Abstract
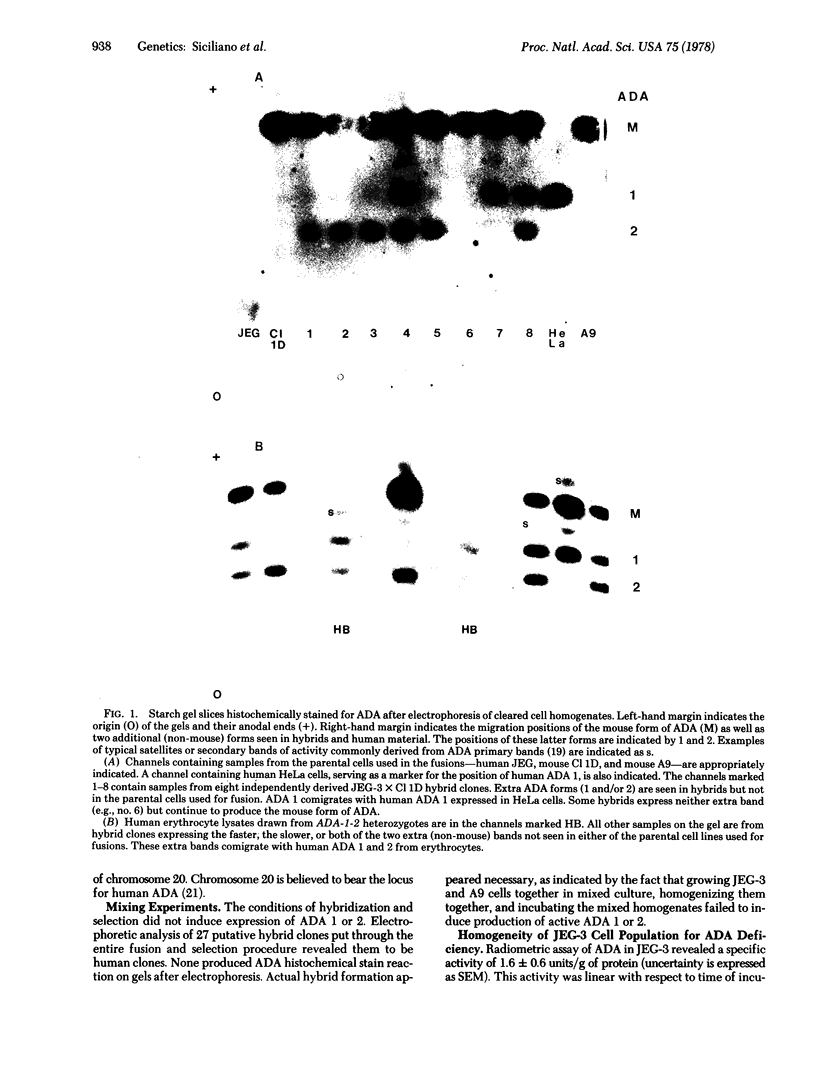
Two human choriocarcinoma cell lines were shown to be deficient in adenosine deaminase (ADA; adenosine aminohydrolase, EC 3.5.4.4) such that they did not produce bands on starch gels after electrophoresis and histochemical staining. Radiometric assay indicated that their ADA specific activity was approximately 2% that of HeLa (human) cell controls. Subclone analysis of one of the lines indicated that this deficiency was representative of individual cells of the line. After fusion of these cells with mouse fibroblasts having high ADA activity, most independently isolated hybrid clones expressed one of two, or both, additional (to the mouse) bands of ADA activity after electrophoresis. The expression of these extra bands in hybrids was dependent upon actual fusion. The phenomenon was observed in 30 of 45 independently derived hybrid clones from four different fusion experiments involving two different parental lines from each species. The pattern of appearance of the extra bands in independent hybrid clones and the tendency of a hybrid clone to lose one of the extra bands through subsequent passages suggests that the bands were the products of human genetic material. The extra bands electrophoretically comigrated with human ADA 1 and 2 from human ADA-1-2 heterozygotes and the faster-migrating of the two extra bands comigrated with human ADA 1 from HeLa cells. Therefore, we suggest that the bands appearing in hybrids are the products of the 1 and 2 alleles of the human ADA locus. The human cells used for fusion were deficient in ADA activity but contained the genetic information for ADA 1 and 2. Fusion with mouse cells having ADA activity resulted in the activation of both human gene products coded for on separate homologous chromosomes. We conclude that the human ADA locus is under manipulatable genetic regulation.
Keywords: genetic regulation, somatic cell hybridization, isozymes
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRADY T. G., O'DONOVAN C. I. A STUDY OF THE TISSUE DISTRIBUTION OF ADENOSINE DEAMINASE IN SIX MAMMAL SPECIES. Comp Biochem Physiol. 1965 Jan;14:101–120. doi: 10.1016/0010-406x(65)90011-3. [DOI] [PubMed] [Google Scholar]
- Bakay B., Nyhan W. L., Croce C. M., Koprowski H. Reversion in expression of hypoxanthine-guanine phosphoribosyl transferase following cell hybridization. J Cell Sci. 1975 May;17(3):567–578. doi: 10.1242/jcs.17.3.567. [DOI] [PubMed] [Google Scholar]
- Benke P. J., Dittmar D. Purine dysfunction in cells from patients with adenosine deaminase deficiency. Pediatr Res. 1976 Jul;10(7):642–646. doi: 10.1203/00006450-197607000-00002. [DOI] [PubMed] [Google Scholar]
- Bordelon M. R., Coon H. G., Kohler P. O. Human glycoprotein hormone production in human-human and human-mouse somatic cell hybrids. Exp Cell Res. 1976 Dec;103(2):303–310. doi: 10.1016/0014-4827(76)90267-6. [DOI] [PubMed] [Google Scholar]
- Boyd Y. L., Harris H. Correction of genetic defects in mammalian cells by the input of small amounts of foreign genetic material. J Cell Sci. 1973 Nov;13(3):841–861. doi: 10.1242/jcs.13.3.841. [DOI] [PubMed] [Google Scholar]
- Chen S. H., Scott C. R., Swedberg D. R. Heterogeneity for adenosine deaminase deficiency: Expression of the enzyme in cultured skin fibroblasts and amniotic fluid cells. Am J Hum Genet. 1975 Jan;27(1):46–52. [PMC free article] [PubMed] [Google Scholar]
- Chen T. R. A simple method to sequentially reveal Q-and C-bands on the same metaphase chromosomes. Chromosoma. 1974;47(2):147–156. doi: 10.1007/BF00331802. [DOI] [PubMed] [Google Scholar]
- Cohen F. Adenosine deaminase and immunodeficiency. Birth Defects Orig Artic Ser. 1975;11(1):124–127. [PubMed] [Google Scholar]
- Croce C. M., Bakay B., Nyhan W. L., Koprowski H. Reexpression of the rat hypoxanthine phosphoribosyltransferase gene in rat-human hybrids. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2590–2594. doi: 10.1073/pnas.70.9.2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. EFFECT OF HALOGENATED PYRIMIDINES AND THYMIDINE ON GROWTH OF L-CELLS AND A SUBLINE LACKING THYMIDINE KINASE. Exp Cell Res. 1964 Jan;33:19–28. doi: 10.1016/s0014-4827(64)81006-5. [DOI] [PubMed] [Google Scholar]
- Darlington G. J., Bernard H. P., Ruddle F. H. Human serum albumin phenotype activation in mouse hepatoma--human leukocyte cell hybrids. Science. 1974 Sep 6;185(4154):859–862. doi: 10.1126/science.185.4154.859. [DOI] [PubMed] [Google Scholar]
- Deisseroth A., Barker J., Anderson W. F., Nienhuis Hemoglobin synthesis in somatic cell hybrids: coexpression of mouse with human or chinese hamster globin genes in interspecific somatic cell hybrids of mouse erythroleukemia cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2682–2686. doi: 10.1073/pnas.72.7.2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards Y. H., Hopkinson D. A., Harris H. Adenosine deaminase isozymes in human tissues. Ann Hum Genet. 1971 Oct;35(2):207–219. doi: 10.1111/j.1469-1809.1956.tb01393.x. [DOI] [PubMed] [Google Scholar]
- Giblett E. R., Anderson J. E., Cohen F., Pollara B., Meuwissen H. J. Adenosine-deaminase deficiency in two patients with severely impaired cellular immunity. Lancet. 1972 Nov 18;2(7786):1067–1069. doi: 10.1016/s0140-6736(72)92345-8. [DOI] [PubMed] [Google Scholar]
- HAYASHI T. T. STUDIES ON PLACENTAL METABOLISM. II. PURINE NUCLEOTIDE CATABOLISM IN EARLY PLACENTA. Am J Obstet Gynecol. 1965 Sep 15;93:266–268. [PubMed] [Google Scholar]
- Hirschhorn R., Beratis N., Rosen F. S. Characterization of residual enzyme activity in fibroblasts from patients with adenosine deaminase deficiency and combined immunodeficiency: evidence for a mutant enzyme. Proc Natl Acad Sci U S A. 1976 Jan;73(1):213–217. doi: 10.1073/pnas.73.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschhorn R., Levytaka V., Pollara B., Meuwissen H. J. Evidence for control of several different tissue-specific isozymes of adenosine deaminase by a single genetic locus. Nat New Biol. 1973 Dec 19;246(155):200–202. doi: 10.1038/newbio246200a0. [DOI] [PubMed] [Google Scholar]
- Hsu S. H., Schacter B. Z., Delaney N. L., Miller T. B., McKusick V. A., Kennett R. H., Bodmer J. G., Young D., Bodmer W. F. Genetic characteristics of the HeLa cell. Science. 1976 Jan 30;191(4225):392–394. doi: 10.1126/science.1246620. [DOI] [PubMed] [Google Scholar]
- Kao F. T., Puck T. T. Genetics of somatic mammalian cells: demonstration of a human esterase activator gene linked to the adeB gene. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3273–3277. doi: 10.1073/pnas.69.11.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Leung W. C., Jorgensen G., Trkula D., Dubbs D. R. Acquisition of chick cytosol thymidine kinase activity by thymidine kinase-deficient mouse fibroblast cells after fusion with chick erythrocytes. J Cell Biol. 1974 Nov;63(2 Pt 1):505–514. doi: 10.1083/jcb.63.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler P. O., Bridson W. E. Isolation of hormone-producing clonal lines of human choriocarcinoma. J Clin Endocrinol Metab. 1971 May;32(5):683–687. doi: 10.1210/jcem-32-5-683. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. SELECTION OF HYBRIDS FROM MATINGS OF FIBROBLASTS IN VITRO AND THEIR PRESUMED RECOMBINANTS. Science. 1964 Aug 14;145(3633):709–710. doi: 10.1126/science.145.3633.709. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. THREE DEGREES OF GUANYLIC ACID--INOSINIC ACID PYROPHOSPHORYLASE DEFICIENCY IN MOUSE FIBROBLASTS. Nature. 1964 Sep 12;203:1142–1144. doi: 10.1038/2031142a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lee P. C. Developmental changes of adenosine deaminase, xanthine oxidase, and uricase in mouse tissues. Dev Biol. 1973 Apr;31(2):227–233. doi: 10.1016/0012-1606(73)90259-5. [DOI] [PubMed] [Google Scholar]
- Meuwissen H. J., Pollara B., Pickering R. J. Combined immunodeficiency disease associated with adenosine deaminase deficiency. Report on a workshop held in Albany, New York, October 1, 1973. J Pediatr. 1975 Feb;86(2):169–181. doi: 10.1016/s0022-3476(75)80463-x. [DOI] [PubMed] [Google Scholar]
- Parkman R., Gelfand E. W., Rosen F. S., Sanderson A., Hirschhorn R. Severe combined immunodeficiency and adenosine deaminase deficiency. N Engl J Med. 1975 Apr 3;292(14):714–719. doi: 10.1056/NEJM197504032921402. [DOI] [PubMed] [Google Scholar]
- Schwaber J. Human lymphocyte-mouse myeloma somatic cell hybrids: selective hybrid formation. Somatic Cell Genet. 1977 May;3(3):295–302. doi: 10.1007/BF01538747. [DOI] [PubMed] [Google Scholar]
- Schwaber J. Immunoglobulin production by a human-mouse somatic cell hybrid. Exp Cell Res. 1975 Jul;93(2):343–354. doi: 10.1016/0014-4827(75)90459-0. [DOI] [PubMed] [Google Scholar]
- Shaw C. R. Electrophoretic variation in enzymes. Science. 1965 Aug 27;149(3687):936–943. doi: 10.1126/science.149.3687.936. [DOI] [PubMed] [Google Scholar]
- Sim M. K., Maguire M. H. Variation in placental adenosine deaminase activity during gestation. Biol Reprod. 1970 Apr;2(2):291–298. doi: 10.1095/biolreprod2.2.291. [DOI] [PubMed] [Google Scholar]
- Tischfield J. A., Creagan R. P., Nichols E. A., Ruddle F. H. Assignment of a gene for adenosine deaminase to human chromosome 20. Hum Hered. 1974;24(1):1–11. doi: 10.1159/000152631. [DOI] [PubMed] [Google Scholar]
- Tischfield J. A., Ruddle F. H. Assignment of the gene for adenine phosphoribosyltransferase to human chromosome 16 by mouse-human somatic cell hybridization. Proc Natl Acad Sci U S A. 1974 Jan;71(1):45–49. doi: 10.1073/pnas.71.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine W. N., Paglia D. E., Tartaglia A. P., Gilsanz F. Hereditary hemolytic anemia with increased red cell adenosine deaminase (45- to 70-fold) and decreased adenosine triphosphate. Science. 1977 Feb 25;195(4280):783–785. doi: 10.1126/science.836588. [DOI] [PubMed] [Google Scholar]
- Weiss M. C., Chaplain M. Expression of differentiated functions in hepatoma cell hybrids: reappearance of tyrosine aminotransferase inducibility after the loss of chromosomes. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3026–3030. doi: 10.1073/pnas.68.12.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss M. C., Green H. Human-mouse hybrid cell lines containing partial complements of human chromosomes and functioning human genes. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1104–1111. doi: 10.1073/pnas.58.3.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]