Abstract
Importance
There is limited information about the effect of erythropoietin or a high transfusion threshold in traumatic brain injury (TBI).
Objective
To compare the effects of erythropoietin and two transfusion thresholds (7 and 10 g/dl) on neurological recovery after TBI.
Design
Randomized trial using a factorial design to test: i.) whether erythropoietin would fail to improve favorable outcomes by 20%, and ii.) whether a transfusion threshold of >10 g/dl would increase favorable outcomes without increasing complications.
Setting
Neurosurgical intensive care units of two Houston level 1 trauma centers
Participants
Between May 2006 and August 2012, 200 patients with closed head injury who were unable to follow commands were enrolled within 6 hours of injury; 102 patients received erythropoetin and 98 received placebo. Erythropoetin or placebo was initially dosed daily for 3 days and then weekly for 2 more weeks (n=74) and then the 24h and 48h doses were dropped for the remainder (n=126). Ninety-nine and 101 patients were assigned to the 7g/dl and 10g/dl transfusion thresholds.
Intervention
Intravenous erythropoietin 500 IU/kg or saline per dose. Transfusion threshold maintained with packed red blood cell transfusion.
Main Outcome
Glasgow Outcome Scale dichotomized as favorable (good recovery and moderate disability) and unfavorable (severe disability, vegetative, or dead) at 6 months post-injury.
Results
There was no erythropoeitin-transfusion threshold interaction. Compared to placebo (favorable outcome rate: 34/89 [38.2%]; 95%CI=28.2-49.1%), both erythropoetin groups were futile (first dosing regimen: 17/35 [48.6%]; 95%CI=31.4-66.0%, p=0.13, and second dosing regimen: 17/57 [29.8%]; 95%CI=18.4-43.4%, p<0.001). Favorable outcome rates were 37/87 (42.5%) and 31/94 (33.0%) in the 7 and 10 g/dl threshold groups (95%CI for the difference = − 0.05 to 0.25, p=0.28). There was a higher incidence of thromboembolic events in the 10 g/dl threshold group (22/101 [21.8%] vs. 8/99 [8.1%], p=0.009).
Conclusions and Relevance
In patients with closed head injury, neither the administration of erythropoietin nor maintaining hemoglobin concentration > 10 g/dl resulted in improved neurological outcome at 6 months and the 10 g/dl threshold was associated with a higher incidence of adverse events.. These findings do not support either approach in this setting.
INTRODUCTION
Patients with severe traumatic brain injury (TBI) commonly develop anemia. For patients with neurological injury, anemia is one potential cause of secondary injury which may worsen neurological outcomes. Treatment of anemia may include transfusions of packed red blood cells (PRBCs) or erythropoietin (Epo).
Epo treatment of anemia after TBI has the additional potential of providing neuroprotection. In experimental models, Epo has improved outcome after TBI. The neuroprotective mechanisms include anti-inflammatory, anti-apoptotic, and vascular actions.1;2 Multicenter trials in critically ill general trauma patients have suggested improved survival with Epo administration,3 but the effects on outcome after TBI are limited to case series and small randomized studies.4-7 The first purpose of the trial was to assess the effect of early administration of Epo on neurological outcome after TBI.
Transfusions of PRBCs restore hematocrit and blood oxygen-carrying capacity but have been associated with increased risk of infection, multi-organ failure including respiratory failure, thromboembolic events, and death. Studies have shown that for most critically ill patients, there is no advantage to maintaining a higher hemoglobin concentration.8-10
Despite these findings in critically ill patients, concern lingers that hemoglobin concentrations as low as 7 g/dl may not be tolerated in patients with severe TBI. Studies in TBI patients have either shown no difference in mortality11 or suggested an association between transfusion and a worse neurological outcome.12;13 A survey in 2009 demonstrated considerable practice variation in the need for transfusions in patients with TBI.14 The second purpose of this trial was to compare the effects of two transfusion thresholds on neurological recovery in TBI patients. The hypothesis was that the benefits of maintaining a hemoglobin concentration of 10 g/dl would exceed the risks of the transfusions required, and neurological outcome would be improved.
METHODS
A randomized trial using a factorial (2 × 2) design compared administration of Epo or placebo and separately compared hemoglobin transfusion thresholds (7 or 10 g/dl). The protocol was approved by the Food and Drug Administration and institutional review boards at all clinical sites. Patients in the first year of the study were enrolled after obtaining written informed consent from their legally authorized representative. In August 2007 after approval of the requirements for emergency research, the study was conducted under regulations for the Exception From Informed Consent for Emergency Research (21 CRF 50.24).15 When families were subsequently located and/or the patient recovered sufficiently to consent, they were asked to sign a consent form to permit continued patient participation in the study.
Patient Population
The study population included patients admitted to the two level 1 trauma centers in Houston with a closed head injury who were not able to follow commands after resuscitation and could be enrolled within 6 hours of injury. Exclusion criteria included Glasgow Coma Score (GCS) of 3 with fixed and dilated pupils, penetrating trauma, pregnancy, life-threatening systemic injuries, and severe pre-existing disease.
Baseline Assessment
Baseline information on age, gender, and type and severity of injury were obtained on admission. Race/ethnicity was also collected as a baseline factor that might affect access to rehabilitation and other resources that could contribute to improved outcome. This designation was based on information from family/significant others, patient, and information given about first, second, and preferred language if available.
The GCS and pupillary reactivity obtained in the emergency center after resuscitation was used for the baseline neurological assessment. When patients were sedated and paralyzed at the time of assessment in the emergency center, the first unsedated examination prior to randomization was used as the enrollment neurological examination. The initial CT scan was classified by the Marshall scoring system,16 and additional factors of basal cistern compression, midline shift, the presence of subarachnoid hemorrhage and epidural hematoma were noted.17 The Injury Severity Score (ISS) was calculated prior to randomization by the research team.18
Randomization and Blinding
A randomization list, stratified by site and using one randomization event to both factors in blocks of 4, was prepared by the study statisticians and kept in each hospital’s research pharmacy. When a new patient was enrolled, the research pharmacist prepared the study drug based on the patient’s weight and treatment assignment from the randomization list and informed the investigators of the transfusion threshold assignment.
Investigators and clinical personnel caring for the patient were blinded to the patients’ treatment with study drug (Epo or placebo) but not to the transfusion threshold assignment. Personnel conducting outcome assessments were blinded to both treatment assignment and transfusion threshold. The clinical personnel were not provided with the outcome assessments.
Study Intervention
Standard management followed a detailed protocol conforming to the Guidelines for the Management of Severe Head Injury.19 Patients received Epo (Epogen, Amgen, Inc., Thousand Oaks, CA) 500 IU/kg or an equal volume of saline intravenous bolus infusion over two minutes for each dose of the study drug. Patients received an initial dosage regimen of the assigned study drug followed by two additional doses, one per week for the next two weeks provided that the patient remained in ICU and hemoglobin concentration remained below 12 g/dl. For the first 74 patients, the initial dosage regimen was one dose given within six hours of injury followed by two additional doses given every 24 hours (Epo 1 regimen). In 2009, the initial dosage regimen was changed for the subsequent 126 patients to one dose given within six hours of injury (Epo 2 regimen). This change was made because of potential safety concerns raised for the initial high dose regimen by the US Food and Drug Administration (FDA) based on an Epo multicenter stroke study. In that study patients who received a dosage regimen similar to the Epo 1 regimen had a higher mortality rate than patients who received placebo (16.4% vs. 9.0%, p=0.01).20
During the acute post-injury recovery period (until intracranial pressure monitoring and ventilatory support were no longer required), the assigned hemoglobin threshold was maintained with transfusion of leukoreduced PRBCs. In patients who were actively bleeding, as may occur in the early post-injury period and during surgical procedures for intracranial injuries, hemodynamic instability was also used as an indication for transfusion in both transfusion threshold arms.
Outcome Measures
The primary outcome was the Glasgow Outcome Scale (GOS) which is a 5 category scale consisting of good recovery, moderate disability, severe disability, vegetative, and dead. The GOS was assessed using a structured interview at 6 months post-injury.21 The GOS was determined either in person in a variety of settings (for example neuropsychology offices, home visit, or work place) or over the telephone by neuropsychology personnel. Information was obtained directly from the patient, next-of-kin, significant others and/or caretakers. If necessary, some information was obtained from records released by other facilities with appropriate consent. The GOS was dichotomized into a prespecified favorable outcome (good recovery or moderate disability) and unfavorable outcome (severe disability, vegetative, or dead). The three primary safety outcomes for the transfusion threshold comparison were mortality, the incidence of Adult Respiratory Distress Syndrome (ARDS), and the incidence of infections (total number of incidences of pneumonia, bacteremia, urinary tract infection, and ventriculitis). The secondary transfusion threshold outcome was the Disability Rating Scale (DRS) which is a 31 point scale ranging from 0 which indicates no disability to 30 which is death. The secondary Epo/placebo outcome was mortality.
Erythropoietin Levels
Plasma and cerebrospinal fluid (CSF) levels of Epo were obtained prior to and one hour after the doses of study drug given within six hours, and at 24 and 48 hours after injury, and then daily for the first ten days after injury. Epo levels were measured using a commercially available solid phase sandwich ELISA (Quantikine IVD erythropoietin ELISA, Cat. DEP00, R& D System Inc., Minneapolis, MN), which detects both native and recombinant Epo to a sensitivity of 0.6 mIU/ml.
Data Analysis
An intent-to-treat statistical analysis was conducted. Baseline characteristics were compared using Fisher’s exact test for categorical variables or a Wilcoxon rank-sum test for continuous variables. Continuous variables were summarized using medians and quartiles. Logistic regression was used to test for an interaction for the primary outcome between the transfusion threshold and the Epo dosing regimen using an alpha of 0.1.
The primary outcome was analyzed using a one-sided and two-sided two-sample test of proportions for the study drug and transfusion threshold comparisons, respectively. The primary futility analysis compared Epo 2 regimen to placebo with alpha = 0.15. If we reject the null hypothesis that the percent of favorable outcomes on the Epo 2 regimen is ≥ to the percent of favorable outcomes on placebo plus 20%, we conclude that studying the drug in a phase III trial would likely be futile. Additional details of the futility analysis are provided in the supplementary material.
As a secondary analysis of GOS, study drug group and transfusion threshold group were separately compared using logistic regression, adjusted for pre-specified covariates of injury severity: the ISS and the IMPACT probability lab model predictions of unfavorable outcome described by Steyerberg et al.22 Post-hoc analyses using a sliding dichotomy23 and using an ordinal logistic regression resulted in similar results and therefore are not presented.
In the absence of evidence to the contrary, multiple imputation for missing 6-month GOS data was performed assuming data were missing at random using chained equations (mice package in R). The imputation was based on a logistic regression model with baseline covariates transfusion threshold group, ISS, the IMPACT lab model score, presence of hypoxia, the treatment group (Epo versus placebo), and presence of epidural hematoma. Results were aggregated over 20 imputed sets using the Rubin variance formula.24
The incidences of secondary binary outcomes were analyzed using a two-sample test of proportions. DRS was compared using a Wilcoxon rank-sum test. The Cox proportional hazard model was used to determine time-to-event hazard ratios and 95% confidence intervals. The proportional hazard assumption was examined using Schoenfeld residual plots and testing a treatment by time (time-dependent) interaction term. The log-rank test was used to compare survival curves. For the primary safety analysis of ARDS, three critical care experts independently determined whether each patient had ARDS by the American-European consensus conference definition.25 Cox regression analyses were performed to determine whether or not transfusion threshold assignment increased the risk of ARDS. Lasso-penalized Cox regression, with the penalty parameter selected by 10-fold cross-validation, was used for feature selection.26 Censor time was defined as date of hospital discharge, withdrawal, or death, whichever occurred first. Generalized estimating equations were used to compare longitudinal hemoglobin levels among treatment groups.
All analyses except the futility analysis (alpha = 0.15) and the tests of interactions for the outcomes between the transfusion threshold and the Epo dosing regimen (alpha = 0.1) were conducted with alpha = 0·05 and two-sided tests. All analyses were conducted using SAS version 9.3 (SAS Institute, Inc., Cary, North Carolina), Stata version 12 (StataCorp LP, College Station, TX), or R version 2.13.1 (R Foundation for Statistical Computing, Vienna, Austria).
Sample Size and Power Calculations
With the change in the initial Epo dosage regimen, the primary Epo analysis plan was changed from a superiority trial to a futility trial of the Epo 2 regimen arm.27 We hypothesized that 30% of subjects in the placebo group would have a favorable outcome at six months and there would be no interaction between the Epo and the transfusion threshold groups. Using a one-sided alpha of 0.15, a sample size of 62 subjects in the Epo 2 regimen group and 100 subjects in the placebo group provided 91% power to test the futility hypothesis described in the analysis.
For the transfusion threshold analysis, we hypothesized that 40% of subjects in the 7 g/dl transfusion threshold group would have a favorable GOS at 6 months and no interaction between the Epo and transfusion threshold groups. Assuming a two-sided test with α = 0.05, we estimated that a sample size of 200 subjects, randomized in a 1:1 ratio to the 7 and 10 g/dl transfusion threshold groups, would provide 80% power to detect a 20% absolute increase in GOS at 6 months post injury in the 10g/dl group.
RESULTS
Interaction of Randomized Factors
A statistically significant interaction between the transfusion threshold and the Epo study drug was not detected for any reported primary, secondary, or safety outcomes; thus, the Epo and placebo groups were combined for the transfusion threshold analyses and the transfusion threshold groups were combined for the Epo study drug analyses described below.
Patient Characteristics
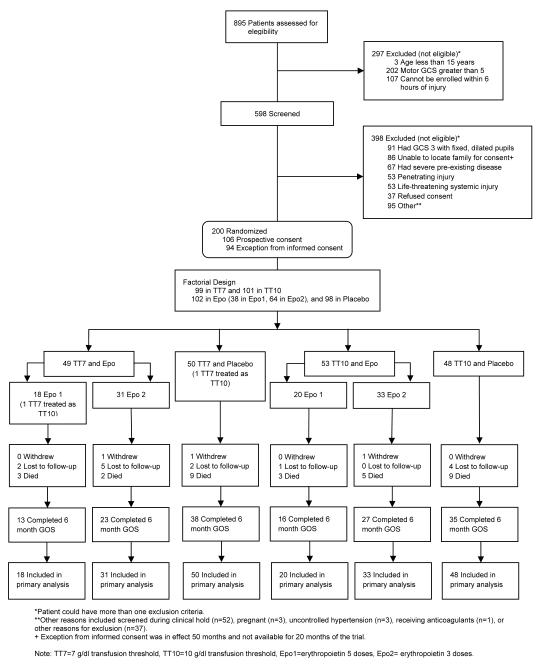
A total of 895 patients were screened for eligibility between May 2006 and August 2012 (Figure 1). Two hundred patients met eligibility criteria and were enrolled. The treatment groups had similar demographic characteristics (Table 1). There were no significant differences in injury characteristics between the study drug treatment groups except that pre-hospital hypoxia was more common in the placebo groups. Except for the incidence of epidural hematoma on the admission CT scan, which was higher in the 10 g/dl threshold group, there were no significant differences detected in the injury characteristics between the two transfusion threshold groups.
Figure 1.
Numbers of patients screened and enrolled in the trial.
Table 1.
Demographic and Injury Characteristics of Patients
| Factor 1 (Epo Study Drug) |
Factor 2a (Transfusion Threshold) |
||||
|---|---|---|---|---|---|
|
| |||||
| Epo 1 Regimen (n=38) |
Epo 2 Regimen (n=64) |
Placebo (n=98) |
Transfusion Threshold 7 g/dl (n=99) |
Transfusion Threshold 10 g/dl (n=101) |
|
|
| |||||
| DEMOGRAPHIC CHARACTERISTICS | |||||
|
| |||||
| Age, median (25th-75th) | 31·5 (23-48) |
29 (23-47) |
30 (22-44) |
28 (21-48) |
31 (24-45) |
|
| |||||
| Gender, n (%) | |||||
| Female | 4 (10·5) | 8 (12·5) | 14 (14·3) | 14 (14.1) | 12 (11.9) |
| Male | 34 (89·5) | 55 (85·9) | 84 (85·7) | 85 (85.9) | 88 (87.1) |
| Male living as female | 1 (1·6) | 1 (1.0) | |||
|
| |||||
| Race/Ethnicity, n (%) | |||||
| Asian | 1 (2·6) | 1 (1·6) | 4 (4·1) | 3 (3.0) | 3 (3.0) |
| Hispanic | 20 (52·6) | 35 (54·7) | 48 (49·0) | 50 (50.5) | 53 (52.5) |
| Black | 7 (18·4) | 10 (15·6) | 26 (26·5) | 20 (20.2) | 23 (22.8) |
| White, non-Hispanic | 10 (26·3) | 18 (28·1) | 20 (20·4) | 26 (26.3) | 22 (21.8) |
|
| |||||
| BASELINE INJURY CHARACTERISTICS | |||||
|
| |||||
| Mechanism of Injury, n (%) | |||||
| Assault | 2 (5·3) | 5 (7·8) | 15 (15·3) | 7 (7.1) | 15 (14.9) |
| Fall/jump | 11 (28·9) | 7 (10·9) | 9 (9·2) | 18 (18.2) | 9 (8.9) |
| Automobile accident | 19 (50) | 41 (64·1) | 56 (57·1) | 58 (58.6) | 58 (57.4) |
| Motorcycle accident | 5 (13·2) | 11 (17·2) | 15 (15·3) | 14 (14.1) | 17 (16.8) |
| Other | 1 (2·6) | 0 | 3 (3·1) | 2 (2.0) | 2 (2.0) |
|
| |||||
| Enrollment motor GCSb, n (%) | |||||
| 4-5 | 26 (68·4) | 38 (59·4) | 65 (66·3) | 63 (63.6) | 66 (65.4) |
| 1-3 | 12 (31·6) | 26 (40·6) | 33 (33·7) | 36 (36.4) | 35 (34.7) |
|
| |||||
| ED pupil reactivity, n (%) | |||||
| Both reactive | 25 (65·8) | 41 (64·1) | 55 (56·1) | 63 (63.6) | 58 (57.4) |
| One reactive | 5 (13·2) | 5 (7·8) | 13 (13·3) | 14 (14.1) | 9 (8.9) |
| Neither reactive | 8 (21·1) | 18 (28·1) | 30 (30·6) | 22 (22.2) | 34 (33.7) |
|
| |||||
| Enrollment sum GCSc, n (%) | |||||
| GCS > 8 | 18 (47.4) | 29 (45.3) | 42 (42.9) | 42 (42.4) | 47 (46.5) |
| GCS 6-8 | 8 (21.1) | 12 (18.8) | 25 (25.5) | 23 (23.2) | 22 (21.8) |
| GCS 3-5 | 12 (31.6) | 23 (35.9) | 31 (31.6) | 34 (34.3) | 32 (31.7) |
|
| |||||
| ED Marshall CT scan category, n (%) |
|||||
| Diffuse injury 1 or 2 | 15 (39·5) | 30 (46·9) | 44 (44·9) | 49 (49.5) | 40 (39.6) |
| Diffuse injury 3 or 4 | 14 (36·8) | 10 (15·6) | 22 (22·5) | 23 (23.2) | 23 (22.8) |
| Mass lesion | 9 (23·7) | 24 (37·5) | 32 (32·7) | 27 (27.3) | 38 (37.6) |
|
| |||||
| ED CT scan, subarachnoid hemorrhage present, n (%) |
26 (68·4) | 44 (68·8) | 68 (69·4) | 71 (71.7) | 67 (66.3) |
|
| |||||
| ED CT scan, epidural hematoma present, n (%) |
6 (15·8) | 12 (18·8) | 14 (14·3) | 10 (10.1) | 22 (21.8) |
|
| |||||
| Surgery on admission, n (%) | 9 (23.7) | 22 (34.4) | 30 (30.6) | 26 (26.3) | 35 (34.7) |
| Epidural hematoma | 0 | 3 (4.7) | 6 (6.1) | 3 (3.0) | 6 (5.9) |
| Subdural hematoma | 7 (18·4) | 19 (29·7) | 20 (20.4) | 20 (20.2) | 26 (25.7) |
| Intracerebral hematoma/contusion |
2 (5·3) | 0 | 2 (2.0) | 2 (2.0) | 2 (2.0) |
| Non-CNS injury | 0 | 0 | 2 (2.0) | 1 (1.0) | 1 (1.0) |
|
| |||||
| Prehospital hypotension, n (%) | 4 (10.5) | 5 (7.8) | 16 (16.3) | 11 (11.1) | 14 (13.9) |
|
| |||||
| Prehospital hypoxia, n (%) | 3 (7.9)** | 7 (10.9) | 29 (29.6) | 18 (18.2) | 21 (20.8) |
|
| |||||
| Injury Severity Score, median (25th-75th) |
27 (26-35) |
29 (25-36.5) |
29 (25-38) |
29 (25-38) |
29 (25-35) |
|
| |||||
| IMPACT probability of poor outcome, mean (sd) |
0.39 (0.3) | 0.40 (0.2) | 0.41 (0.3) | 0.43 (0.3) | 0.39 (0.3) |
|
| |||||
| ED Hemoglobin (g/dl), median (25th-75th) |
14.7 (13.5-15.6) |
14.6 (12.8-15.5) |
14.2 (12.7-15.6) |
14.4 (13-15.6) |
14.6 (12.8-15.5) |
|
| |||||
| ED Glucose (mmol/L), median (25th-75th) |
8.5 (7.2-10.7) |
8.8 (7.1-10.1) |
8.2 (6.9-10.1) |
8.7 (7.3-10.4) |
8.0 (6.-10.0) |
Factors 1 and 2 include the same patients
motor GCS = motor component of the Glasgow Coma Score, which ranges from 1 (no motor response) to 6 (follows commands)
sum GCS = sum of the eye, motor, and verbal components of the Glasgow Coma Score, which ranges from 3 (no responses) to 15 (normal responses)
Adherence to Protocol and Protocol-related Factors
Epo Study Drug Protocol
All patients received the initial dose of the assigned study drug (eTable 1). The average time of the first study drug dose was 5.2 (standard deviations=0.8) hours after injury with 187 (93.5%) given within six hours of injury. See supplemental material for additional dosing information.
At enrollment prior to receiving the initial dose of study drug, the median plasma Epo levels were 15.7 mIU/ml (normal range 4-27 mIU/ml, eTable 1). In the placebo treated group, the median plasma Epo levels gradually increased over time, peaking at 111.6 mIU/ml at 48 hours after injury. In the patients who received Epo, the median plasma levels of Epo increased by 12 hours after injury to 1,745.0 mIU/ml. These elevated plasma levels of Epo were sustained for a longer time in the patients receiving the Epo 1 regimen compared to those receiving the Epo 2 regimen (eFigure 2-left graph).
The CSF levels of Epo followed the same pattern (eFigure 2-right graph). At six hours prior to receiving the initial dose of study drug, Epo was undetectable in most of the patients. In the patients receiving Epo, the median CSF levels of Epo increased to 11.8 mIU/ml at 12 hours after injury, and remained elevated above baseline values through 96 hours.
There were no differences in the number of transfusions required in the Epo study drug groups. The hemoglobin concentration was less than 10 g/dl for a shorter time in the patients receiving the Epo 1 dose regimen, compared to the placebo group (Table 2).
Table 2.
Transfusion Characteristics
| Transfusion Variable | Factor 1a (Epo Study Drug) |
Factor 2 (Transfusion Threshold) |
|||
|---|---|---|---|---|---|
|
| |||||
| Epo 1 Regimen (n=38) |
Epo 2 Regimen (n=64) |
Placebo (n=98) |
Transfusion Threshold 7 g/dl (n=99) |
Transfusion Threshold 10 g/dl (n=101) |
|
|
| |||||
| Patients with at least one unit PRBCs required, n(%) |
23 (60.5) | 39 (60.9) | 63 (64.3) | 52 (52.5) | 73 (72.3) |
| Units of PRBCs required, n mean per patient (range) |
162 7 (1-15) |
195 5 (1-17) |
407 6.5 (1-22) |
243 4.7 (1-22) |
521 7.1 (1-21) |
| Given to keep hemoglobin above assigned threshold |
83 4.4 (1-7) |
109 3.8 (1-11) |
228 3.9(1-16) |
87 2.4(1-5) |
333 4.7 (1-16) |
| Given for active bleeding | 79 5.3 (1-11) |
82 3.2 (1-10) |
167 4.3(1-18) |
144 3.8(1-18) |
184 4.4 (1-12) |
| Given after acute care per clinical decision |
0 | 4 2 (2-2) |
8 2 (1-3) |
8 2 (2-2) |
4 2 (1-3) |
| Given in violation of protocol | 0 | 0 | 4 2 (2-2) |
4 2 (2-2) |
0 |
|
| |||||
| Time (hours) that hemoglobin was < 10 g/dl, median (25th-75th) |
8.3 * (.3-16.4) |
13.4 (3.5-39.7) |
18.9 (5.7-48.1) |
33.9 (4.0-60.8) |
10.5 ** (1.1-19.0) |
|
| |||||
| Time (hours) that hemoglobin was < 7 g/dl, median (25th-75th) |
0 (0-0) |
0 (0-0) |
0 (0-0.2) |
0.0 (0-.6) |
0.0 ** (0-0) |
Factors 1 and 2 include the same patients
different from Placebo group (p<0.05)
different from 7 g/dl threshold group (p<0.001)
Transfusion Threshold Protocol
Adherence to the protocol throughout the study was good with a few exceptions. Two patients who were assigned to the 7 g/dl group were mistakenly managed as if their assigned hemoglobin threshold was 10 g/dl. In addition, there were two patients who were assigned to and managed according to the 7 g/dl group protocol but received transfusions on one occasion, that were not according to the protocol.
The number of units of PRBCs required to maintain the assigned transfusion threshold and the hemoglobin concentrations over time in the treatment groups are detailed in Tables 2 and 3. The number of transfusions given for active bleeding (due to traumatic injuries or during surgical procedures) was similar in the two groups and the major difference was in the number of transfusions required in hemodynamically stable patients to maintain the assigned hemoglobin concentration. The length of time that the hemoglobin concentration was less than 10 g/dl was greater in the 7 g/dl threshold group (eFigure 1), and the average hemoglobin concentration over time was higher in the 10 g/dl group (Table 3).
Table 3.
Hemoglobin Concentrations Over Time
| Treatment Group | Time After Injury | ||||
|---|---|---|---|---|---|
| Enrollment | Day 9 | Day 16 | Day 23 | Day 30 | |
| Factor 1 (Epo Study Drug)a | |||||
| Epo 1 Regimen, median (25th- 75th) |
14·7 (13.5-15.6) |
10·9 (10.3-12.3) |
11·0 (9.4-12.1) |
11·6 (10.9-12.3) |
11·6 (11.3-12.3) |
| N | 38 | 35 | 27 | 16 | N=11 |
| Epo 2 Regimen, median (25th- 75th) |
14·6 (12.8-15.5) |
10·6 (9.5-11.9) |
10·6 (9.1-11.7) |
11·2 (10.3- 12.8) |
10·8 (9.7-12.2) |
| N | 64 | 57 | 47 | 36 | 23 |
| Placebo, median (25th-75th) | 14·2 (12.7-15.6) |
10·9 (9.4-11.7) |
10·4 (9.6-11.8) |
10·9 (9.8-12.1) |
11·5 (9.7-12.2) |
| N | 98 | 80 | 66 | 49 | 34 |
| Factor 2 (Transfusion Threshold) | |||||
| Transfusion Threshold 7 g/dl, median (25th-75th) |
14·4 (13-15.6) |
9·7 (8.6-10.9) |
9·6 (8.8-10.6) |
10·7 (9.6-11.5) |
10·8 (9.5-11.5) |
| N | 99 | 85 | 65 | 48 | 28 |
| Transfusion Threshold 10 g/dl, median (25th-75th) |
14·6 (12.8-15.5) |
11·4 (10.7-12.2) |
11·2 (10.4-12.2) |
11·9 (10.9-12.8) |
11·7 (10.8-12.4) |
| N | 101 | 87 | 75 | 53 | 40 |
Factors 1 and 2 include the same patients
Primary Outcome - Neurological Recovery at 6 Months
Epo Study Drug Analysis
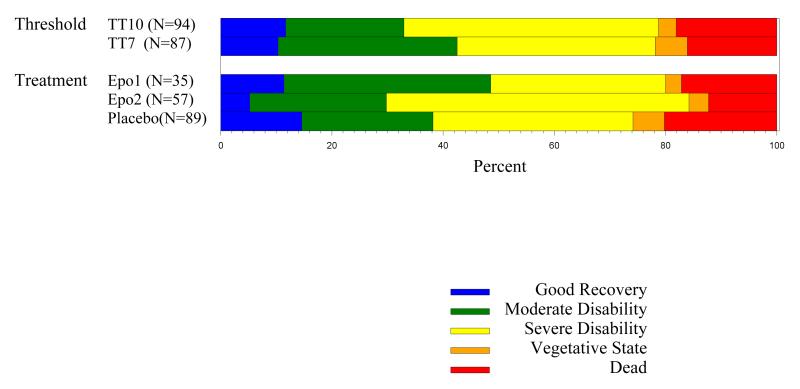
A difference in proportion of six-month favorable GOS outcome could not be detected between the placebo patients enrolled during the Epo 1 (36%) and Epo 2 (39%) regimens (95% confidence interval [CI] for difference = −26.1%-20.3%, p=0.96). These two groups were combined into a single placebo group for analyses. The primary outcome was available in 35 (92%) Epo 1 regimen patients, 57 (89%) Epo 2 regimen patients and 89 (91%) placebo patients. The distribution of missing outcome data was similar among the three treatment groups (p=0.90).
In the placebo group, 34 (38.2%, 95% CI = 28.1%-49.1%) of the patients recovered to a favorable outcome, compared to 17 (48.6%, 95% CI = 31.4%-66.0%) in the Epo 1 regimen group and 17 (29.8%, 95% CI = 18.4%-43.4%) in the Epo 2 regimen group (Figure 2). Table 4 details the results of the logistic regression analyses where the GOS was adjusted for pre-specified covariates and for the presence of prehospital hypoxia, which was more common in the placebo treated patients. Treatment with Epo was not significant in any of the models.
Figure 2.
Glasgow Outcome Scale at 6 months (complete cases). For the primary outcome, good recovery and moderate disability were combined as favorable outcome. Severe disability, vegetative, and dead were combined as unfavorable outcome.
Table 4.
Details for Analysis of Primary Outcome Adjusting for Pre-specified Covariates and Baseline Variables that were not Balanceda.
| Multiple Imputation pooled estimates | Odds ratios | 95% Confidence Interval |
p-Value |
|---|---|---|---|
| Factor 1: Epo 2 Regimen Analysis | |||
| Epo 2 Regimen - Logistic Regression Model Adjusted for Pre-specified Covariates | |||
| Intercept | 9.48 | 1.58-57.03 | 0.01 |
| Epo 2 (compared to Placebo) | 0.63 | 0.27-1.48 | 0.29 |
| Injury Severity Score | 0.99 | 0.94-1.04 | 0.63 |
| IMPACT probability of unfavorable GOS (per 10% unit increment) |
0.53 | 0.41-0.67 | <0.001 |
|
Epo 2 Regimen - Logistic Regression Model Adjusted for Pre-specified Covariates and for Baseline
Variables that were not Balanced | |||
| Intercept | 9.73 | 1.62-58.56 | 0.01 |
| Epo 2 (compared to Placebo) | 0.69 | 0.29-1.65 | 0.40 |
| Injury Severity Score | 0.99 | 0.94-1.04 | 0.60 |
| IMPACT probability of unfavorable GOS (per 10% unit increment) |
0.50 | 0.38-0.64 | <0·001 |
| Prehospital hypoxia present | 2.55 | 0.78-8.28 | 0.12 |
| Factor 1: Epo 1 Regimen Analysis | |||
| Epo 1 Regimen - Logistic Regression Model Adjusted for Pre-specified Covariates | |||
| Intercept | 9.09 | 1.44-57.52 | 0.02 |
| Epo 1 (compared to Placebo) | 1.56 | 0.60-4.08 | 0.36 |
| Injury Severity Score | 0.99 | 0.93-1.04 | 0.58 |
| IMPACT probability of unfavorable GOS (per 10% unit increment) |
0.55 | 0.44-0.69 | <0.001 |
|
Epo 1 Regimen - Logistic Regression Model Adjusted for Pre-specified Covariates and for Baseline
Variables that were not Balanced | |||
| Intercept | 8.84 | 1.39-56.41 | 0·01 |
| Epo 1 (compared to Placebo) | 1.78 | 0.66-4.83 | 0·24 |
| Injury Severity Score | 0.99 | 0.93-1.04 | 0·51 |
| IMPACT probability of unfavorable GOS (per 10% unit increment) |
0.52 | 0.41-0.66 | <0·001 |
| Prehospital hypoxia present | 2.28 | 0.64-8.06 | 0·16 |
| Factor 2: Transfusion Threshold Analysis | |||
| Transfusion Threshold: GOS logistic regression model adjusted for pre-specified covariates | |||
| Intercept | 11.10 | 2.10-58.60 | 0.005 |
| 10 g/dl threshold (compared to 7 g/dl threshold) | 0.75 | 0.36-1.55 | 0.43 |
| Injury Severity Score | 0.98 | 0.94-1.03 | 0.43 |
| IMPACT probability of poor outcome (per 10% unit increment) |
0.54 | 0.44-0.66 | <0.001 |
|
Transfusion Threshold: GOS logistic regression adjusted model adjusted for pre-specified covariates
and baseline variables that were not balanced | |||
| Intercept | 11.47 | 2.06-63.84 | 0.006 |
| 10 g/dl threshold (compared to 7 g/dl threshold) | 0.61 | 0.28-1.30 | 0.20 |
| Injury Severity Score | 0.98 | 0.93-1.02 | 0.33 |
| IMPACT probability of poor outcome (per 10% unit increment) |
0.54 | 0.44-0.67 | <0.001 |
| Presence of epidural hematoma | 4.74 | 1.63-13.73 | 0.004 |
Pre-specified covariates were injury severity score and IMPACT probability of poor outcome. Baseline covariates that were not balanced were prehospital hypoxia for the Epo comparison and presence of epidural hematoma for the trigger comparison.
In the primary futility analysis, the null hypothesis was that the percentage of patients with favorable outcome in the Epo 2 regimen group would be greater than 0.2 + the percentage in the placebo group. The null hypothesis was rejected at the 0.15 level (p<0.001). In a similar futility analysis for the Epo 1 regimen group, the null hypothesis was rejected at the 0.15 level (p=0.13). It is unlikely that either dosage regimen for Epo has at least a 20% higher favorable outcome compared to the placebo group.
Transfusion Threshold Analysis
The 6 month GOS was available in 87 (87.9%) patients in the 7 g/dl threshold group and 94 (93.1%) patients in the 10 g/dl threshold group (Figure 2). The distribution of missing outcome data was similar among the two transfusion threshold groups (odds ratio [OR]=1.85, 95% CI=0.64-5.80, p=0.24). In the 7 g/dl threshold group, 37 (42.5%) recovered to a favorable outcome, compared to 31 (33.0%) in the 10 g/dl threshold group (95% CI for difference =−0.06-0.25), In the primary analysis using multiple imputation of missing GOS scores, there was no significant difference in outcome detected between the two treatment groups (95% CI for difference=−0.07-0.20, p=0.34).
After adjustment for prespecified covariates (Table 4), a transfusion threshold association with the GOS outcome was not detected (OR=0.75, 95% CI=0.36-1.55, p=0.43). In a post-hoc analysis adjusting for incidence of epidural hematoma as an additional covariate in the logistic regression model, a transfusion threshold association with GOS was also not detected (OR=0.61, 95% CI=0.28-1.30, p=0.20).
Secondary Outcome – DRS
The median 6-month DRS score was 5 (25th-75th = 1.25-12.75) in the Epo 1 regimen (p=0.52 compared with Placebo), 7 (25th-75th = 4-12) in the Epo 2 regimen (p=0.97 compared with Placebo), and 6.5 (25th-75th = 3-18.75) in the placebo group. The median 6-month DRS score was 5 (25th-75th = 2.25-9.75) in the 7 g/dl threshold group and 8 (25th-75th = 4-17) in the 10 g/dl threshold group (p=0.06). A higher DRS score represents a worse outcome.
Safety and Secondary Outcomes
Mortality Analysis
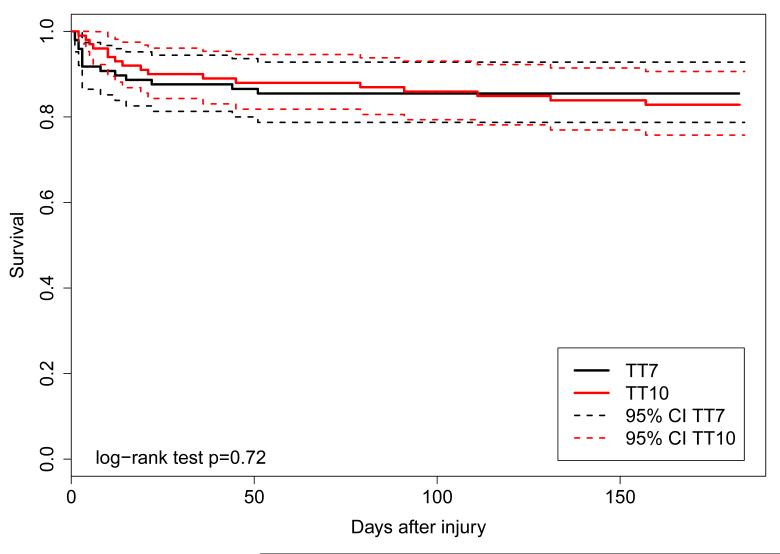
Information about survival to six months was available for 190 (95%) of the patients enrolled in the study. Six patients in the Epo 1 regimen group, seven patients in the Epo 2 regimen group, and 18 patients in the placebo group died during the six months of follow-up. Kaplan-Meier survival curves for the Epo 1 regimen group (p=0.75) and for the Epo 2 regimen group (p=0.25) were not significantly different from the placebo group (Figure 3).
Figure 3.
Kaplan-Meier survival curves for the transfusion threshold groups (left) and the Epo dosing regimen groups (right).
Fourteen patients in the 7g/dl threshold group and 17 patients in the 10 g/dl threshold group died during the 6 months of follow-up. Kaplan-Meier survival curves for the two threshold groups are illustrated in Figure 3. The overall log rank test was not significant (p=0.72).
ARDS Analysis
A total of 16 (16.2%) patients in the 7 g/dl threshold group and 25 (24.7%) patients in the 10 g/dl threshold group developed ARDS (p=0.16). In the final Cox regression model (Table 5), the 10 g/dl transfusion threshold was not significantly associated with ARDS (HR=1.79, 95% CI=0.93-3.45, p=0.08).
Table 5.
Details of Cox proportional hazard model for ARDS analysis
| n | # events |
Hazard Ratio |
95% Confidence Interval |
p-Value | |
|---|---|---|---|---|---|
|
| |||||
| Transfusion threshold | 1.79 | 0.93-3.45 | 0.08 | ||
| 10 g/dl | 101 | 25 | |||
| 7 g/dl (reference) | 99 | 16 | |||
|
| |||||
| Enrollment GCS-sum (per 1 unit of increment) |
-- | -- | 0.84 | 0.74-0.96 | 0.01 |
|
| |||||
| Injury Severity Score (per 1 unit of increment) |
-- | -- | 1.06 | 1.03-1.10 | <0.001 |
|
| |||||
| Hypotension | 0.6 | 0.23-1.59 | 0.3 | ||
| Yes | 25 | 5 | |||
| No (reference) | 175 | 36 | |||
|
| |||||
| Place of intubation | 3.48 | 1.40-8.65 | 0.007 | ||
| ER | 152 | 34 | |||
| ICU or field (reference) | 48 | 7 | |||
|
| |||||
| CT category | 0.61 | 0.32-1.19 | 0.15 | ||
| High-risk | 111 | 21 | |||
| Low-risk (reference) | 89 | 20 | |||
Infections Analysis
The most common infection was pneumonia, which occurred in 33 (17%) of the patients. The second most common infection was urinary tract infection, occurring in 21 (11%) of patients, followed by ventriculitis and bacteremia. There were a total of 27 patients in the 7 g/dl threshold group and 36 patients in the 10 g/dl threshold group who had one or more infectious complications (95% CI for difference in proportions = −0.22-0.05, p=0.26).
Thromboembolic Events Analysis
The incidence of thromboembolic events was examined because a higher overall incidence was observed in the 10 g/dl threshold group and a higher incidence of upper extremity deep venous thrombosis (DVT) was found in the Epo treated groups in the reporting of adverse and serious adverse events (eTable 2).
A total of 30 patients developed one or more thromboembolic events during the 6 months of follow-up. All of the thromboembolic events occurred after three days post-injury, and three events occurred after 30 days post-injury. Twenty-five (12.5%) of the 200 patients developed DVT. Nine patients (4.5%) developed pulmonary embolus. Four patients had multiple thromboembolic events. The patients in the 10 g/dl threshold group had a significantly greater incidence of one or more thromboembolic events (22 [21.8%] patients compared to 8 [8.1%] patients in the 7 g/dl threshold group [OR=0.32, 95% CI=0.12-0.79, p=0.009]). No statistically significant differences for other adverse events except anemia could be detected between the two transfusion threshold treatment groups (eTable 2).
During the first 30 days after injury, deep venous thrombosis (DVT) occurred in five (13.2%) patients in the Epo 1 regimen group, 11 (17.1%) in the Epo 2 regimen group, and seven (7.1%) of the placebo group. The incidence of the subcategory of upper extremity DVT was significantly higher in the Epo 2 regimen group as compared to the placebo group (OR=13.7, 95% CI=1.76-619.09, p<0.01). Pulmonary embolus occurred in none of the patients in the Epo 1 group, but in 4 (6.3%) of the Epo 2 group, and 3 (3.1%) of the placebo group. The incidence of other cardiovascular complications was also significantly higher in the Epo 1 group than the placebo group (eTable 2, OR=10.6, 95% CI=1.89-109.9, p=.002).
DISCUSSION
Maintaining a hemoglobin concentration of approximately 10 g/dl has long been a management strategy to improve cerebral oxygenation in TBI patients. In studies of anemic TBI patients, transfusion does improve brain oxygenation in some patients.28;29 Other potentially beneficial effects of maintaining a higher hemoglobin concentration are to avoid increased ICP induced by anemia,30 and to provide a higher blood pressure and therefore better cerebral perfusion pressure.
This transfusion practice was expected to reduce neurological injury particularly during the acute recovery period when the brain is most vulnerable to ischemic insults. Instead, no long-term benefit on neurological outcome was detected in the 10 g/dl threshold group, and a greater incidence of thromboembolic events was observed in the 10 g/dl threshold group.
There were several limitations in the design of the study. The trial was powered for a relatively large difference in outcome for the transfusion threshold factor because it was thought that maintaining an adequate oxygen delivery to the injured brain was an important critical care principle for TBI patients. A small decrease in the percentage of favorable outcomes in either transfusion threshold group cannot be excluded by the results. However, it is unlikely that an increase in the percentage of favorable outcomes in the 10 g/dl transfusion threshold group would have been detected even with a larger sample size.
Secondly, the trial was conducted at only 2 clinical sites, which could limit the ability to generalize the results, and required 6 years to complete enrollment. Two additional factors contributed to the lengthy recruitment. First, enrollment under the Exception From Informed Consent was not allowed in the early months of the study, and it was difficult to recruit subjects within the 6 hour window.15 Second, the trial was on clinical hold for approximately a year over safety concerns about the initial Epo dosage regimen. There were no changes in patient management at the two sites over the time period of the trial, but systematic changes in patient characteristics cannot be excluded.
Translating preclinical studies with Epo to a clinical trial design had some limitations. The effective time window for Epo neuroprotection is six hours in experimental TBI.31;32 This timeframe is feasible clinically and almost all enrolled patients received their first dose of study drug within six hours of injury. However, the dose of Epo that is safe in patients is at the lower end of the dosage range that has been found to be effective in rodent models of injury. The most effective Epo dose in experimental models (5000 IU/kg) is ten times the dose used in this study.33 In addition, an initial dosage regimen of three daily doses has been more effective than a single initial dose in experimental studies.34 Based on the experience of a multicenter stroke trial reported to FDA in 2008,20 there was concern that the initial regimen of three daily doses of Epo (Epo 1 dose regimen) would impose a greater risk of death. This concern resulted in a modified study design after approximately one third of the patients had been enrolled in the trial. We did not detect an increased mortality rate with the Epo 1 dose regimen, and the neurological outcome results were more promising than with the Epo 2 dose regimen. However, because this dose regimen was stopped early, the numbers of cases are very small to draw any conclusions.
CONCLUSIONS
Among patients with closed head injury, neither the administration of Epo nor maintaining hemoglobin concentration at least 10 g/dl resulted in improved neurological outcome at 6 months. These findings do not support either approach in TBI patients.
Supplementary Material
ACKNOWLEDGMENTS
Funding/Support: This study was supported by National Institute of Neurological Disorders and Stroke (grant #P01-NS38660).
Role of the funding source: The National Institute of Neurological Disorders and Stroke had no role in the design and conduct of the study; the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Robertson had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Study concept and design: Claudia S. Robertson, Alex Valadka, Shankar Gopinath, H. Julia Hannay, J. Clay Goodman
Acquisition of data: Claudia S. Robertson, Shankar Gopinath, Alex Valadka, Imoigele Aisiku, Pratik Doshi, H. Julia Hannay, J. Clay Goodman, Athena Baldwin, Lucia Rivera Lara, Hector Saucedo Crespo, Osama Ahmed, Santhosh Sadasivan, Luciano Ponce, Jovany Cruz-Navarro, Hazem Shahin, Leslie Neipert, Jace Waguspack
Analysis and interpretation of data: Claudia S. Robertson, Jose-Miguel Yamal, H. Julia Hannay, Barbara Tilley
Drafting of manuscript: Claudia S. Robertson, Jose-Miguel Yamal, H. Julia Hannay, Barbara Tilley
Critical review of manuscript for important intellectual content: All authors
Statistical analysis: Jose-Miguel Yamal, Barbara Tilley, M. Laura Rubin, Julia S. Benoit
Obtained funding: Claudia S. Robertson, Alex Valadka, Shankar Gopinath, H. Julia Hannay, J. Clay Goodman
Administrative, technical, or material support: Claudia S. Robertson, Shankar Gopinath, Alex Valadka, Imoigele Aisiku, H. Julia Hannay, J. Clay Goodman, Jose-Miguel Yamal, Barbara Tilley
Study supervision: Claudia S. Robertson, Jose-Miguel Yamal
The Epo Severe TBI Trial Investigators: Baylor College of Medicine: Claudia S. Robertson (intensivist), Shankar Gopinath (neurosurgeon), J. Clay Goodman (neuropathologist), Athena Baldwin, Lucia Rivera Lara, Hector Saucedo Crespo, Osama Ahmed, Santhosh Sadasivan, Luciano Ponce, Jovanny Navarro Cruz, Hazem Shahin (research coordinators); University of Texas Health Science Center Houston: Imoigele P. Aisiku, Pratik Doshi (intensivists), Alex Valadka (neurosurgeon); University of Houston (Outcomes Core): H. Julia Hannay, Leslie Neipert, Jace M. Waguspack; University of Texas School of Public Health (Statistical Center): Jose-Miguel Yamal, Barbara Tilley, M. Laura Rubin, Julia S. Benoit, Paul Swank.
Conflict of Interest Disclosures: Dr. Robertson, Dr. Doshi, and Jace Wagaspack report receiving grants from NIH NINDS during the conduct of the study. None of the other authors report any disclosures.
Data and Safety Monitoring Committee: Charles Contant (chair), Ramon Diaz-Arrastia, Geoffrey Manley, Kyra Becker, Daniel Hanley
ARDS consensus committee: Venkata Bandi, Imoigele P. Aisiku, Bradford Scott
NINDS Project Scientist: Ramona Hicks
Additional Contributions: We thank the two clinical sites for their participation in the study: Ben Taub General Hospital and Memorial Hermann Hospital. We also thank Michael O. Gonzalez MS and Xuemei Xi from University of Texas School of Public Health for their programming in the Statistical Center and Jeannie P. Tamez PhD, Brenda Lopez BA, Afife D. Batarse BS, Larissa Gonzalez BS, Laura O’Rosky BS, Michelle C. Munguia BA from University of Houston for their work in performing the outcome assessments. These participants received salary support from the grant funding the study.
Online-Only Material: Statistical methods supplement, eTables 1 and 2, eFigure 1 and 2, diagnostic criteria for the expected adverse events, and a justification of the Exception From Informed Consent
Trial Registration: clinicaltrials.gov Identifier: NCT00313716.
REFERENCES
- (1).Siren AL, Fratelli M, Brines M, et al. Erythropoietin prevents neuronal apoptosis after cerebral ischemia and metabolic stress. Proc Natl Acad Sci U S A. 2001;98(7):4044–4049. doi: 10.1073/pnas.051606598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Villa P, Bigini P, Mennini T, et al. Erythropoietin selectively attenuates cytokine production and inflammation in cerebral ischemia by targeting neuronal apoptosis. J Exp Med. 2003;198(6):971–975. doi: 10.1084/jem.20021067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Napolitano LM, Fabian TC, Kelly KM, et al. Improved survival of critically ill trauma patients treated with recombinant human erythropoietin. J Trauma. 2008;65(2):285–297. doi: 10.1097/TA.0b013e31817f2c6e. [DOI] [PubMed] [Google Scholar]
- (4).Talving P, Lustenberger T, Kobayashi L, et al. Erythropoiesis stimulating agent administration improves survival after severe traumatic brain injury: a matched case control study. Ann Surg. 2010;251(1):1–4. doi: 10.1097/SLA.0b013e3181b844fa. [DOI] [PubMed] [Google Scholar]
- (5).Talving P, Lustenberger T, Inaba K, et al. Erythropoiesis-stimulating agent administration and survival after severe traumatic brain injury: a prospective study. Arch Surg. 2012;147(3):251–255. doi: 10.1001/archsurg.2011.1838. [DOI] [PubMed] [Google Scholar]
- (6).Nirula R, Diaz-Arrastia R, Brasel K, Weigelt JA, Waxman K. Safety and efficacy of erythropoietin in traumatic brain injury patients: a pilot randomized trial. Crit Care Res Pract. 2010;2010 doi: 10.1155/2010/209848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Abrishamkar S, Safavi M, Honarmand A. Effect of erythropoietin on Glasgow Coma Scale and Glasgow Outcome Sale in patient with diffuse axonal injury. J Res Med Sci. 2012;17(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- (8).Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–417. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- (9).Lacroix J, Hebert PC, Hutchison JS, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–1619. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- (10).Hajjar LA, Vincent JL, Galas FR, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–1567. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- (11).McIntyre LA, Fergusson DA, Hutchison JS, et al. Effect of a liberal versus restrictive transfusion strategy on mortality in patients with moderate to severe head injury. Neurocrit Care. 2006;5(1):4–9. doi: 10.1385/ncc:5:1:4. [DOI] [PubMed] [Google Scholar]
- (12).Warner MA, O’Keeffe T, Bhavsar P, et al. Transfusions and long-term functional outcomes in traumatic brain injury. J Neurosurg. 2010;113(3):539–546. doi: 10.3171/2009.12.JNS091337. [DOI] [PubMed] [Google Scholar]
- (13).Elterman J, Brasel K, Brown S, et al. Transfusion of red blood cells in patients with a prehospital Glasgow Coma Scale score of 8 or less and no evidence of shock is associated with worse outcomes. J Trauma Acute Care Surg. 2013;75(1):8–14. doi: 10.1097/TA.0b013e318298492e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Sena MJ, Rivers RM, Muizelaar JP, Battistella FD, Utter GH. Transfusion practices for acute traumatic brain injury: a survey of physicians at US trauma centers. Intensive Care Med. 2009;35(3):480–488. doi: 10.1007/s00134-008-1289-z. [DOI] [PubMed] [Google Scholar]
- (15).Yamal J-M, Robertson CS, ubin ML, Benoit JS, Hannay HJ, Tilley BC. Enrollment of racially/ethnically diverse participants in traumatic brain injury trials: Effect of availability of exception from informed consent. Clinical Trials. doi: 10.1177/1740774514522560. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Marshall LF, Marshall SB, Klauber MR, et al. The diagnosis of head injury requires a classification based on computed axial tomography. J Neurotrauma. 1992;9(Suppl 1):S287–S292. [PubMed] [Google Scholar]
- (17).Maas AI, Hukkelhoven CW, Marshall LF, Steyerberg EW. Prediction of outcome in traumatic brain injury with computed tomographic characteristics: a comparison between the computed tomographic classification and combinations of computed tomographic predictors. Neurosurgery. 2005;57(6):1173–1182. doi: 10.1227/01.neu.0000186013.63046.6b. [DOI] [PubMed] [Google Scholar]
- (18).Baker SP, O’Neill B, Haddon W, Jr., Long WB. The injury severity score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- (19).Guidelines for the management of severe traumatic brain injury. J Neurotrauma. 2007;24(Suppl 1):S1–106. doi: 10.1089/neu.2007.9999. [DOI] [PubMed] [Google Scholar]
- (20).Ehrenreich H, Weissenborn K, Prange H, et al. Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- (21).Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: guidelines for their use. J Neurotrauma. 1998;15(8):573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- (22).Steyerberg EW, Mushkudiani N, Perel P, et al. Predicting outcome after traumatic brain injury: development and international validation of prognostic scores based on admission characteristics. PLoS Med. 2008;5(8):e165. doi: 10.1371/journal.pmed.0050165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Murray GD, Barer D, Choi S, et al. Design and analysis of phase III trials with ordered outcome scales: the concept of the sliding dichotomy. J Neurotrauma. 2005;22(5):511–517. doi: 10.1089/neu.2005.22.511. [DOI] [PubMed] [Google Scholar]
- (24).Rubin DB. Multiple Imputation for Nonresponse in Surveys. John Wiley & Sones, Inc.; New York: 1987. [Google Scholar]
- (25).Bernard GR, Artigas A, Brigham KL, et al. Report of the American-European consensus conference on ARDS: definitions, mechanisms, relevant outcomes and clinical trial coordination. The Consensus Committee. Intensive Care Med. 1994;20(3):225–232. doi: 10.1007/BF01704707. [DOI] [PubMed] [Google Scholar]
- (26).Tibshirani R. The lasso method for variable selection in the Cox model. Stat Med. 1997;16(4):385–395. doi: 10.1002/(sici)1097-0258(19970228)16:4<385::aid-sim380>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- (27).Tilley BC, Palesch YY, Kieburtz K, et al. Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66(5):628–633. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- (28).Zygun DA, Nortje J, Hutchinson PJ, Timofeev I, Menon DK, Gupta AK. The effect of red blood cell transfusion on cerebral oxygenation and metabolism after severe traumatic brain injury. Crit Care Med. 2009;37(3):1074–1078. doi: 10.1097/CCM.0b013e318194ad22. [DOI] [PubMed] [Google Scholar]
- (29).Figaji AA, Zwane E, Kogels M, et al. The effect of blood transfusion on brain oxygenation in children with severe traumatic brain injury. Pediatr Crit Care Med. 2010;11(3):325–331. doi: 10.1097/PCC.0b013e3181b80a8e. [DOI] [PubMed] [Google Scholar]
- (30).Tango HK, Schmidt AP, Mizumoto N, Lacava M, Cruz RJ, Jr., Auler JO., Jr. Low hematocrit levels increase intracranial pressure in an animal model of cryogenic brain injury. J Trauma. 2009;66(3):720–726. doi: 10.1097/TA.0b013e3181719b35. [DOI] [PubMed] [Google Scholar]
- (31).Brines ML, Ghezzi P, Keenan S, et al. Erythropoietin crosses the blood-brain barrier to protect against experimental brain injury. Proc Natl Acad Sci U S A. 2000;97(19):10526–10531. doi: 10.1073/pnas.97.19.10526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Cherian L, Goodman CJ, Robertson CS. Neuroprotection with Erythropoietin Administration Following Controlled Cortical Impact Injury in Rats. J Pharmacol Exp Ther. 2007;322(2):789–794. doi: 10.1124/jpet.107.119628. [DOI] [PubMed] [Google Scholar]
- (33).Meng Y, Xiong Y, Mahmood A, Zhang Y, Qu C, Chopp M. Dose-dependent neurorestorative effects of delayed treatment of traumatic brain injury with recombinant human erythropoietin in rats. J Neurosurg. 2011;115(3):550–560. doi: 10.3171/2011.3.JNS101721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Xiong Y, Mahmood A, Meng Y, et al. Delayed administration of erythropoietin reducing hippocampal cell loss, enhancing angiogenesis and neurogenesis, and improving functional outcome following traumatic brain injury in rats: comparison of treatment with single and triple dose. J Neurosurg. 2010;113(3):598–608. doi: 10.3171/2009.9.JNS09844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.