Abstract
We reported that AS101 (organotellurium compound, trichloro(dioxoethylene-O,O′) tellurate) inhibited the differentiation of Th17 cells and reduced the production of IL-17 and GM-CSF. In addition, AS101 promoted the production of IL-2 in activated T cells. Flow cytometric analysis showed that AS101 inhibited Th17 cell proliferation. AS101 blocked the activation of transcriptional factor NFAT, Stat3, and RORγt, and increased activation of Erk1/2, suggesting a mechanism of action of AS101. We further demonstrated that AS101 was effective in amelioration of experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis. Finally, by real-time PCR analysis we showed that AS101 reduces the IL-17, IFN-γ, GM-CSF, and IL-6 mRNA expression in inflammatory cells of spinal cords. Additionally, flow cytometry analysis also indicated that the CD4+ T cells and IL-17 and GM-CSF-producing cells were reduced in the spinal cords of AS101 treated mice compared to those treated with PBS.
Keywords: Inflammation, GM-CSF, IL-17, signal transduction, Th17 cells, Stat3
1. Introduction
Multiple sclerosis (MS) is a debilitating autoimmune disease characterized by both inflammation and axonal degeneration. The resulting demyelination and subsequent degeneration of axons account for the disability of patients with MS (Calabresi 2002). MS is estimated to affect 400,000 persons in the United States and 2 million people worldwide (Calabresi 2002). Drugs that are commonly used for MS include: corticosteroids, interferons, glatiramer (Copaxone), natalizumab (Tysabi), mitoxantrone (Novantrone), fingolimod (Gilenya), and teriflunomide (Aubagio). The limited effectiveness of these approved treatments for MS, as well as reports of adverse events and toxicity, such as severe liver damage, shortness of breath after injection, brain infection and cardiopathology following a mitoxantrone treatment, emphasize the need for the development of new therapies with improved efficacy and reduced side-effects (Calabresi 2002; Ulzheimer et al., 2010; Friese et al., 2006). Hence, there is a need for additional treatment options in MS.
Experimental autoimmune encephalomyelitis (EAE) is an animal model of human MS. Increased understanding of the underlying pathophysiology of the disease and advances in developing new biotechnology has led to new therapeutic approaches to the treatment of MS. Early studies indicated that CD4+ T cells are effectors for disease progression (Hohlfeld and Wekerle 2001) and EAE can be induced by adoptive transfer of myelin-specific CD4+ Th1 cells into naïve recipients (Waldburger et al., 1996). However, cytokine IFN-γ is not required for development of EAE since IFN-γ−/− or IFN-γR−/− mice develop more severe EAE than wild type counterparts (Ferber et al., 1996). It is postulated that the differentiation pathway that generate Th1 cells may be important in encephalitogenicity, but the downstream production of IFN-γ by myelin-specific T cells is not critical (Lovett-Rack et al., 2011). Further animal model study showed that IFN-γ and central nervous system (CNS) able to respond to IFN-γ is important for inflammation of the spinal cord (Lees et al. 2008), which is consistent with the clinical trial that treatment of MS with IFN-γ exacerbates the severity of MS (Pantich et al. 1987).
In addition, recent studies identified additional contributions to disease pathogenesis, including IL-17-producing T cells, B cells, and CD8+ T cells (Kang et al., 2010; Huseby et al., 2001; Langrish et al., 2005). Mounting evidence showed that IL-17 plays a pathogenic role in several autoimmune diseases, including EAE and MS (Calabresi 2002; Kang et al., 2010; Sifftin et al., 2010). IL-17 is produced by Th17 cells and other immune cells such as γδ T cells, CD8+ T cells and NKT cells (Langrish et al., 2005, Mangan et al., 2006; Sutton et al., 2012). IL-6 together with TGF-β is critical for naive T cell differentiation into T helper type 17 (Th17) cells (Mangan et al., 2006). IFN-γ and IL-2 inhibit IL-17 production by Th17 cells (Mangan et al., 2006, Bettelli et al., 2006; Laurence et al., 2007). More recent work has shown that GM-CSF is essential for these effector T cells to induce autoimmune neuroinflammation and RORγt drives production of the cytokine GM-CSF in activated T helper cells (Cordarri et al., 2011; El-Behi et al., 2011). Therefore, targeting the differentiation pathway of IL-17 and GM-CSF-producing cells should be an ideal therapeutic intervention for EAE and MS. It should be noted that utilizing IL-17 deficient mice, it was demonstrated that IL-17 was not necessary for the development of EAE. Collectively, studies suggest that both Th1 and Th17 cells contribute to the development of EAE (Lovett-Racke et al., 2011).
Previous work demonstrated that AS101 is an immunomodulator, promoting IL-2 production by lymphocytes (Sredni et al., 1987), inhibiting IL-6 production by macrophages, and reducing the size of tumors in mice (Sredni et al, 1997; Kalechman et al., 2004; Brodsky et al., 2010). AS101 also enhances neuronal survival and rescues neurons from apoptotic death (Okun et al, 2007; Sredni et al., 2007). Studies of AS101 in mice and cancer patients have shown that AS101 is a non-toxic immunomodulator, suggesting that it may be an appropriate treatment of MS (Kalechman et al., 2004; Okun et al., 2007; Sredni et al., 2007; Sredni 2012). Supporting this notion, a more recent report showed that AS101 ameliorates EAE by VLA-4 inhibition and suppression of monocyte and T cell infiltration into the central nervous system (CNS) (Lee et al., 2014). Our current study provides new evidence that AS101 downregulates the production of IL-17 and GM-CSF in activated T cells by blocking activation of NFAT, Stat3, and RORγt, and that administration of AS101 to mice reduced the severity of EAE. These studies further suggest that AS101 inhibits development of EAE through multiple mechanisms and may be effective in the treatment of MS.
2. Materials and Methods
2.1 Mice
Eight to ten week old female C57BL/6 mice were bred in Arkansas Biosciences Institute animal facility under specific pathogen-free conditions. The animal protocol was approved by the Arkansas State University Animal Care and Use Committees.
2.2 CD4+ T cell polarization and AS101 treatment
For the generation of GM-CSF and IL-17 secreting cells, splenocytes were isolated from naïve female C57BL/6 mice. The purified naïve CD4+ T cells were isolated by negative selection by kits (Stem cells Inc, Vancouver, BC, Canada) or BD FACSAira (BD Bioscience, San Diego, CA, USA) according to the manufacture’s instruction. Cells were stimulated with plate-coated anti-CD3 (10μg/ml) and anti-CD28 (3μg/ml) plus cytokines and neutralizing antibodies for the desired polarization as follows: for Th17 cells, cells were stimulated with anti-IFN-γ (10μg/ml) and anti-IL-4 (5μg/ml) together with a combination of the cytokines TGF-β (2.0ng/ml), IL-6 (20ng/ml), and IL-23 (20ng/ml) or TGF-β (2.0ng/ml) and IL-21 (50ng/ml) (Cordarri et al., 2011; El-Behi et al., 2011). GM-CSF secreting cells were generated in the presence of anti-IFN-γ (10μg/ml), anti-IL-4 (5μg/ml), IL-1β (15 ng/ml), and IL-23(20ng/ml) (El-Behi et al., 2011). Th1 cells were generated with IL-12 (10ng/ml) and anti-IL-4 (10μg/ml). AS101 was supplied by M. Albeck from the Department of Chemistry at Bar-Ilan University or purchased from Santa Cruz Biotechnology Inc (Santa Cruz, CA). Cells were analyzed by intracellular staining after 3 days in culture. Cytokines were obtained from PeproTech (Rocky Hill, NJ, USA), except IL-23 which was obtained from eBioscience (San Diego, CA, USA)
2.3 Flow cytometry
Collected splenocytes were treated with ammonium chloride lysis buffer to remove red blood cells and washed twice with RPMI medium. Isolated CD4+ T cells were cultured as described above. 3 days after culture, PMA (phorbol-12-myristate-13-acetate) (100ng/ml) and ionomycin (0.5μg/ml) (Sigma, St Louis, MO, USA) and Brefeldin A(10μg/ml) (eBioscience) were added to the culture at the last 5–6 hours before harvesting. The cells were first stained with allophycocyamin- or FITC-conjugated with anti-CD4 (Biolegend, San Diego, CA, USA) and followed with fixation/permeabilization solution (eBioscience). After 2 washes, cells were incubated with PE-conjugated anti-IL-17 or anti-GM-CSF (Biolegend). Acquisitions were made with a BD FACSCalibur (BD Biosciences, San Diego, CA, USA). Cell Quest software was used for data analysis.
2.4 Lymphocyte proliferation assay
T cells were labeled with CFSE (carboxyfluorescein succinimidyl ester) (Molecular Probes, Inc. Eugene, OR, USA) before culture. CFSE labeled T cells were cultured for 3 days under Th17 or GM-CSF secreting cell polarizing conditions (as described above) in the presence and/or absence of AS101. CFSE dilution assay was performed using CFSE Cell Proliferation Kit according to manufacturer’s instruction (Molecular Probes).
2.5 Western blot
Whole-cell lysates and nuclear extracts were prepared and Western blot analysis was performed as previously described (Zhang et al., 2013). The concentration of protein was determined with BCA protein assay reagent (Thermo Scientific Pierce, Rockford, IL, USA). Protein samples were separated on 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane (Bi-Rad, Hercules, CA, USA). The primary antibodies (Abs) for p-Akt, p-Stat3, NFAT, and p-ERK1/2 were from Cell Signaling Inc (Danvers, MA, USA). The antibodies for Beta-Actin, Stat3, and ERK1/2 were purchased from Santa Cruz Biotech Inc (Santa Cruz, CA, USA). Anti-RORγt was obtained from Biolegend. Blots were developed by SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific Pierce, Rockford, IL, USA).
2.6 Induction of EAE and treatment
Eight to ten week old female C57BL/6 (B6) mice were immunized by s.c. injection with 200μg myelin oligodendrocyte glycoprotein (MOG) residues 35–55 (GenScript, Piscataway, NJ, USA). Immunization was performed by mixing MOG protein in CFA (Complete Freund’s Adjuvant) (Sigma, St Louis, MO, USA). Pertussis toxin (200ng, Sigma) in PBS was administrated i.p. on the day of immunization and 48 hours later. Mice were examined daily and scored for disease severity using the standard scale: 0, no clinical signs; 1, limp tail; 2, paraparesis (weakness, incomplete paralysis of one or two hind limbs; 3, paraplegia (completely paralysis of two hind limbs); 4, paraplegia with forelimb weakness or paralysis; and 5, moribund or death. For the treatment, AS101 (10μg/mouse) or vehicle (PBS) was administrated i.p. every 48 hours, starting from day 1 after EAE immunization and ending at the termination of the experiments. Disease scores over the course of the 35 day experiments were totaled for each animal, and the mean for both experimental and control groups expressed as a cumulative EAE score (Matsushita et al., 2010).
2.7 Histology
For analysis of CNS histopathology, mice were perfused with PBS as described (Miller et al., 2007), and spinal cord with bone were fixed immediately in 4% (wt/vol) paraformaldehyde after perfusion. Spinal cords were removed from bone for paraffin section at 3 day of fixation. Paraffin-embedded 7μm sections of spinal cord were stained with H & E by IDEXX RADIL Lab, Animal Materials Diagnostic Testing (Columbia, MO, USA) and then examined by light microscopy.
2.8 Immunohistochemical (IHC) staining
Paraffin sections of spinal cord from mice with EAE were deparaffinized in xylene and hydrated in graded alcohol as previously described (Yu et al., 2008). The slides were washed in PBS (0.1 M, pH 7.6). Pre-treatment of tissue with heat-induced epitope retrieval was done by use of microwave. The slides were blocked for 1 h with 1.5% normal goat serum. Anti-CD3 (Dako North America, Inc. CA) was used as primary antibody (1:50–1:100 dilution), isotype rabbit IgG was used as a negative control. Biotinylated goat-anti-rabbit IgG (Jackson Immunoresearch, West Grove, PA) was used as secondary antibody, followed by incubation with Vectastain Elite avidin-biotin complex (Vector Laboratories, Burlingame, CA). Peroxidase activity was visualized using Nova Red substrate (Vector) (Yu et al., 2008). Cell nuclei were counter-stained with hematoxylin (Vector).
2.9 Luxol fast blue staining for demyelination analysis
Paraffin sections of spinal cord from EAE mice treated with PBS and AS101 were stained with Luxol fast blue staining kit according to the manufacture’s instruction (IHC World LLC, Woodstock, MD).
2.10 Isolation of mononuclear cells from spinal cords
Mononuclear cells were extracted from inflamed CNS tissue as previously described (Chen et al., 2013). Mice were perfused with cold PBS to remove blood from internal organs. The spinal cord was flushed out by hydrostatic pressure and cut into small pieces and digested in a solution with 0.2 U/ml Liberase DL (Roche) and 1mg/ml DNAse I (Roche) in DMEM at 37°C for 45 min. A single cell suspension was prepared by passing through a 70–μm cell strainer. The cells were washed once in PBS, placed in 37% Percoll solution, and overlaid with 70% solution, then centrifuged at 1800 rpm for 20 min. The mononuclear cells in the interphase layer of the Percoll gradient were transferred into a fresh tube and used for subsequent experiments.
2.11 RNA isolation and quantitative RT-PCR
Cells were collected, and total RNA was extracted using TRIzol (Invitrogen, Life Technology). A total of 500ng RNA was reverse transcribed into cDNA using Super Script III first-strand synthesis kit (Invitrogen) according to the manufacture’s protocols. The resulting cDNA template was subjected to real-time PCR using BioRad CFX96 Real-Time PCR detection system with SYBR Green Reagent Kit (Invitrogen). The target mRNA levels were normalized to GAPDH levels for each sample run in triplicate. The IL-17, GM-CSF, IL-6, IFN-γ, and GAPDH primer sequences are described in previous report (Chen et al., 2013).
2.12 Statistical analysis
The student t-test was used to analyze the differences between two groups for the in vitro experiments. The data are expressed as the mean ± SEM. A p value < 0.05 was considered statistically significant. Statistics on EAE clinical scores were evaluated by Mann-Whitney-Wilcoxon non-parametric analysis to determine the significance of difference between AS101- and vehicle PBS-treated mice.
3. Results
3.1 AS101 inhibits production of IL17 by activated CD4+ T cells
In the presence of antigen stimulation, TGF-β and IL-6 promote naive CD4+ T cell differentiation to produce IL-17 (Mangan et al., 2006; Sutton et al., 2012). However, IL-2 and IFN-γ inhibit Th17 cell differentiation (Laurence et al., 2007; Park et al., 2005). IL-17 has been shown to have a pathogenic role in several autoimmune diseases (Kang et al., 2010; Kroenke et al., 2008; Ciric et al., 2009; Doodes et al., 2010; Wilson et al., 2007). It is therefore important to find novel therapeutic agent to inhibit IL-17 production. AS101 promotes lymphocytes to secret IL-2, and reduces IL-6 production (Sredni et al 1987; Sredni et al., 1997; Kalechman et al., 2004; Brodsky et al., 2010), suggesting that AS101 may have the potential to inhibit IL-17 production.
To address this question, we did in vitro cell culture using splenocytes from 8–10 week old C57BL/6 mice. We isolated naïve CD4+ T cells by using magnetic beads (Stem Cell Biotech. Inc.). Flow cytometric analysis showed the purity of isolated CD4+ was above 93% (data not shown). We cultured the isolated CD4+ T cells under Th17 polarization conditions in the presence or absence of AS101. After 3–4 days in culture, cells were harvested for cytokine intracellular staining, and the supernatants were collected for ELISA assay. We used flow cytometry to analyze the intracellular cytokine production by CD4+ T cells.
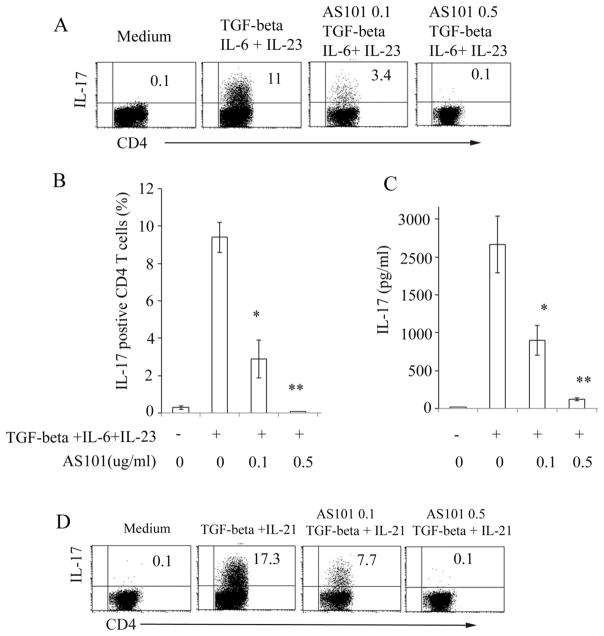
The results showed that CD4+ T cells produce considerable amounts of IL-17 when cells were stimulated with TGF-β +IL-6+IL-23(Fig. 1A). Interestingly, adding AS101 markedly reduced IL-17 production by CD4+ T cells compared to cells cultured under the same Th17 polarizing conditions in the absence of AS101 (Fig. 1A, B). ELISA analysis showed that the level of IL-17 in Th17 cell supernatants was significantly reduced following treatment with AS101 (Fig. 1C).
Figure 1. AS101 inhibits production of IL-17 by CD4+ T cells.
Isolated CD4+ T cells from 8 to 12 week old naïve C57BL/6 mice were cultured in 24 well plates coated with anti-CD3 (10μg/ml) under Th17 polarizing conditions (TGF-β 2 ng/ml +IL-6 20ng/ml + IL-23 20ng/ml) in the presence or absence of AS101 (0.1 to 0.5μg/ml). Anti-IFN-γ (10μg/ml), anti-IL-4 (5μg/ml), and anti-CD28 (3μg/ml) were added to the cultures. Intracellular cytokine staining was performed following 5 hrs restimulation with PMA/ionomycin/brefeldin A. FACSCalibur was used for the data collection (A). Summary of three representative experiments (Mean ± SEM) is shown (B). C. CD4+ T cell culture supernatants were harvested after 72 hrs culture and analyzed by IL-17 ELISA kits (eBiosciences). Summary of 3 individual experiments is shown (Mean ± SEM). D. Isolated CD4+ T cells were stimulated with different Th17 polarizing cytokines (TGF-β (2 ng/ml), IL-21 (50 ng/ml) in the presence or absence of AS101(0.1, 0.5μg/ml) as in Fig. 1A. *, p<0.05; **, p<0.01.
Flow cytometric analysis also confirmed that AS101 inhibited production of IL-17 by CD4+ T cells when stimulated by different combinations of Th17 polarization cytokines (TGF-β+IL-21) (Fig. 1D). In addition, when total splenocytes were cultured under Th17 cell polarization conditions, AS101 still significantly inhibited production of IL-17 compared to that without AS101 in the culture (data not shown).
3.2 IL-2 partially contributes to AS101 inhibition of IL-17 production
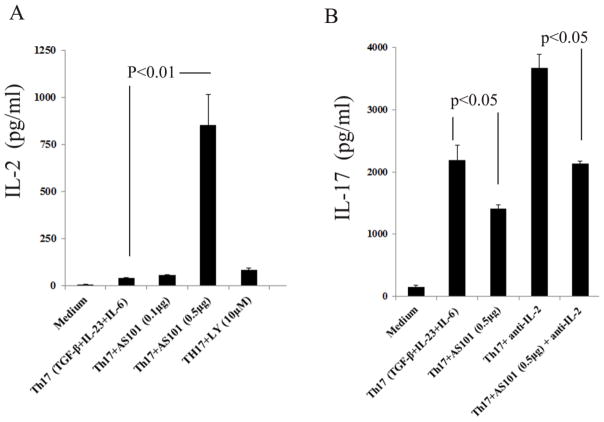
Previous work revealed that AS101 promotes production of IL-2 and expression of IL-2R by lymphocytes (Sredni et al., 1987). ELISA analysis also showed that AS101 promotes IL-2 production by CD4+ T cells under Th17 polarization conditions (Fig. 2A). Because IL-2 has been shown to inhibit IL-17 production (Laurence et al., 2007), we hypothesized that AS101 inhibition of IL-17 production in T cells is partially dependent on IL-2. To test this hypothesis, we used anti-IL-2 to neutralize IL-2 in the culture and determined whether AS101 is still able to reduce IL-17 production. As expected, neutralization of IL-2 by anti-IL-2 significantly enhanced IL-17 production by Th17 cells compared to that of Th17 cells without anti-IL-2 (Fig. 2B). However, neutralization of IL-2 only partially reduces AS101-mediated inhibition of IL-17 production (Fig. 2B), suggesting that AS101 inhibits production of IL-17 is partially dependent on IL-2.
Figure 2. AS101 promotes IL-2 production by lymphocytes cultured under Th17 polarizing condition.
A. CD4+ T cells were cultured under Th17 (TGF-β, IL-6, and IL-23) polarizing conditions in the presence or absence of AS101 as described in Fig. 1A. Cell culture supernatants were harvested and analyzed by IL-2 ELISA (Biolegend). Compared to cells stimulated with Th17 polarizing cytokines, AS101 (0.5μg/ml) significantly increased production of IL-2 in Th17 cells (p<0.01). B. CD4+ T cells were cultured in the presence or absence of anti-IL-2 (20μg/ml) under Th17 polarization condition with AS101. Neutralization of IL-2 partially reduced the effects of AS101 on inhibition of IL-17 production. Summary of 3 individual experiments is shown (Mean ± SEM). P<0.05.
3.3 AS101 inhibits activation of Akt, Stat3 and RORγt, but not of ERK1/2
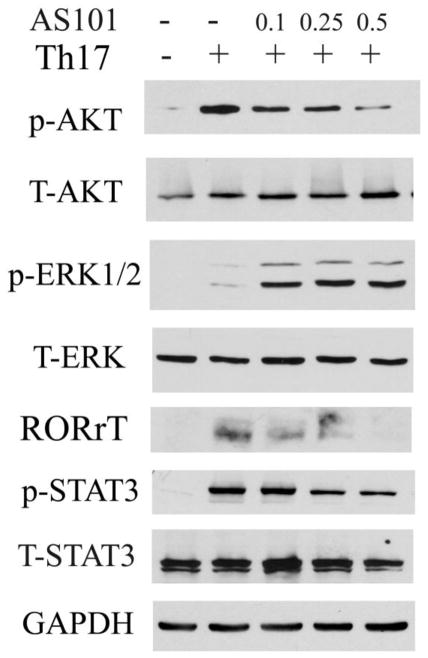
Cytokine-directed in vitro differentiation of Th17 cells and in vivo Th17-mediated inflammatory disease require induction of RORγt (Ivanov et al., 2006). In Th17 culture condition, CD4+ T cells from RORγt−/− mice displayed a marked reduction in IL-17+ cells compared to the wild type CD4+ T cells. In addition, RORγt can be induced by TGF-β and IL-6. IL-6 has been shown to play a key role in the generation of Th17 cells (Ivanov et al., 2006; Zhou et al., 2007). Stat3 is an important transcription factor for IL-6-mediated effects. The production of IL-17 was much reduced in Stat3−/− T cells compared to wild type T cells, which further supports the role of Stat3 in Th17 cell differentiation (Mathur et al., 2007). Previous studies showed that AS101 could reduce the activation of Stat3 by inhibiting phosphorylation of Akt in tumor cells (Kalechman et al., 2004, Hayun et al., 2006), and IL-6 production by macrophages (Brodsky et al., 2010). Moreover, PI3 Kinase inhibitor (LY290042) prevents phosphorylation of Akt and inhibits Th17 cell differentiation (Kim et al., 2005). We hypothesized that AS101 inhibits production of IL-17 at multiple checkpoints including reducing function of Stat3 and RORγt. In addition, because previous work showed that activation of ERK1/2 are implicated in regulating IL-2 production (Kogkopoulu et al., 2006), we hypothesized that AS101 promotes IL-2 production by increasing activation of ERK1/2, which partially contribute to AS101 inhibition of IL-17 production (Fig. 2). We performed the following experiments to test this hypothesis. CD4+ T cells were cultured under Th17 polarizing condition in the presence or absence of AS101. Whole cell lysates or cell nuclear protein extracts were isolated at different time points after culture. We did Western blots to examine the activation of Akt, Stat3, RORγt, and ERK1/2. The results showed that treatment with AS101 inhibited the activation of Akt, p-Stat3 and RORγt, but not of ERK1/2. In fact, AS101 promoted the phosphorylation of ERK1/2 (Fig. 3). As expected the activation of Stat3 and RORγt in cell nucleus extracts was much reduced by AS101 compared to that cells cultured under same Th17 polarization condition without AS101 (data not shown). Therefore, these results suggest that AS101 inhibits production of IL-17 in activated T cells via blocking the activation of Akt, Stat3 and RORγt, and promoting activation of ERK1/2.
Figure 3. AS101 inhibits activation of Akt, Stat3, and RORγt, but upregulates activation of ERK1/2.
CD4+ T cell were cultured under Th17 polarization condition (TGF-β, IL-6, and IL-23) with or without different dosage of AS101 as Fig. 1. Western blots were used to measure the activation of STAT3, AKT, RORγt, and ERK1/2 in Th17 cell lysates. GAPDH represents a loading control. The data are representative of 3 similar experiments.
3.4 AS101 reduces production of GM-CSF in activated CD4+ T cells
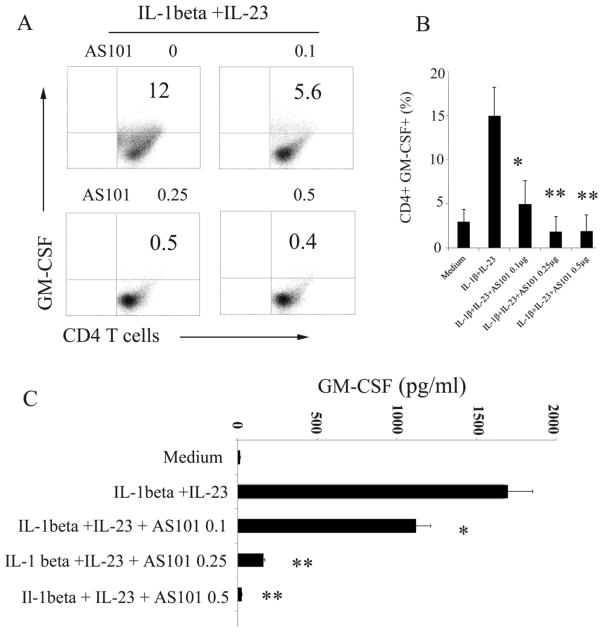
Th17 cells are able to recruit other inflammatory cells into CNS lesions through the production of multiple proinflammatory cytokines including IL-1β and GM-CSF (Korn et al., 2009). GM-CSF is required for development of EAE and GM-CSF−/− mice are resistant to development of EAE (McQuater et al., 2001; Ponomarev et al., 2007). More recent work indicated that encephalitogenic Th17 cell induction of EAE is dependent on GM-CSF, and RORγt transcription factor is required for the production of GM-CSF (Cordarri et al., 2011; El-Behi et al., 2011). Because AS101 inhibits activation of RORγt in T cells under Th17 polarization conditions (Fig. 3), we were interested in determining whether AS101 is able to inhibit production of GM-CSF in activated T cells. To answer this question, isolated CD4+ T cells from naïve mice were cultured with anti-CD3, anti-CD28, IL-1β and IL-23 (El-Behi et al., 2011) in the presence or absence of AS101. We used flow cytometry to examine the GM-CSF production in activated CD4+ T cells. As expected, flow cytometric analysis showed that IL-1β and IL-23 stimulate CD4+ T cells to produce GM-CSF (Fig. 4), which is in line with previous report (El-Behi et al., 2011). However, AS101 reduces the production of GM-CSF in CD4+ T cells under similar culturing conditions in a dose dependent manner (Fig. 4A, B). We performed ELISAs to measure the levels of GM-CSF in the culture supernatant. The results are consistent with the data obtained by flow cytometry (Fig. 4A, B). The level of GM-CSF in culture supernatants was much reduced by AS101 compared to that polarized cells without AS101 (Fig. 4C). Results in Fig. 4C are a summary of 3 individual experiments. The statistical analysis reveals that there were significant differences in the production of GM-CSF between the groups treated with and without AS101 (P<0.01), which indicated that AS101 inhibits production of GM-CSF in activated CD4+ T cells. In addition, although Th17 cells produce less GM-CSF than activated CD4+ T cells, AS101 also significantly reduces the production of GM-CSF by Th17 cells (data not shown).
Figure 4. AS101 reduces production of GM-CSF in activated CD4+ T cells.
CD4+ T cells were cultured under GM-CSF producing cell polarizing conditions (IL-1β and IL-23) in the presence or absence of AS101 as described in Fig. 1. Flow cytometry was used for analysis of GM-CSF in CD4+ T cells (A), and summary of 3 individual experiments in B. Cell culture supernatants were analyzed by GM-CSF ELISA kit (Biolegend) (C). Results in B and C (Mean ± SEM) represents summary of 3 individual experiments. Compared to GM-CSF-producing cells generated with IL-1β and IL-23 in the absence of AS101, adding AS101 in the similar culture system significantly reduces GM-CSF production by activated CD4+ T cells. *, p< 0.05, ** p< 0.01.
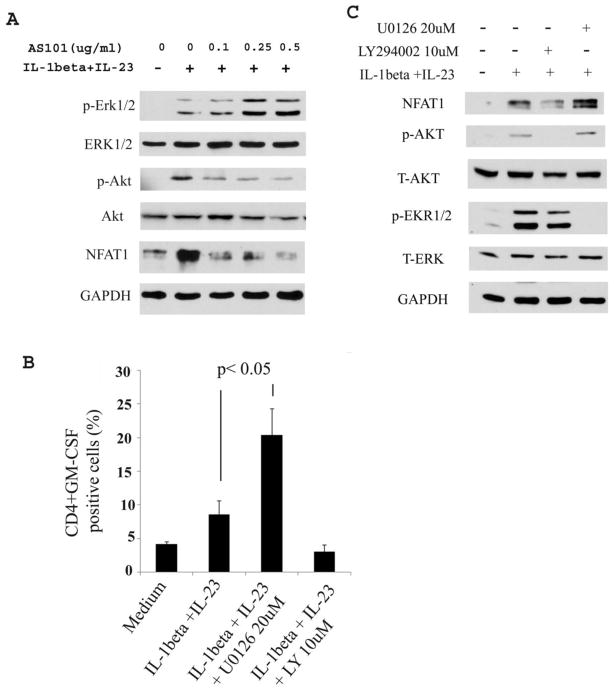
3.5 AS101 inhibits NFAT in activated CD4+ T cells
GM-CSF is the one of many cytokines produced following T cell activation (Shi et al., 2006). The GM-CSF gene contains NFAT and AP-1 families of transcriptional control regions (Shi et al., 2006; Shang et al., 1999). Recent work has indicated that RORγt transcriptional factor promotes the production of GM-CSF in Th17 cells (El-Behi et al., 2011). To further determine the underlying mechanisms by which AS101 reduces the production of GM-CSF in activated CD4+ T cells, we did Western blots to assess the transcriptional factors in CD4+ T cell extracts with or without AS101. Expression of NFAT1 and phosphorylation of Akt were detected in activated T cells stimulated by IL-β and IL-23. However, treatment with AS101 reduced the level of the transcriptional factors NFAT1 and activation of Akt, but it increased activation of ERK1/2 (Fig. 5A). To further determine the role of Akt and ERK1/2 in GM-CSF production in T cells activated by IL-β and IL-23, we pharmacologically inhibited activation of Akt1 and ERK1/2 by LY290042 (LY) and U0126, respectively. The results showed that inhibition of activation of Akt1 by LY reduced production of GM-CSF by activated T cells, but inhibition of activation of ERK1/2 by U0126 dramatically enhanced GM-CSF production (Fig. 5B). We did western blot to confirm that LY and U0126 inhibits the phosphorylation of Akt and ERK1/2, respectively (Fig. 5C). As expected, inhibiting the activation of Akt by LY correlated well with the reduction of NFAT1. In contrast, reducing activation of ERK1/2 by U0126 increased the expression of NFAT1 (Fig. 5C). These results suggested that AS101 inhibits the production of GM-CSF in activated CD4+ T cells by blocking activation of Akt and transcriptional factor NFAT1 and promoting activation of ERK1/2.
Figure 5. AS101 inhibits activation of NFAT1, Akt, but increase activation of Erk1/2 in activated CD4+ T cells resulting in reduction of GM-CSF.
A. CD4+ T cells were cultured as described in Fig. 4. The cell lysates were used for Western blot to detect activation of ERK1/2, Akt, and NFAT1. One of 3 representative experiments is shown. B. CD4+ T cells were induced to produce GM-CSF as described in Fig. 4 with or without U0126 (20μM) and LY294002 (10μM). The GM-CSF producing cells were detected by flow cytometry. Results are summary of 3 representative experiments (Mean ± SEM). P< 0.05. C. CD4+ T cells were cultured as described in Fig. 5B. Western blots were done to detect NFAT1, activation of ERK1/2 and Akt in cell lysates. The results showed that blocking activation of ERK1/2 by U0126 promotes the expression of NFAT1. In contrast, blocking the activation of Akt by LY 294002 dramatically reduced the expression of NFAT1. One of 3 representative experiments is shown.
3.6 AS101 inhibits Th17 cell proliferation
Since AS101 inhibits production IL-17 and GM-CSF, we were interested to determine if AS101 inhibits Th17 cell proliferation. To address this question, naïve CD4+ T cells were first labeled with CFSE and cultured under Th17 polarizing condition. We used flow cytometric analysis to measure cell proliferation. The results showed that AS101 significantly reduces lymphocyte proliferation under Th17 polarization conditions (Supple. Fig. 1). In addition, AS101 is able to suppress GM-CSF producing cell proliferation stimulated by IL-1β and IL-23 (data not shown).
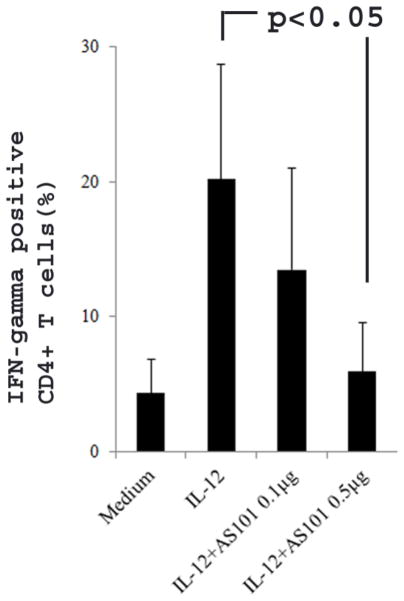
3.7. AS101 reduces production of IFN-γ by Th1 cells
Although IFN-γ has been reported to play both a protective and pathogenic roles in EAE (Ferber et al., 1996; Lees et al. 2008), administration of IFN-γ to MS patients exacerbates the diseases (Pantich et al. 1987), suggesting that IFN-γ promotes pathogenesis of MS. To further evaluate the effect of AS101 on Th1 cells, we culture naïve CD4+ T cells under Th1 cell polarizing conditions with or without AS101. Three day after culture, flow cytometry was used to measure IFN-γ production. The results showed that low dose of AS101 (0.1μg/ml) did not reduce IFN-γ production (Fig. 6), which is different from the effect of low dose of AS101 on IL-17 and GM-CSF production (Fig. 1 and 4). However, high dose of AS101 (0.5μg/ml) dramatically reduced IFN-γ production by Th1 cells (Fig. 6), suggesting that AS101 may have a beneficial effect for MS patients.
Figure 6. AS101 reduces IFN-γ production by Th1 cells.
Isolated naïve CD4+ T cells were cultured under Th1 polarization condition as described in Materials and Methods in the presence or absence of AS101 for 72 hours. The flow cytometry was used for measuring IFN-γ-producing cells. The results showed that high dosage of AS101 (0.5μg/ml) significantly reduced the IFN-γ production by Th1 cells. Summary of 3 representative experiments is shown (Mean ± SEM).
3.8 AS101 ameliorates the pathogenesis of EAE
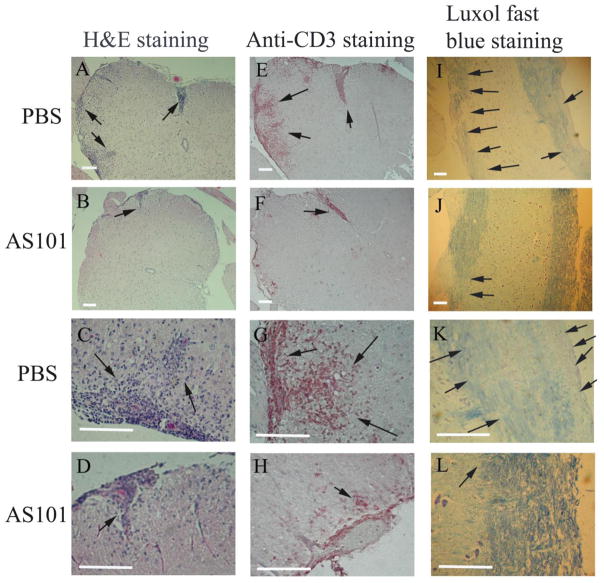
To determine the function of AS101 for the treatment of EAE, we used MOG-induced EAE in 8 to 12 week old female C57BL/6 mice. The induction of EAE was done according to the methods described previously (Matsushita et al., 2010; Miller et al., 2007; Xu and Drew, 2007). Treatment with AS101 and PBS was administrated at the first day of immunization ip and the treatments were repeated every other day. As expected, PBS treated control group of mice began developing EAE at day 7 postimmunization, reaching the peak of disease around day 14, and continued to exhibit chronic symptoms until around day 34 at the end of the experiment. However, AS101 treatment delayed the onset of EAE and significantly inhibited the development of EAE compared to the PBS treated control group, which developed severe chronic autoimmune neuroinflammation (Fig. 7). The group of mice treated with AS101 recovered sooner than the control group of mice treated with PBS (Fig. 7). Histological analysis also showed that AS101 reduced the inflammatory cell infiltration of spinal cord compared to the PBS treated group, where there was an abundance of inflammatory cell infiltration of spinal cord (Fig. 8A, C vs B, D). This was further confirmed by anti-CD3 immunohistochemical staining in spinal cord section of EAE mice treated with PBS (Fig. 8E, G) and AS101 (Fig. 8F, H). In line with the findings that AS101reduced the severity of EAE, large areas of demyelination of the white matter was present in the spinal cord of EAE mice treated with PBS, but not of the mice treated with AS101 (arrows in Fig. 8, I, K vs J, L).
Figure 7. AS101 inhibits development of EAE.
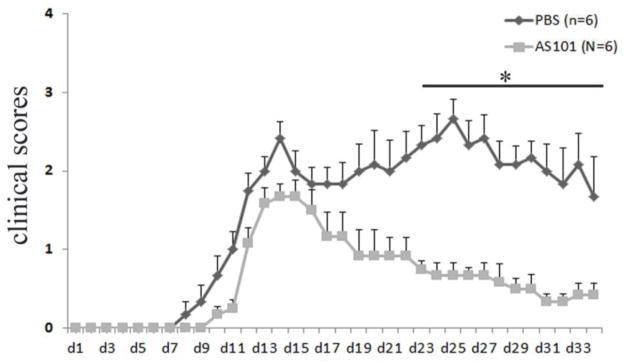
Eight to ten week old female C57BL/6 mice were immunized with MOG (200μg) plus CFA and received PTX on the day of immunization and 48 hours later. 6 mice received 10μg AS101 (in 200μl volume) i.p. and 6 mice got vehicle PBS (200μl) beginning the day of immunization. The injections were repeated every other day. Mice were monitored daily for clinical disease. Experiments were terminated 34 days after immunization. The results showed that AS101 treatment ameliorates the development of EAE compared to PBS treated mice. Disease scores were for total 6 mice per group (Mean ± SEM). Results are representative of two independent experiments. *, p < 0.05 for AS101-treated versus vehicle PBS-treated mice.
Figure 8. Less severe inflammatory cell infiltration and demyelination in spinal cords of AS101 treated EAE mice compared to those treated with PBS.
Spinal cords from PBS and AS101 treated EAE mice were sectioned and stained by H & E staining (A–D), anti-CD3 immunohistochemical staining (E–H), and luxol fast blue staining (I–L). The inflammatory cell infiltration in spinal cord was high-lighted by arrows. The results showed that AS101 treatment much reduced the inflammatory cell infiltrations of spinal cords and less demyelination compared to that of treated with PBS (arrows). Representative histology is shown. Scale bars: 100μm.
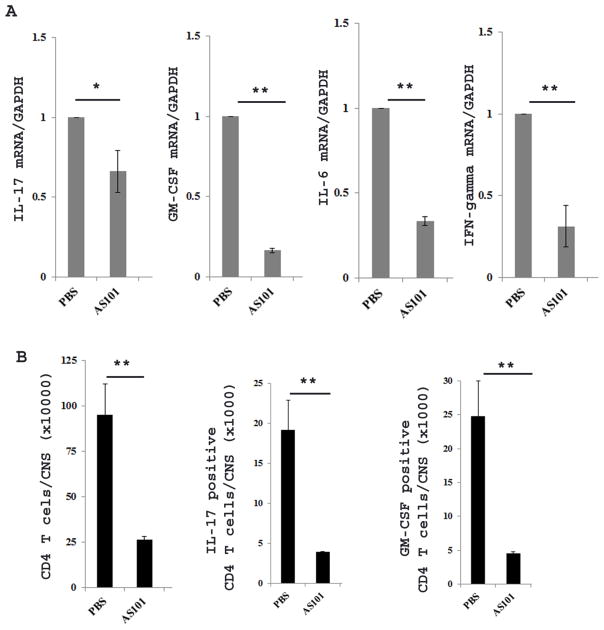
We further isolated mononuclear cells from spinal cords of EAE mice treated with AS101 or PBS at day 19 postimmunization to quantitate inflammatory cytokine production by real-time PCR and flow cytometry. The results showed that the expression of IL-17, GM-CSF, IFN-γ, and IL-6 mRNA was much reduced in AS101 treated mice compared to PBS treated mice (Fig. 9A). In addition, flow cytometric analysis of CD4+ T cells demonstrated that GM-CSF-, and IL-17 producing cells were significantly reduced in the spinal cords of mice treated with AS101 compared with those treated with PBS (Fig. 9B), suggesting that AS101 also plays a role in vivo to reduce these inflammatory cytokines that are important for development of EAE.
Figure 9. AS101 reduces the inflammatory cytokine production by mononuclear cells in spinal cords.
At 19 day of postimmunization, mononuclear cells were isolated from spinal cords of EAE mice treated with PBS and AS101 as described in materials and methods. Real-time PCR and flow cytometry were used to quantify the inflammatory cytokine mRNA (A) and protein expression (B). One of two repeat experiments is shown. *, p< 0.05, ** p< 0.01.
Taken together, these results suggested that AS101 has the potential as a novel therapeutic regent for treatment of MS.
4. Discussion
Although AS101 is an immunomodulator that has anti-cancer and anti-inflammation functions, we have shown here that AS101 inhibits the differentiation of Th17 cells and reduces the production of IL-17 and GM-CSF by blocking the activation of transcriptional factor NFAT, Stat3, and RORγt. By using a mouse model of multiple sclerosis, we showed that AS101 is effective in the amelioration of EAE and reduces inflammatory cell migration to the spinal cord. These results suggest that AS101 may have potential therapeutic intervention for human multiple sclerosis.
Growing evidence showed that IL-17 and GM-CSF play a role in the pathogenesis of EAE and MS (Cordarri et al., 2011; El-Behi et al., 2011; Ponomarev et al., 2007). Targeting both IL-17 and GM-CSF is ideal for therapeutic intervention of autoimmune neuroinflammation. However, so far to the best of our knowledge, there are no reported agents that could target both IL-17 and GM-CSF inflammatory cytokines by the same agent. Here we provide evidence that AS101 inhibits differentiation of Th17 cells and reduces production of GM-CSF. Interestingly, AS101 is not simply an immunosuppressor. Consistent with previous reports, AS101 could promote production of IL-2 (Sredni et al., 1987; Fig. 2). It is well known that activation of ERK1/2 is important for IL-2 gene expression (Kogkopoulou et al., 2006). Therefore, AS101 enhances IL-2 production by promoting phosphorylation of ERK1/2 (Fig. 3). Since IL-2 could inhibit Th17 cell differentiation (Laurence et al., 2007), AS101 inhibition of IL-17 production is partially dependent on regulation of IL-2. As expected, when we neutralized IL-2, this partially reversed AS101-mediated inhibition on production of IL-17 by activated T cells (Fig. 2B).
To further validate the mechanisms by which AS101 reduces production of IL-17 and GM-CSF, we did western blot. We demonstrated that AS101 inhibits activation of Stat3, Akt and RORγt. Previous work showed that NFAT is involved in GM-CSF expression (Shang et al., 1999; Xu and Drew, 2007). To determine the underlying mechanism by which AS101 reduces GM-CSF production, Western blots were done to detect cell lysates of T cells activated by IL-1β and IL-23. The results showed that AS101 down regulates expression of NFAT and up regulates activation of Erk1/2. Mounting evidence shows that Stat3, RORγt, and NFAT are crucial transcription factors for IL-17 and GM-CSF production (Laurence et al., 2007; Cordarri et al., 2011; El-Behi et al., 2011; Zhou et al., 2007; Mathur et al., 2007; Hayun et al., 2006; Korn et al., 2009; Shi et al., 2006 Shang et al., 1999). Our studies demonstrated that AS101 down regulates production of IL-17 and GM-CSF by blocking activation of Stat3, RORγt, and NFAT.
Although some reports showed that IFN-γ−/− mice develop severe EAE suggesting that IFN-γ has a protective role in EAE (Ferber et al, 1996, Willenborg et al, 1996), clinical and hematological symptoms are exacerbated in relapsing/remitting MS patients following the administration of IFN-γ (Panitch et al. 1987). Interestingly, not low dosage of AS101 but high dosage of AS101 did reduce IFN-γ production by CD4+ T cells under Th1 polarization conditions (Fig. 6). Because the increased levels of Th1 cytokines are particularly evident during EAE/MS relapse (Imam et al, 2007) and self-reactive Th1 clones derived in vitro are capable of adoptively transferring EAE to naïve recipients (Anderson et al. 2000), it is likely that AS101 may have a function to suppress EAE and/or MS, in part, by modulating the differentiation of Th1 cells.
EAE is valuable in investigating basic immunological mechanism of MS and as an animal model for novel therapy and drug mechanism elucidation. Early studies showed that both Th1 and Th17 cells in MS patients express high level of VLA-4, and VLA-4 was identified as the primary adhesion molecule mediating the attachment of lymphocytes to the CNS vascular endothelium (Baron et al., 1993). A new report showed that AS101 inhibits VLA-4 expression to ameliorate EAE (Lee et al. 2014). Our current study showed that AS101, a non-toxic small molecule immunomodulator, exerts potent anti-inflammatory actions including suppression of IL-17 and GM-CSF expression by Th17 cells, suggesting that AS101 is an immunomodulator with multiple effects to suppress the development of EAE. These studies suggest that AS101 has the potential for therapeutic intervention of multiple sclerosis.
Supplementary Material
Isolated naïve CD4+ T cells were labeled with CFSE and cultured under Th17 polarization condition (TGF-beta, IL-6 + IL-23) in the presence or absence of AS101 for 72 hours. The flow cytometry was used to monitor cell proliferation. The results showed that AS101 dramatically reduced Th17 cell proliferation. One of 3 representative experiments is shown.
Highlights.
Immunomodulator AS101 inhibited the differentiation of Th17 and Th1 cells.
AS101 reduced the production of IL-17, GM-CSF, and IFN-γ in activated T cells.
But, AS101 promoted the production of IL-2 in activated T cells.
AS101 blocked the activation of NFAT, Stat3, and RORγt, and increased activation of Erk1/2.
Acknowledgments
This study was supported by Arkansas Biosciences Institute (ABI) Fund, Arkansas State University Faculty Fund, NIH Grant Number p20GM103429 from the IDeA Networks of Biomedical Research Excellence (INBRE) Program of the National Center for Research Resources, and NSF MRI. N. Awar was an ABI Summer Biotechnology Internship Recipient.
Abbreviations
- AS101
trichloro(dioxoethylene-O,O′) tellurate
- EAE
Experimental autoimmune encephalomyelitis
- MOG
myelin oligodendrocyte glycoprotein
Footnotes
Authorship
SY designed the study. LX performed some flow analysis and most the western blots. JC, AM and SY did the EAE study and JC did mononuclear cell isolation and Real-time PCR analysis. NA performed the ELISA assay. SN performed some flow analysis. BS, PD and SY analyzed and interpreted the data. SY, LX, JC, AM, and PD wrote the manuscript.
Conflict of interest
The authors have no financial conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson AC, Nicholson LB, Legge KL, Turchin V, Zaghouani H, Kuchroo VK. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naïve mice: mechanisms of selection of the self-reactive repertoire. J Exp Med. 2000;191:761–770. doi: 10.1084/jem.191.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron JL, Madri JA, Ruddle NH, Hashim G, Janeway CA. Surface expression of alpha 4 integrin by CD4 T cells is required for their entry into brain parenchyma. J Exp Med. 1993;177:57–68. doi: 10.1084/jem.177.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelli F, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal development pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- Brodsky M, Halpert G, Albeck M, Sredni B. The anti-inflammatory effects of the tellurium redox modulating compound, AS101, are associated with regulation of NFkappaB signaling pathway and nitric oxide induction in macrophages. J Inflamm. 2010;7:3. doi: 10.1186/1476-9255-7-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi PA. Consideration in the treatment of relapsing-remitting multiple sclerosis. Neurol. 2002;58:S10–S22. doi: 10.1212/wnl.58.8_suppl_4.s10. [DOI] [PubMed] [Google Scholar]
- Ciric B, El-Behi M, Cabrera R, Zhang GX, Rostami A. IL-23 drives pathogenic IL-17-producing CD8+ T cells. J Immunol. 2009;182:5296–5305. doi: 10.4049/jimmunol.0900036. [DOI] [PubMed] [Google Scholar]
- Cordarri L, Gyulveszi G, Tosevski V, Hesske L, Fontana A, Magnetat L, Suter T, Becher B. ROR gamma t drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- Doodes PD, Cao Y, Hamel KM, Wang Y, Rodeghero RL, Mikecz K, Glant TT, Iwakura Y, Finnegan A. IFN-gamma regulates the requirement for the IL-17 in proteoglycan-induced arthritis. J Immunol. 2010;184:1552–1559. doi: 10.4049/jimmunol.0902907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Behi M, Ciric B, Dai H, Yan Y, Cullimore M, Safavi F, Zhang GX, Dittel BN, Rostami A. The encephalitogenicity of T(H)17 cells is dependent on IL-1 and IL-23-induced production of the cytokine GM-CSF. Nat Immunol. 2011;12:568–575. doi: 10.1038/ni.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferber IA, Brock S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman GC. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis. J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Friese MA, Montalba X, Willcox N, Bell JI, Martin R, Fugger L. The value of animal models for drug development in multiple sclerosis. Brain. 2006;129:1940–1952. doi: 10.1093/brain/awl083. [DOI] [PubMed] [Google Scholar]
- Hayun A, Naor Y, Weil M, Albeck M, Peled A, Don J, Haran-Ghera N, Sredni B. The immunomodulator AS101 induces growth arrest and apoptosis in multiple myeloma: association with the Akt/surviving pathway. Biochem Pharmacol. 2006;72:1423–1431. doi: 10.1016/j.bcp.2006.06.015. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R, Wekerle H. Immunological update on multiple sclerosis. Curr Opin Neurol. 2001;14:299–304. doi: 10.1097/00019052-200106000-00006. [DOI] [PubMed] [Google Scholar]
- Huseby ES, Liggitt D, Brabb T, Schnabel B, Ohlen C, Goverman JA. A pathogenic role for myelin-specific CD8 (+) T cells in a model for multiple sclerosis. J Exp Med. 2001;194:669–676. doi: 10.1084/jem.194.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam SA, Guyton MK, Haque A, Vandenbark A, Tyor WR, Ray SK, Banik NL. Increased calpain correlates with Th1 cytokine profile in PBMCs from MS patients. J Neuroimmunol. 2007;190:139–145. doi: 10.1016/j.jneuroim.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- Kalechman Y, Gafter U, Weinstein T, Chagnac A, Freidkin I, Tobar A, Albeck M, Sredni B. Inhibition of interleukin-10 by the immunomodulator AS101 reduces mesangial cell proliferation in experimental mesangioproliferative glomerulonephritis: association with dephosphorylation of STAT3. J Biol Chem. 2004;279:24724–24732. doi: 10.1074/jbc.M312006200. [DOI] [PubMed] [Google Scholar]
- Kang Z, Altuntas CZ, Gulen MF, Liu C, Giltiay N, Qin H, Liu L, Qian W, Ransohoff RM, Bergmann C, Stohlman S, Tuohy VK, Li X. Astrocyte-restricted ablation of interleukin-17-induced Act1-mediated signaling ameliorates autoimmune encephalomyelitis. Immunity. 2010;32:414–425. doi: 10.1016/j.immuni.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KW, Cho ML, Park MK, Yoon CH, Park SH, Lee SH, Kim HY. Increased interleukin-17 production via a phosphoinositide 3-kinase/Akt and nuclear factor kappaB-dependent pathway in patients with rheumatoid arthritis. Arthritis Res Ther. 2005;7:R139–148. doi: 10.1186/ar1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogkopoulou O, Tzakos E, Mavrothalassitis G, Balari CT, Paliogianni F, Young HA, Thyphronitis G. Conditional up-regulation of IL-2 production by p38 MAPK inactivation is mediated by increased Erk1/2 activity. J Leukoc Biol. 2006;79:1052–1060. doi: 10.1189/jlb.0705418. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurence A, Tato CM, Davidson TS, Kanno Y, Chen Z, Yao Z, Blank RB, Meylan F, Siegel R, Hennighausen L, Shevach EM, O’shea JJ. Interlieukin-2 signaling via STAT5 constrains T helper 17 cell generation. Immunity. 2007;26:371–378. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Lee JH, Halperin-Sheinfeld M, Baatar D, Mughal MR, Tae HJ, Kim JW, Carter A, Lustig A, Snir O, Lavie G, Okun E, Mattson MP, Sredni B, Taub DD. Tellurium compound AS101 ameliorates experimental autoimmune encephalomyelitis by VLA-4 inhibition and suppression of monocytes and T cell infiltration into CNS. Neuromolecular Med. 2014 doi: 10.1007/s12017-013-8277-3. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH. Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med. 2008;205:2633–2642. doi: 10.1084/jem.20080155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett-Racke AE, Yang Y, Racke MK. Th1 versus Th17: are T cell cytokines relevant in multiple sclerosis? Biochemical Biophysica Acta. 2011;1812:246–251. doi: 10.1016/j.bbadis.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangan PR, Harrington LE, O’Quinn DB, Helms WS, Bullard DC, Elson CO, Hatton RD, Wahl SM, Schoeb TR, Weaver CT. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- Mathur AN, Chang HC, Zisoulis DG, Stritesky GL, Yu Q, O’Malley JT, Kepur R, Levy DE, Kansas GS, Kaplan MH. Stat3 and Stat4 direct development of IL-17-screening T cells. J Immunol. 2007;178:4901–4907. doi: 10.4049/jimmunol.178.8.4901. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Horikawa M, Iwata Y, Tedder TF. Regulatory B cells (B10 cells) and regulatory t cells have independent roles in controlling experimental autoimmune encephalomyelitis initiation and late-phase immunopathogenesis. J Immunol. 2010;185:2240–2252. doi: 10.4049/jimmunol.1001307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuater JL, Darwiche R, Ewing C, Onuki M, Kay TW, Hamilton JA, Reid HH, Bernard CC. Granulocyte macrophage colony-stimulating factor: a new putative therapeutic target in multiple sclerosis. J Exp Med. 2001;194:873–882. doi: 10.1084/jem.194.7.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SD, Karpus WJ, Davidson TS. Curr Protoc Immunol. Unit-15.1. 2007. Experimental autoimmune encephalomyelitis in the mouse. Chapter. [DOI] [PubMed] [Google Scholar]
- Okun E, Arumugam TV, Tang SC, Gleichmann M, Albeck M, Sredni B, Mattson MP. The organotellurium compound ammonium triloro(dioxoethylene-0,0′) tellurate enhances neuronal survival and improves functional outcome in an ischemic stroke model in mice. J Neurochem. 2007;102:1232–1241. doi: 10.1111/j.1471-4159.2007.04615.x. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarev ED, Shriver LP, Maresz K, Pedras-Vasconcelos J, Verthelyi D, Dittel BN. GM-CSF production by autoreactive T cells is required for the activation of microglial cells and the onset of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:39–48. doi: 10.4049/jimmunol.178.1.39. [DOI] [PubMed] [Google Scholar]
- Shang C, Attema J, Cakouros D, Cockerill PN, Shannon MF. Nuclear factor of activated T cells contributes to the function of the CD28 response region of the granulocyte macrophage-colony stimulating factor promoter. Int Immunol. 1999;11:1945–1956. doi: 10.1093/intimm/11.12.1945. [DOI] [PubMed] [Google Scholar]
- Shi Y, Liu CH, Roberts AI, Das J, Xu G, Ren G, Zhang Y, Zhang L, Yuan ZY, Tan HS, Das G, Devadas S. Granulocyte-macrophage colony-stimulating factor (GM-CSF) and T-cell responses: what we do and don’t know. Cell Research. 2006;16:126–133. doi: 10.1038/sj.cr.7310017. [DOI] [PubMed] [Google Scholar]
- Siffrin V, Radbruch H, Glumm R, Niesner R, Paterka M, Herz J, Leuenberger T, Lehmann SM, Leuenstedt S, Rinnenthal JL, Laube G, Luche H, Lehnardt S, Fehling HJ, Griesbeck O, Zipp F. In Vivo imaging of partially reversible the Th17 cell-induced neuronal dysfunction in the course of encephalomyelitis. Immunity. 2010;33:424–436. doi: 10.1016/j.immuni.2010.08.018. [DOI] [PubMed] [Google Scholar]
- Sredni B, Caspi RR, Klein A, Kalechman Y, Danziger Y, Ben Ya’akov M, Tamari T, Shalit F, Albeck M. A new immunomodulating compound (AS101) with potential therapeutic application. Nature. 1987;330:173–176. doi: 10.1038/330173a0. [DOI] [PubMed] [Google Scholar]
- Sredni B, Gafter U, Da JP, Albeck M, Alarcon-Segovia D, Sredni B. Delay in the onset of systemic lupus erythematous following treatment with the immunomodulator AS101: association with IL-10 inhibition and increase in TNF-alpha levels. J Immunol. 1997;159:2658–2667. [PubMed] [Google Scholar]
- Sredni B, Geffen-Aricha R, Duan W, Albeck M, Shalit F, Lander HM, Kinor N, Sagi O, Albeck A, Yosef S, Brodsky M, Sredni-Kenigsbuch D, Sonino T, Longo DL, Mattson MP, Yadid G. Multifunctional tellurium molecule protects and restores dopaminergic neurons in Parkinson’s disease models. FASEB J. 2007;21:1870–1883. doi: 10.1096/fj.06-7500com. [DOI] [PubMed] [Google Scholar]
- Sredni B. Immunomodulating tellurium compounds as anti-cancer agents. Semin Cancer Biol. 2012;22:60–69. doi: 10.1016/j.semcancer.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Sutton CE, Mielke LA, Mills KH. IL-17-producing γδ T cells and innate lymphoid cells. Eur J Immunol. 2012;42:2221–2231. doi: 10.1002/eji.201242569. [DOI] [PubMed] [Google Scholar]
- Ulzheimer JC, Meuth SG, Bittner S, Kleinschnitz C, Kieseier BC, Wiendl H. Therapeutic approaches to multiple sclerosis: an updated on failed, interrupted, or inconclusive trials of immunomodulatory treatment strategies. Biodrug. 2010;24:349–274. doi: 10.2165/11537160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Willenborg DO, Fordham S, Bernard CC, Cowden WB, Ramshaw IA. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J Immunol. 1996;157:3223–3227. [PubMed] [Google Scholar]
- Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, Basham B, Smith K, Chen T, Morel F, Lecron JC, Kastelein RA, Cua DJ, McClanahan TK, Bowman EP, de Waal Malefyt R. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol. 2007;8:950–957. doi: 10.1038/ni1497. [DOI] [PubMed] [Google Scholar]
- Xu J, Drew PD. Peroxisome proliferation-activated receptor-γ agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu S, Sharp GC, Braley-Mullen H. TGF-β promotes thyroid epithelial cell hyperplasia and fibrosis in IFN-γ deficient NOD. H-2h4 mice. J Immunol. 2008;181:2238–2245. doi: 10.4049/jimmunol.181.3.2238. [DOI] [PubMed] [Google Scholar]
- Zhang H, Chen J, Liu X, Awar L, McMickle A, Bai F, Nagarajan S, Yu S. IL-17 induces expression of vascular cell adhesion molecule through signaling pathway of NF-κB, but not Akt1 and TAK1 in vascular smooth muscle cells. Scand J Immunol. 2013;77:230–237. doi: 10.1111/sji.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Ivanov II, Spolski R, Min R, Henderov SK, Egawa T, Levy DE, Leonard WJ, Littman DR. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathway. Nat Immunol. 2007;8:967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Isolated naïve CD4+ T cells were labeled with CFSE and cultured under Th17 polarization condition (TGF-beta, IL-6 + IL-23) in the presence or absence of AS101 for 72 hours. The flow cytometry was used to monitor cell proliferation. The results showed that AS101 dramatically reduced Th17 cell proliferation. One of 3 representative experiments is shown.