Abstract
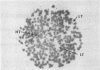
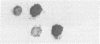
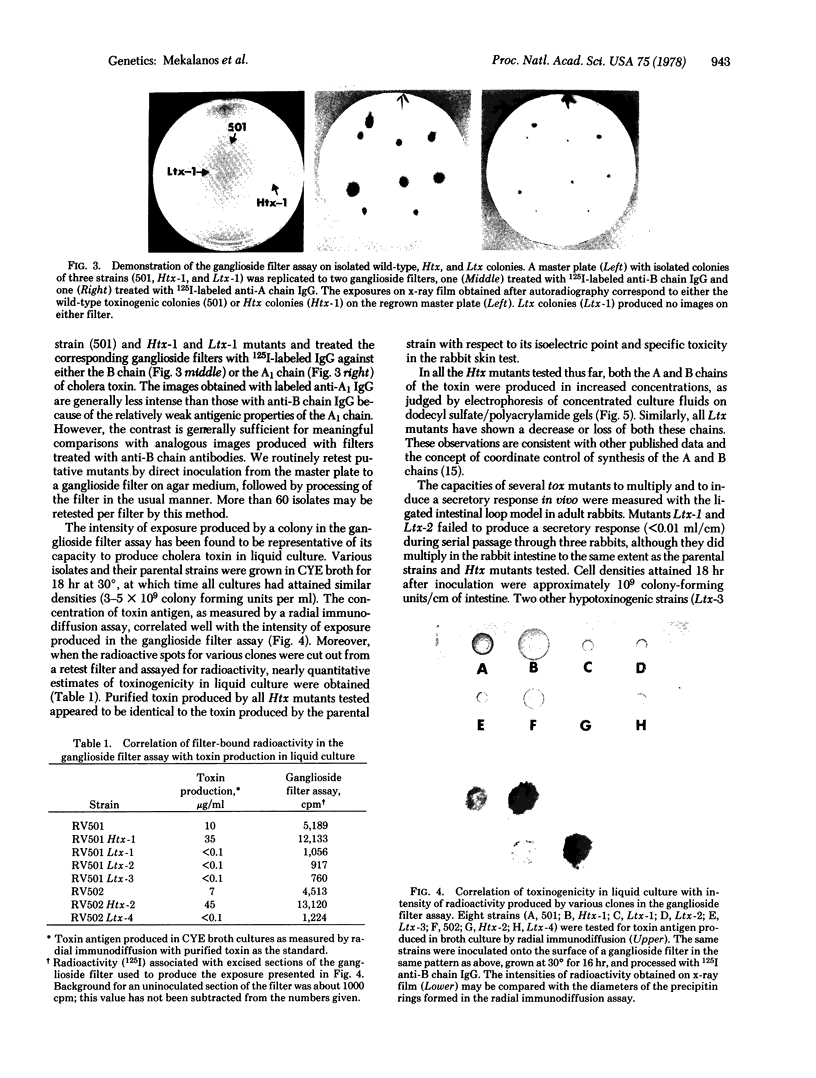
We have devised a novel plate assay method for detecting mutants of Vibrio cholerae altered in the production of cholera toxin (tox mutants). Colonies replicated from a master plate are grown on the surface of a cellulose filter disc to which ganglioside-albumin conjugates have been attached. Toxin secreted by the colonies is tightly bound to the ganglioside filters. After removal of the cells by washing, the bound toxin may be detected by treating the filters with radioactively labeled antibodies against either whole toxin or one of its constituent polypeptide chains, followed by autoradiography. Colonies producing significantly greater of lesser amounts of toxin than the parental type are easily recognized and can be shown in liquid culture to have the corresponding hypertoxinogenic or hypotoxinogenic phenotype. This method, termed "the ganglioside filter assay," is applicable to screening large numbers of colonies and should facilitate isolation of various specific classes of mutants in cholera toxin production. In modified form the method will be applicable to various systems in which mutants of secreted proteins are sought.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burrows W., Musteikis G. M. Cholera infection and toxin in the rabbit ileal loop. J Infect Dis. 1966 Apr;116(2):183–190. doi: 10.1093/infdis/116.2.183. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Vibrio cholerae choleragenoid. Mechanism of inhibition of cholera toxin action. Biochemistry. 1973 Aug 28;12(18):3577–3581. doi: 10.1021/bi00742a034. [DOI] [PubMed] [Google Scholar]
- David G. S. Solid state lactoperoxidase: a highly stable enzyme for simple, gentle iodination of proteins. Biochem Biophys Res Commun. 1972 Jul 25;48(2):464–471. doi: 10.1016/s0006-291x(72)80074-3. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Gill D. M. The arrangement of subunits in cholera toxin. Biochemistry. 1976 Mar 23;15(6):1242–1248. doi: 10.1021/bi00651a011. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Immunological study of the heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. Infect Immun. 1974 Mar;9(3):564–570. doi: 10.1128/iai.9.3.564-570.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Vasil M. L., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. III. Characterization of nontoxinogenic mutants in vitro and in experimental animals. J Clin Invest. 1975 Mar;55(3):551–560. doi: 10.1172/JCI107962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mekalanos J. J., Collier R. J., Romig W. R. Simple method for purifying choleragenoid, the natural toxoid of Vibrio cholerae. Infect Immun. 1977 Jun;16(3):789–795. doi: 10.1128/iai.16.3.789-795.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]