Brains of patients with schizophrenia (SZ) show both anatomical and functional deficits that suggest aberrant glutamate neurotransmission. Historically, many studies have addressed neurochemical changes in glutamate and its metabolites in various brain regions and cerebrospinal fluid in patients with SZ and compared them with to those in normal controls. Alteration in molecular disposition associated with glutamate signaling has also been addressed by receptor binding assays. Although all such results are not perfectly in complete accord, an approach with 1H magnetic resonance spectroscopy (MRS) indicates more consistently that the levels of N-acetyl-aspartate (NAA), a marker associated with glutamate synapses and signaling, are decreased in SZ brains, especially in the frontal cortex, temporal cortex, and thalamus (1). More recent genetic studies and molecular expression profiling have supported the notion of disturbed neurotransmission and synaptic connectivity (2). Genetic susceptibility factors for SZ identified through association study and cytogenetic approaches are enriched in the dendritic spine, the key postsynaptic structure, including Neuregulin-1, ErbB4, and regulator of G-protein signaling 4. Microarray analysis, an unbiased method for gene expression profiling, also provides evidence that expression of synaptic molecules is decreased in SZ. Consistent with these findings, decrease in dendritic spines is reported in SZ (2, 3).
Glutamate Dysregulation in Schizophrenia: Sole or Multiple Mechanisms?
Provided that there is intrinsic susceptibility and abnormalities in glutamatergic neurotransmission in SZ patients, what is (are) their mechanism(s) underlying SZ pathology: is there a single mechanism or are there multiple independent mechanisms via the same neurotransmitter? To address this question, it is clear that glutamate is the most abundant neurotransmitter and has multiple roles in the brain. Thus, it is crucial for neuronal connectivity at the functional level in adult brains; furthermore, it plays a key role in plasticity by triggering neuronal activity-dependent (that is, glutamate-mediated) synaptic reorganization, which is especially crucial in neurodevelopment. Excess glutamate results in toxicity to neurons designatedexcitotoxicity. In order to obtain mechanistic insight into the role of glutamate and SZ in depth, it is particularly important to consider cell-type, regional, and temporal specificities. It is also important to consider how glutamate signaling “cross-talks” with other brain factors.
Phenotypic disturbances clearly suggest that SZ has deficits associated with neuronal circuitry involving the cerebral cortex, especially the prefrontal cortex. As noted above, changes in dendritic spines and theirarborization are reported in SZ brains (2, 3). The synaptic spine is a unique and crucial structure that conveys plasticity in the “pyramidal neurons.” Because glutamate and its NMDA-type receptor have critical roles in the maintenance and reorganization of the synaptic spine, the abnormalities found in SZ are likely to be associated with glutamatergic deficits. In contrast, there is convincing psychopharmacological evidence of psychostimulant phencyclidine (PCP), an antagonist of the NMDA glutamate receptor, induces dysfunction in gamma-aminobutyric acid (GABA)-ergic “interneurons” (4). Thus, the hypo-NMDA receptor function reduces the excitation of last-spiking interneurons, which, in turn, results in disinhibition of pyramidal cells. What are possible relationships between these deficits (synaptic changes in pyramidal neurons and hypofunction of interneurons) and, if they exist, which one is primary? This issue remains currently unresolved.
The thalamus is another key region that is considered to be involved in glutamate and SZ. This brain region has bidirectional projections with the cortex and plays a key role in information processing, which is impaired in SZ. Furthermore, decrease in the volume of thalamus in SZ has been reported by neuropathological studies and brain imaging. Glutamatergic signaling is modulated by many other soluble fractions, such as other neuromodulators, neurotransmitters, and growth factors. Accumulating evidence has suggests that D-serine may be an endogenous co-agonist of the NMDA receptor at the glycine site. D-Serine is synthesized by serine racemase, which converts L-serine to D-serine in brain. There is good genetic support that genes coding for regulation of biosynthesis and degradation of D-serine are associated with SZ. Thus, D-serine may be a key modulatory factor for glutamatergic dysfunction in SZ. Dopamine regulates firing of pyramidal neurons and surrounding GABAergic inhibitory interneurons principally via dopamine D1 and D2 receptors. Growth factors, such as brain-derived neurotrophic factor (BNDF), also modulate glutamatergic neurotransmission. Taken together, deficits in these factors can also underlie mechanisms for glutamatergicdysregulation.
Glutamate Dysregulation in Cortical Development: Future Implications in Therapeutic Strategies for Schizophrenia?
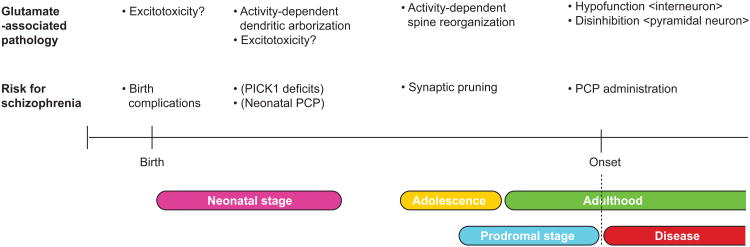
The onset of SZ is primarily in adolescence and young adulthood. Nonetheless, epidemiological data indicate that risks during neurodevelopment play important roles. Thus, if intrinsic glutamatergicdysregulation exists, this may play a significant role in perinatal and neonatal stages, and in adolescence and young adulthood (corresponding to the prodromal stage of SZ), and then at and after the onset of SZ, respectively. Because the glutamate receptor composition and circuitry property are very different during these stages, biological mechanisms of how glutamate dysregulation leads to SZ pathology at each stage may be distinct, although some common mechanisms are likely to exist as well.
In the perinatal stage, a major risk for SZ is birth complications, especially birth hypoxia. It is possible that glutamate-associated excitotoxicity may play a role in this process.
In the neonatal stage, there is a good model to address role for glutamate and D-serine in this stage. Mice deficient in Protein Interacting with C-Kinase-1(PICK1), a modulator of the D-serine synthesizing enzyme serine racemase, display a transient knockdown of D-serine in the forebrain (5). In contrast to serine racemase knockout mice in which there is continuous knockdown of D-serine, this PICK knockout model may be useful in clarifying a role for D-serine and glutamatergic neurotransmission during the specific neonatal stage. Indeed, the mice show behavioral deficits in adulthood, including abnormalities in prepulse inhibition. Several groups have also reported that neonatal injection of PCP can result in behavioral alteration in adulthood, relevant to the endophenotypes of SZ. Because the receptor composition and circuitry property involving glutamate are different between neonatal stages and adulthood, it is likely that the pathological mechanisms elicited by neonatal injection of PCP and those by PCP psychosis in adulthood are also different. Nonetheless, mice with PICK1 knockout as well as those with neonatal injection of PCP can provide insight into how neonatal disturbance of glutamate might be associated with SZ endophenotype-associated developmental defects of the cortex.
In adolescence, dynamic reorganization of brain networks occurs. One of the most marked changes among them is reorganization of synapses, so-called synaptic pruning (2). Recent study shows that the levels of key synaptic proteins, such as postsynaptic density protein 95 and synaptophysin, are decreased in late adolescence and early adulthood (6). In a systematic study of postmortem brains (the middle frontal gyrus) showed that synaptic density increases after birth until 2-5 years of age, and decreases robustly in early adolescence, and synapses eliminated in this process of “synaptic pruning” are mainly glutamatergic(7). Thus, intrinsic deficits of glutamatergic neurotransmission, probably in part due to genetic variations in synaptic susceptibility factors for SZ, can directly underlie aberrant pruning, which results in dendritic changes in the pyramidal neurons from SZ patients.
Finally, an important question is whether we can treat SZ by normalizing glutamatergic and associated dysregulationin the future. Current strategies of modulating glutamatergicdysregulation in adulthood, especially for interneuron deficits and disinhibition of pyramidal neurons, will make substantial progress in sympotomatic relief in near future. Beyond this, if we wish to treat “at-risk” patients at the most earliest stage even by preventing disease onset, it becomes very crucial to understand roles for glutamate in each developmental stage of SZ pathology.
This Issue
In this issue of Biological Psychiatry, Stone et al. (8) report glutamate dysfunction in people with prodromal symptoms of psychosis in association with gray matter volume. In this report, the authors measured the levels of glutamate and metabolites in the thalamus and anterior cingulate from at-risk mental state subjects by 1H-MRS, in correlation with gray matter volume, reporting decreased levels of glutamate in these subjects compared to those in controls and the correlation of the level of thalamic glutamate with gray matter volume in the medial temporal cortex and insula. Although the exact mechanisms that link these two remain to be elucidated, one or more than one of the mechanisms addressed above, such as disinhibition of glutamatergic projection and glutamate-associated cytotoxicity, might be involved in. In this issue, Vinogradov et al. (9) and Sambataro et al. (10) also report correlation of measures for BDNF and COMT with cognitive functions associated with SZ, respectively. As described above, neurotrophic factors and key non-glutamatergic neurotransmitters, such as dopamine and serotonin, could be examined in relation to glutamate at mechanistic levels, which would provide a more complete overall picture of the pathology of SZ.
Figure.
Possible links between risk of schizophrenia and glutamate-associated brain pathology from perinatal stages to adulthood. There are many types of risk factors for schizophrenia in each stage, but only risks mentioned in the text are depicted as representative examples. Risks known in preclinical models are indicated in parentheses.
Acknowledgments
Dr. Sawa reports research funding from the following industries (Wyeth, Astellas, Eisai, Taisho, Otsuka, Tanabe-Mitsubishi, and Janssen), as well as that from NIH and nonprofit foundations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McCullumsmith RE, Clinton SM, Meador-Woodruff JH. Schizophrenia as a disorder of neuroplasticity. Int Rev Neurobiol. 2004;59:19–45. doi: 10.1016/S0074-7742(04)59002-5. [DOI] [PubMed] [Google Scholar]
- 2.Jaaro-Peled H, Hayashi-Takagi A, Seshadri S, Kamiya A, Brandon NJ, Sawa A. Neurodevelopmental mechanisms of schizophrenia: understanding disturbed postnatal brain maturation through Neuregulin-1/ErbB4 and DISC1. Trens in Neurosciences. 2009 doi: 10.1016/j.tins.2009.05.007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 4.Lisman JE, Coyle JT, Green RW, Javitt DC, Benes FM, Heckers S, et al. Circuit-based framework for understanding neurotransmitter and risk gene interactions in schizophrenia. Trends Neurosci. 2008;31:234–242. doi: 10.1016/j.tins.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hikida T, Mustafa AK, Maeda K, Fujii K, Barrow RK, Saleh M, et al. Modulation of D-serine levels in brains of mice lacking PICK1. Biol Psychiatry. 2008;63:997–1000. doi: 10.1016/j.biopsych.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourgeois JP, Rakic P. Changes of synaptic density in the primary visual cortex of the macaque monkey from fetal to adult stage. J Neurosci. 1993;13:2801–2820. doi: 10.1523/JNEUROSCI.13-07-02801.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glantz LA, Gilmore JH, Hamer RM, Lieberman JA, Jarskog LF. Synaptophysin and postsynaptic density protein 95 in the human prefrontal cortex from mid-gestation into early adulthood. Neuroscience. 2007;149:582–591. doi: 10.1016/j.neuroscience.2007.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stone JM, Day F, Tsagaraki H, Valli I, McLean MA, Lythgoe DJ, et al. Glutamate dysfunction in people with prodromal symptoms of psychosis: relationship to gray matter volume. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Vinogradov S, Fisher M, Holland C, Shelly W, Wolkowitz O, Mellon SH. Is serum brain-derived neurotrophic factor a biomarker for cognitive enhancement in schizophrenia? Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sambatano F, Reed JD, Murty VP, Das S, Tan HY, Callicott JH, et al. Catechol-O-Methyltransferase valine 158 methionine polymorphism modulates brain networks underlying working memory across adulthood. Biological Psychiatry. 2009 doi: 10.1016/j.biopsych.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]