioral consequences this sex difference remain to be determined but the results underscore the importance of assessment of EAL in both male and female patients
Acknowledgments
Acknowledgements/Funding Support: Supported by NIH grants R01 DK048351, P50 DK064539, P30 DK041301, R03 DK084169, K01 DK085133. Pilot scanning provided by UCLA Ahmanson-Lovelace Brain Mapping Center
Footnotes
Disclosures: No conflicts of interest exist
References
- 1.Heitkemper M, Jarrett M, Jun SE. Update on irritable bowel syndrome program of research. Journal of Korean Academy of Nursing. 2013;43:579–86. doi: 10.4040/jkan.2013.43.5.579. [DOI] [PubMed] [Google Scholar]
- 2.Labus JS, Naliboff BN, Fallon J, Berman SM, Suyenobu B, Bueller JA, Mandelkern M, Mayer EA. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. NeuroImage. 2008;41:1032–43. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradford K, Shih W, Videlock EJ, Presson AP, Naliboff BD, Mayer EA, Chang L. Association Between Early Adverse Life Events and Irritable Bowel Syndrome. Clin Gastroenterol H. 2012;10:385–U159. doi: 10.1016/j.cgh.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Malley D, Quigley EM, Dinan TG, Cryan JF. Do interactions between stress and immune responses lead to symptom exacerbations in irritable bowel syndrome? Brain, behavior, and immunity. 2011;25:1333–41. doi: 10.1016/j.bbi.2011.04.009. Review. [DOI] [PubMed] [Google Scholar]
- 5.Lackner JM, Gudleski GD, Blanchard EB. Beyond abuse: the association among parenting style, abdominal pain, and somatization in IBS patients. Behaviour Research and Therapy. 2004;42:41–56. doi: 10.1016/s0005-7967(03)00069-x. [DOI] [PubMed] [Google Scholar]
- 6.Talley NJ, Boyce PM, Jones M. Is the association between irritable bowel syndrome and abuse explained by neuroticism? A population based study. Gut. 1998;42:47–53. doi: 10.1136/gut.42.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dai C, Jiang M. The Incidence and Risk Factors of Post-Infectious Irritable Bowel Syndrome: A Meta-Analysis. Hepato-Gastroenterol. 2012;59:67–72. doi: 10.5754/hge10796. [DOI] [PubMed] [Google Scholar]
- 8.Fukudo S, Kanazawa M. Gene, environment, and brain-gut interactions in irritable bowel syndrome. J Gastroen Hepatol. 2011;26:110–5. doi: 10.1111/j.1440-1746.2011.06631.x. [DOI] [PubMed] [Google Scholar]
- 9.Olden KW, Drossman DA. Psychologic and psychiatric aspects of gastrointestinal disease. Med Clin N Am. 2000;84:1313. doi: 10.1016/s0025-7125(05)70288-1. + [DOI] [PubMed] [Google Scholar]
- 10.Cohen H, Jotkowitz A, Buskila D, Pelles-Avraham S, Kaplan Z, Neumann L, Sperber AD. Post-traumatic stress disorder and other co-morbidities in a sample population of patients with irritable bowel syndrome. Eur J Intern Med. 2006;17:567–71. doi: 10.1016/j.ejim.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 11.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 12.Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, Cole S, Kobor MS. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. P Natl Acad Sci USA. 2009;106:14716–21. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cole SW, Conti G, Arevalo JM, Ruggiero AM, Heckman JJ, Suomi SJ. Transcriptional modulation of the developing immune system by early life social adversity. Proc Natl Acad Sci U S A. 2012;109:20578–83. doi: 10.1073/pnas.1218253109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tillisch K, Labus J, Nam B, Bueller J, Smith S, Suyenobu B, Siffert J, McKelvy J, Naliboff B, Mayer E. Neurokinin-1-receptor antagonism decreases anxiety and emotional arousal circuit response to noxious visceral distension in women with irritable bowel syndrome: a pilot study. Aliment Pharmacol Ther. 2012;35:360–7. doi: 10.1111/j.1365-2036.2011.04958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hall GBC, Kamath MV, Collins S, Ganguli S, Spaziani R, Miranda KL, Bayati A, Bienenstock J. Heightened central affective response to visceral sensations of pain and discomfort in IBS. Neurogastroent Motil. 2010;22 doi: 10.1111/j.1365-2982.2009.01436.x. [DOI] [PubMed] [Google Scholar]
- 16.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–9. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 17.Berman SM, Naliboff BD, Suyenobu B, Labus JS, Stains J, Ohning G, Kilpatrick L, Bueller JA, Ruby K, Jarcho J, Mayer EA. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:349–59. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayer EA, Aziz Q, Coen S, Kern M, Labus JS, Lane R, Kuo B, Naliboff B, Tracey I. Brain imaging approaches to the study of functional GI disorders: a Rome working team report. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2009;21:579–96. doi: 10.1111/j.1365-2982.2009.01304.x. Research Support, N.I.H., Extramural Research Support, U.S. Gov't, Non-P.H.S. Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. Meta-Analysis Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sheehan J, Gaman A, Vangel M, Kuo B. Pooled analysis of brain activity in irritable bowel syndrome and controls during rectal balloon distension. Neurogastroenterology and motility: the official journal of the European Gastrointestinal Motility Society. 2011;23:336–46, e158. doi: 10.1111/j.1365-2982.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, Feier N, Bueller J, Stains J, Smith S, Suyenobu B, Naliboff B, Mayer EA. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain. 2013 doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Labus JS, Mayer EA, Jarcho J, Kilpatrick LA, Kilkens TO, Evers EA, Backes WH, Brummer RJ, van Nieuwenhoven MA. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60:1196–203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ellingson BM, Mayer E, Harris RJ, Ashe-McNally C, Naliboff BD, Labus JS, Tillisch K. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013 doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang Z, Dinov ID, Labus J, Shi Y, Zamanyan A, Gupta A, Ashe-McNalley C, Hong JY, Tillisch K, Toga AW, Mayer EA. Sex-Related Differences of Cortical Thickness in Patients with Chronic Abdominal Pain. Plos One. 2013;8:e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Ebrat B, Tillisch K, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA. Irritable Bowel Syndrome in female patients is associated with alterations in structural brain networks. Pain. 2013 doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Seminowicz DA, Labus JS, Bueller JA, Tillisch K, Naliboff BD, Bushnell MC, Mayer EA. Regional Gray Matter Density Changes in Brains of Patients With Irritable Bowel Syndrome. Gastroenterology. 2010;139:48–U82. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.LeResche L. Defining Gender Disparities in Pain Management. Clin Orthop Relat R. 2011;469:1871–7. doi: 10.1007/s11999-010-1759-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chang L, Mayer EA, Labus JS, Schmulson M, Lee OY, Olivas TI, Stains J, Naliboff BD. Effect of sex on perception of rectosigmoid stimuli in irritable bowel syndrome. Am J Physiol-Reg I. 2006;291:R277–R84. doi: 10.1152/ajpregu.00729.2005. [DOI] [PubMed] [Google Scholar]
- 29.Berman S, Suyenobu B, Naliboff BD, Bueller J, Stains J, Wong H, Mandelkern M, Fitzgerald L, Ohning G, Gupta A, Labus JS, Tillisch K, Mayer EA. Evidence for alterations in central noradrenergic signaling in irritable bowel syndrome. NeuroImage. 2012;63:1854–63. doi: 10.1016/j.neuroimage.2012.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hong JY, Kilpatrick LA, Labus J, Gupta A, Jiang ZG, Ashe-McNalley C, Stains J, Heendeniya N, Ebrat B, Smith S, Tillisch K, Naliboff B, Mayer EA. Patients with Chronic Visceral Pain Show Sex-Related Alterations in Intrinsic Oscillations of the Resting Brain. Journal of Neuroscience. 2013;33:11994–2002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kilpatrick LA, Ornitz E, Ibrahimovic H, Treanor M, Craske M, Nazarian M, Labus JS, Mayer EA, Naliboff BD. Sex-related differences in prepulse inhibition of startle in irritable bowel syndrome (IBS) Biological psychology. 2010;84:272–8. doi: 10.1016/j.biopsycho.2010.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Drossman DA. Introduction. The Rome Foundation and Rome III. Neurogastroent Motil. 2007;19:783–6. doi: 10.1111/j.1365-2982.2007.01001.x. [DOI] [PubMed] [Google Scholar]
- 33.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. The Journal of clinical psychiatry. 1998;59(Suppl 20):22–33. Comparative Study Historical Article Research Support, Non-U.S. Gov't Review. quiz 4-57. [PubMed] [Google Scholar]
- 34.Bremner JD, Bolus R, Mayer EA. The early trauma inventory self report (ETI-SR) Gastroenterology. 2005;128:A340-A. [Google Scholar]
- 35.Bremner JD, Bolus R, Mayer EA. Psychometric properties of the Early Trauma Inventory-Self Report. J Nerv Ment Dis. 2007;195:211–8. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costa PT, Mccrae RR. Domains and Facets - Hierarchical Personality-Assessment Using the Revised Neo Personality-Inventory. J Pers Assess. 1995;64:21–50. doi: 10.1207/s15327752jpa6401_2. [DOI] [PubMed] [Google Scholar]
- 37.McCrae RR, Kurtz JE, Yamagata S, Terracciano A. Internal consistency, retest reliability, and their implications for personality scale validity. Pers Soc Psychol Rev. 2011;15:28–50. doi: 10.1177/1088868310366253. Comparative Study Research Support, N.I.H., Intramural Validation Studies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 39.Mykletun A, Jacka F, Williams L, Pasco J, Henry M, Nicholson GC, Kotowicz MA, Berk M. Prevalence of mood and anxiety disorder in self reported irritable bowel syndrome (IBS). An epidemiological population based study of women. Bmc Gastroenterology. 2010;10 doi: 10.1186/1471-230X-10-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Biswal BB, Mennes M, Zuo XN, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski AM, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kotter R, Li SJ, Lin CP, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts SARB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng GJ, Veijola J, Villringer A, Walter M, Wang LH, Weng XC, Whitfield-Gabrieli S, Williamson P, Windischberger C, Zang YF, Zhang HY, Castellanos FX, Milham MP. Toward discovery science of human brain function. P Natl Acad Sci USA. 2010;107:4734–9. doi: 10.1073/pnas.0911855107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Labus JS, Ebrat B, Jiang ZG, Stains J, Tillisch K, Naliboff BD, Mayer EA. The Effect of Cognitive Load on Interoceptive Processing. Gastroenterology. 2011;140:S368–S9. [Google Scholar]
- 42.Tillisch K, Labus J, Kilpatrick L, Jiang Z, Stains J, Ebrat B, Guyonnet D, Legrain-Raspaud S, Trotin B, Naliboff B, Mayer EA. Consumption of Fermented Milk Product With Probiotic Modulates Brain Activity. Gastroenterology. 2013;144:1394–U136. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurth F, Labus JS, Stains J, Ebrat B, Vianna E, Jiang ZG, Tillisch K, Naliboff BD, Mayer EA. Mild Visceral Stimuli Interfere With Attentional Processes in IBS but Not Healthy Control Subjects. Gastroenterology. 2012;142:S553-S. [Google Scholar]
- 44.Naliboff BD, Waters AM, Labus JS, Kilpatrick L, Craske MG, Chang L, Negoro H, Ibrahimovic H, Mayer EA, Ornitz E. Increased Acoustic Startle Responses in IBS Patients During Abdominal and Nonabdominal Threat. Psychosomatic Medicine. 2008;70:920–7. doi: 10.1097/PSY.0b013e318186d858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y, A T, Calhoun VD. Sample Dependence correction for order selection in fMRI analysis. Washington D.C.: 2006. [Google Scholar]
- 46.Bell AJ, Sejnowski TJ. An Information Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995;7:1129–59. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- 47.Himberg J, Hyvarinen A, Esposito F. Validating the independent components of neuroimaging time series via clustering and visualization. NeuroImage. 2004;22:1214–22. doi: 10.1016/j.neuroimage.2004.03.027. [DOI] [PubMed] [Google Scholar]
- 48.Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Filippini N, Watkins KE, Toro R, Laird AR, Beckmann CF. Correspondence of the brain's functional architecture during activation and rest. P Natl Acad Sci USA. 2009;106:13040–5. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008;21:424–30. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 50.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: A tutorial and review. NeuroImage. 2011;56:455–75. doi: 10.1016/j.neuroimage.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 51.McIntosh AR, Bookstein FL, Haxby JV, Grady CL. Spatial pattern analysis of functional brain images using partial least squares. NeuroImage. 1996;3:143–57. doi: 10.1006/nimg.1996.0016. [DOI] [PubMed] [Google Scholar]
- 52.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. NeuroImage. 2004;23:S250–S63. doi: 10.1016/j.neuroimage.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 53.McIntosh AR. Tracing the route to path analysis in neuroimaging. NeuroImage. 2012;62:887–90. doi: 10.1016/j.neuroimage.2011.09.068. Historical Article Research Support, Non-U.S. Gov't Review. [DOI] [PubMed] [Google Scholar]
- 54.Benjamini Y. Discovering the false discovery rate. J R Stat Soc B. 2010;72:405–16. [Google Scholar]
- 55.Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor KS, Seminowicz DA, Davis KD. Two Systems of Resting State Connectivity Between the Insula and Cingulate Cortex. Human brain mapping. 2009;30:2731–45. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bingel U, Glascher J, Weiller C, Buchel C. Somatotopic representation of nociceptive information in the putamen: An event-related fMRI study. Cerebral cortex. 2004;14:1340–5. doi: 10.1093/cercor/bhh094. [DOI] [PubMed] [Google Scholar]
- 58.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic Brain Connectivity in Fibromyalgia Is Associated With Chronic Pain Intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Baliki MN, Baria AT, Apkarian AV. The Cortical Rhythms of Chronic Back Pain. Journal of Neuroscience. 2011;31:13981–90. doi: 10.1523/JNEUROSCI.1984-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Starr CJ, Sawaki L, Wittenberg GF, Burdette JH, Oshiro Y, Quevedo AS, McHaffie JG, Coghill RC. The contribution of the putamen to sensory aspects of pain: insights from structural connectivity and brain lesions. Brain. 2011;134:1987–2004. doi: 10.1093/brain/awr117. Research Support, N.I.H., Extramural. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia-Garcia I, Jurado MA, Garolera M, Segura B, Sala-Llonch R, Marques-Iturria I, Pueyo R, Sender-Palacios MJ, Vernet-Vernet M, Narberhaus A, Ariza M, Junque C. Alterations of the salience network in obesity: a resting-state fMRI study. Human brain mapping. 2013;34:2786–97. doi: 10.1002/hbm.22104. Research Support, Non-U.S. Gov't. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Craig AD. Emotional moments across time: a possible neural basis for time perception in the anterior insula. Philos Trans R Soc Lond B Biol Sci. 2009;364:1933–42. doi: 10.1098/rstb.2009.0008. Research Support, Non-U.S. Gov't Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lanius RA, Vermetten E, Pain C. The impact of early life trauma on health and disease: the hidden epidemic. Cambridge, UK; New York: Cambridge University Press; 2010. [Google Scholar]
- 64.Baldacara L, Jackowski AP, Schoedl A, Pupo M, Andreoli SB, Mello MF, Lacerda ALT, Mari JJ, Bressan RA. Reduced cerebellar left hemisphere and vermal volume in adults with PTSD from a community sample. J Psychiat Res. 2011;45:1627–33. doi: 10.1016/j.jpsychires.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 65.Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ. Resting state cortico-cerebellar functional connectivity networks: a comparison of anatomical and self-organizing map approaches. Front Neuroanat. 2012;6 doi: 10.3389/fnana.2012.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9:463–84. doi: 10.1016/j.ejpain.2004.11.001. Meta-Analysis Review. [DOI] [PubMed] [Google Scholar]
- 67.Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. Meta-Analysis. [DOI] [PubMed] [Google Scholar]
- 68.Pitel AL, Chanraud S, Muller-Oehring EM, Pfefferbaum A, Sullivan EV. Modulation of limbic-cerebellar functional connectivity enables alcoholics to recognize who is who. Brain Struct Funct. 2013;218:683–95. doi: 10.1007/s00429-012-0421-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang YR, Zhu J, Li Q, Li W, Wu N, Zheng Y, Chang HF, Chen JJ, Wang W. Altered Fronto-Striatal and Fronto-Cerebellar Circuits in Heroin-Dependent Individuals: A Resting-State fMRI Study. Plos One. 2013;8 doi: 10.1371/journal.pone.0058098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma QM, Zeng LL, Shen H, Liu L, Hu DW. Altered cerebellar-cerebral resting-state functional connectivity reliably identifies major depressive disorder. Brain research. 2013;1495:86–94. doi: 10.1016/j.brainres.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 71.Liu L, Zeng LL, Li YM, Ma QM, Li BJ, Shen H, Hu DW. Altered Cerebellar Functional Connectivity with Intrinsic Connectivity Networks in Adults with Major Depressive Disorder. Plos One. 2012;7 doi: 10.1371/journal.pone.0039516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cauda F, Cavanna AE, D'agata F, Sacco K, Duca S, Geminiani GC. Functional Connectivity and Coactivation of the Nucleus Accumbens: A Combined Functional Connectivity and Structure-Based Meta-analysis. J Cognitive Neurosci. 2011;23:2864–77. doi: 10.1162/jocn.2011.21624. [DOI] [PubMed] [Google Scholar]


