Abstract
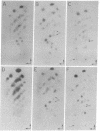
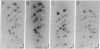
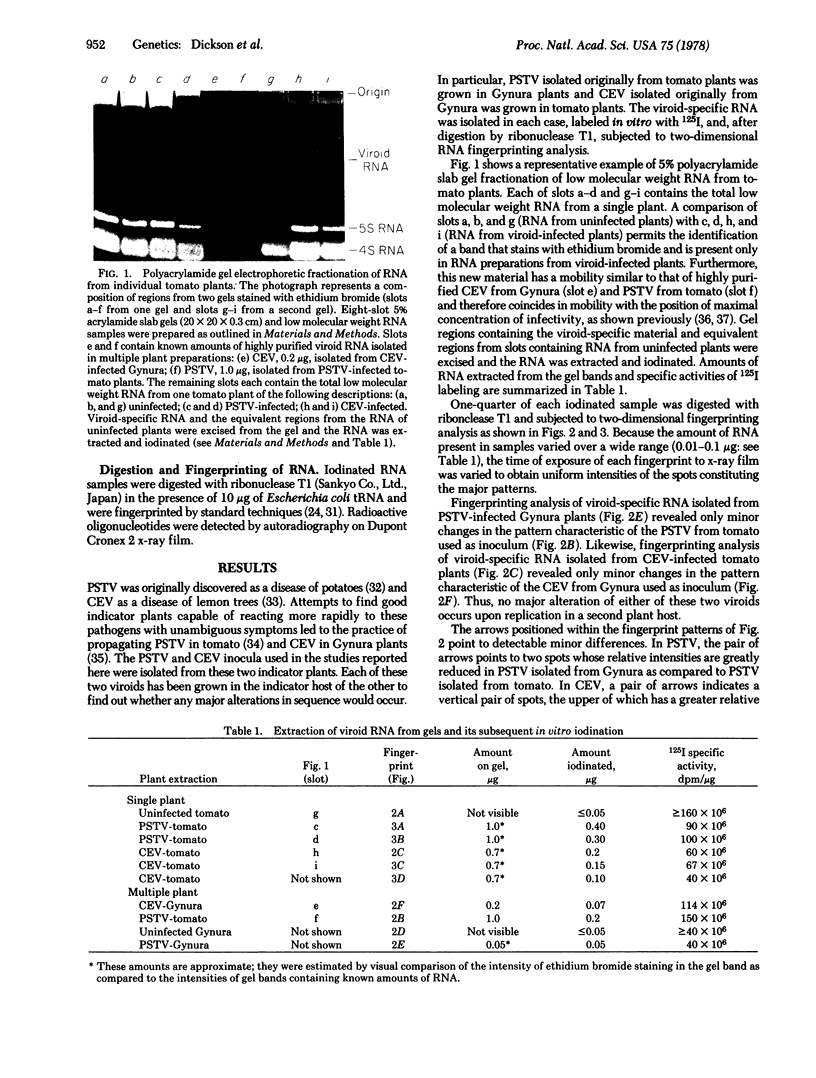
Potato spindle tuber viroid and citrus exocortis viroid, each purified from tomato (Lycopersicon esculentum) and from Gynura aurantiaca, were iodinated in vitro with 125I, digested with ribonuclease T1, and subjected to two-dimensional RNA fingerprinting analysis. With the exception of minor variations, each viroid retained its distinctive fingerprint pattern irrespective of the host species from which it was isolated. We conclude that the nucleotide sequences of these viroids are principally determined by the infecting viroid and not by the host.
Keywords: RNA fingerprinting, iodine-125, viroid diseases
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Commerford S. L. Iodination of nucleic acids in vitro. Biochemistry. 1971 May 25;10(11):1993–2000. doi: 10.1021/bi00787a005. [DOI] [PubMed] [Google Scholar]
- Davies J. W., Kaesberg P., Diener T. O. Potato spindle tuber viroid. XII. An investigation of viroid RNA as a messenger for protein synthesis. Virology. 1974 Sep;61(1):281–286. doi: 10.1016/0042-6822(74)90262-1. [DOI] [PubMed] [Google Scholar]
- Dickson E., Prensky W., Robertson H. D. Comparative studies of two viroids: analysis of potato spindle tuber and citrus exocortis viroids by RNA fingerprinting and polyacrylamide-gel electrophoresis. Virology. 1975 Dec;68(2):309–316. doi: 10.1016/0042-6822(75)90274-3. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber "virus". IV. A replicating, low molecular weight RNA. Virology. 1971 Aug;45(2):411–428. doi: 10.1016/0042-6822(71)90342-4. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber viroid. 8. Correlation of infectivity with a UV-absorbing component and thermal denaturation properties of the RNA. Virology. 1972 Nov;50(2):606–609. doi: 10.1016/0042-6822(72)90412-6. [DOI] [PubMed] [Google Scholar]
- Diener T. O. Potato spindle tuber virus: a plant virus with properties of a free nucleic acid. 3. Subcellular location of PSTV-RNA and the question of whether virions exist in extracts or in situ. Virology. 1971 Jan;43(1):75–89. doi: 10.1016/0042-6822(71)90226-1. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Schneider I. R., Smith D. R. Potato spindle tuber viroid. XI. A comparison of the ultraviolet light sensitivities of PSTV, tobacco ringspot virus, and its satellite. Virology. 1974 Feb;57(2):577–581. doi: 10.1016/0042-6822(74)90198-6. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Smith D. R. Potato spindle tuber viroid. XIII. Inhibition of replication by actinomycin D. Virology. 1975 Feb;63(2):421–427. doi: 10.1016/0042-6822(75)90314-1. [DOI] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross H. J., Domdey H., Sänger H. L. Comparative oligonucleotide fingerprints of three plant viroids. Nucleic Acids Res. 1977 Jun;4(6):2021–2028. doi: 10.1093/nar/4.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadidi A., Jones D. M., Gillespie D. H., Wong-Staal F., Diener T. O. Hybridization of potato spindle tuber viroid to cellular DNA of normal plants. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2453–2457. doi: 10.1073/pnas.73.7.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall T. C., Wepprich R. K., Davies J. W., Weathers L. G., Semancik J. S. Functional distinctions between the ribonucleic acids from citrus exocortis viroid and plant viruses: cell-free translation and aminoacylation reactions. Virology. 1974 Oct;61(2):486–492. doi: 10.1016/0042-6822(74)90284-0. [DOI] [PubMed] [Google Scholar]
- Henco K., Riesner D., Sanger H. L. Conformation of viroids. Nucleic Acids Res. 1977 Jan;4(1):177–194. doi: 10.1093/nar/4.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W. L., Kaesberg P. Size and secondary structure of potato spindle tuber viroid. Virology. 1977 Feb;76(2):477–484. doi: 10.1016/0042-6822(77)90230-6. [DOI] [PubMed] [Google Scholar]
- Owens R. A., Erbe E., Hadidi A., Steere R. L., Diener T. O. Separation and infectivity of circular and linear forms of potato spindle tuber viroid. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3859–3863. doi: 10.1073/pnas.74.9.3859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prensky W. The radioiodination of RNA and DNA to high specific activities. Methods Cell Biol. 1976;13:121–152. doi: 10.1016/s0091-679x(08)61800-2. [DOI] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E., Model P., Prensky W. Application of fingerprinting techniques to iodinated nucleic acids. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3260–3264. doi: 10.1073/pnas.70.11.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson H. D., Dickson E. RNA processing and the control of gene expression. Brookhaven Symp Biol. 1975 Jul;(26):240–266. [PubMed] [Google Scholar]
- Robertson H. D., Jeppesen P. G. Extent of variation in three related bacteriophage RNA molecules. J Mol Biol. 1972 Jul 28;68(3):417–428. doi: 10.1016/0022-2836(72)90096-4. [DOI] [PubMed] [Google Scholar]
- Sanger H. L., Klotz G., Riesner D., Gross H. J., Kleinschmidt A. K. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976 Nov;73(11):3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semancik J. S., Magnuson D. S., Weathers L. G. Potato spindle tuber disease produced by pathogenic RNA from citrus exocortis disease: evidence for the identity of the causal agents. Virology. 1973 Mar;52(1):292–294. doi: 10.1016/0042-6822(73)90419-4. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Morris T. J., Weathers L. G. Structure and conformation of low molecular weight pathogenic RNA from exocortis disease. Virology. 1973 Jun;53(2):448–456. doi: 10.1016/0042-6822(73)90224-9. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Exocortis disease: evidence for a new species of "infectious" low molecular weight RNA in plants. Nat New Biol. 1972 Jun 21;237(77):242–244. doi: 10.1038/newbio237242a0. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Weathers L. G. Pathogenic 10 S RNA from exocortis disease recovered from tomato bunchy-top plants similar to potato spindle tuber virus infection. Virology. 1972 Aug;49(2):622–625. doi: 10.1016/0042-6822(72)90518-1. [DOI] [PubMed] [Google Scholar]
- Takahashi T., Diener T. O. Potato spindle tuber viroid. XIV. Replication in nuclei isolated from infected leaves. Virology. 1975 Mar;64(1):106–114. doi: 10.1016/0042-6822(75)90083-5. [DOI] [PubMed] [Google Scholar]
- Zaitlin M., Hariharasubramanian V. A gel electrophoretic analysis of proteins from plants infected with tobacco mosaic and potato spindle tuber viruses. Virology. 1972 Feb;47(2):296–305. doi: 10.1016/0042-6822(72)90265-6. [DOI] [PubMed] [Google Scholar]