Abstract
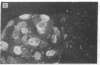
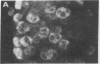
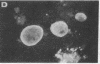
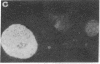
Mouse two-cell embryos, morulae, and blastocysts were killed when infected in vitro with simian virus 40 (SV40) at high multiplicities of infection. Polyoma virus was not deleterious for preimplantation embryos, even at a very high multiplicity of infection; however, the outgrowths of polyoma-infected blastocysts disintegrated after several days of culture. Indirect immunofluorescence tests revealed the presence of SV40 T and V antigens and polyoma virus V antigen in the nuclei of trophoblastic cells. Virus-specific antigens were not found in the nuclei of cells forming inner cell masses of blastocysts or in inner cell mass-derived cells in blastocyst out-growths. The appearance of SV40 T and V antigens in the nuclei was inhibited by αamanitin, a RNA polymerase II inhibitor. The amount of infectious virus recovered from cultures of morulae or blastocysts on subsequent days after infection with SV40 initially declined but later increased. These points of evidence indicate that some cells of early mouse embryos are permissive for the expression of early and late functions of SV40 genome and that susceptibility to infection with polyoma virus and/or permissiveness for the expression of polyoma virus late functions develop gradually between the two-cell and blastocyst stages. Electron microscope observations showed the presence of specific complexes of membranes and virions in the cytoplasm of trophoblastic cells. Single viral particles could be found in the nuclei and also in mitochondria.
Keywords: papova viruses, T and V antigens, α-amanitin, virus—membrane interactions, permissiveness
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biczysko W., Solter D., Pienkowski M., Koprowski H. Interactions of early mouse embryos with oncogenic viruses--simian virus 40 and polyoma. I. Ultrastructural studies. J Natl Cancer Inst. 1973 Dec;51(6):1945–1954. doi: 10.1093/jnci/51.6.1945. [DOI] [PubMed] [Google Scholar]
- Black P. H. Transformation of mouse cell line 3T3 by SV40: dose response relationship and correlation with SV40 tumor antigen production. Virology. 1966 Apr;28(4):760–763. doi: 10.1016/0042-6822(66)90262-5. [DOI] [PubMed] [Google Scholar]
- Calarco P. G., Brown E. H. An ultrastructural and cytological study of preimplantation development of the mouse. J Exp Zool. 1969 Jul;171(3):253–283. doi: 10.1002/jez.1401710303. [DOI] [PubMed] [Google Scholar]
- Graessmann M., Graessmann A. Regulation mechanism of simian virus 40 late gene expression in primary kidney cells and simian virus 40 transformed 3T3 cells. Virology. 1975 Jun;65(2):591–594. doi: 10.1016/0042-6822(75)90066-5. [DOI] [PubMed] [Google Scholar]
- Graham R. C., Jr, Karnovsky M. J. The early stages of absorption of injected horseradish peroxidase in the proximal tubules of mouse kidney: ultrastructural cytochemistry by a new technique. J Histochem Cytochem. 1966 Apr;14(4):291–302. doi: 10.1177/14.4.291. [DOI] [PubMed] [Google Scholar]
- Jackson A. H., Sugden B. Inhibition by -amanitin of simian virus 40-specific ribonucleic acid synthesis in nuclei of infected monkey cells. J Virol. 1972 Nov;10(5):1086–1089. doi: 10.1128/jvi.10.5.1086-1089.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Mintz B. Simian virus 40 DNA sequences in DNA of healthy adult mice derived from preimplantation blastocysts injected with viral DNA. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1250–1254. doi: 10.1073/pnas.71.4.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Byrne J. C., Martin M. A. Patterns of Simian Virus 40 DNA transcription after acute infection of permissive and nonpermissive cells. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1925–1928. doi: 10.1073/pnas.69.7.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Huebner K. Effect of cell chromosome number on simian virus 40 replication. Exp Cell Res. 1973 Sep;81(1):120–126. doi: 10.1016/0014-4827(73)90118-3. [DOI] [PubMed] [Google Scholar]
- Robb J. A., Martin R. G. Genetic analysis of simian virus 40. I. Description of microtitration and replica-plating techniques for virus. Virology. 1970 Aug;41(4):751–760. doi: 10.1016/0042-6822(70)90439-3. [DOI] [PubMed] [Google Scholar]
- Sawicki W., Baranska W., Koprowski H. Susceptibility of unfertilized and fertilized mouse eggs to simian virus 40 and Moloney sarcoma virus. J Natl Cancer Inst. 1971 Nov;47(5):1045–1051. [PubMed] [Google Scholar]
- Solter D., Knowles B. B. Immunosurgery of mouse blastocyst. Proc Natl Acad Sci U S A. 1975 Dec;72(12):5099–5102. doi: 10.1073/pnas.72.12.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan K. B., Sokol F. Structural proteins of simian virus 40: phosphoproteins. J Virol. 1972 Nov;10(5):985–994. doi: 10.1128/jvi.10.5.985-994.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WINOCOUR E. Purification of polyoma virus. Virology. 1963 Feb;19:158–168. doi: 10.1016/0042-6822(63)90005-9. [DOI] [PubMed] [Google Scholar]