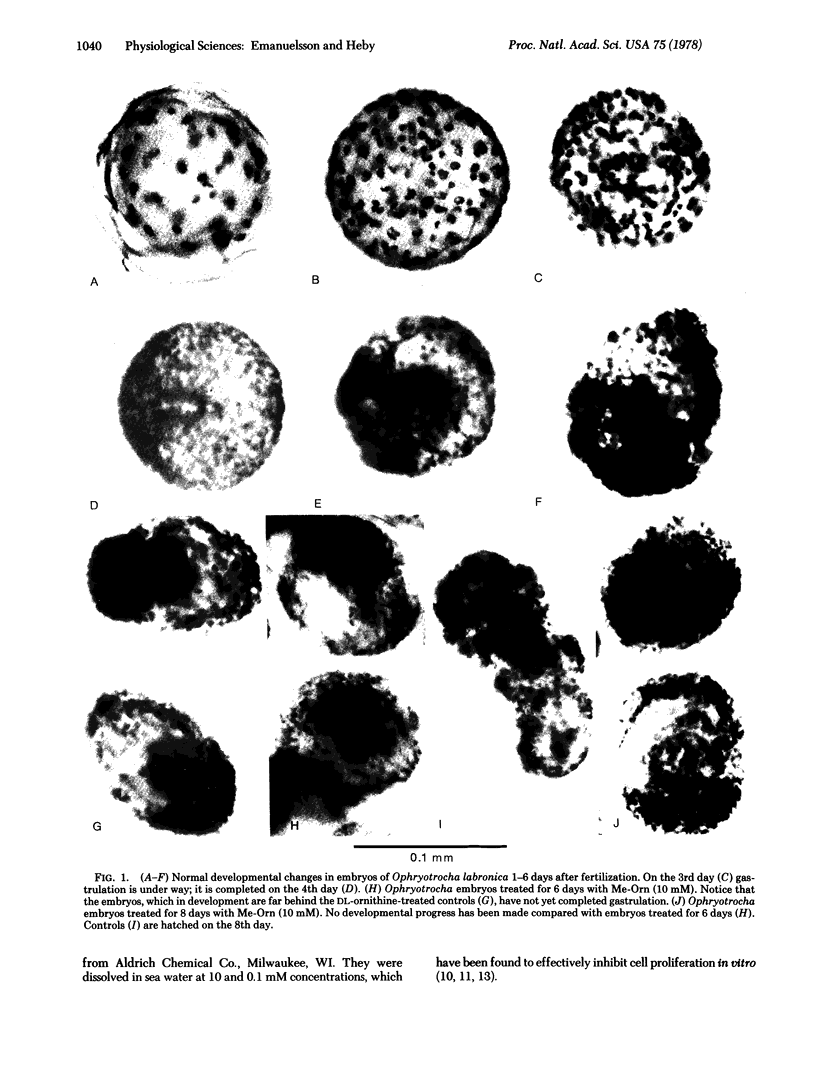
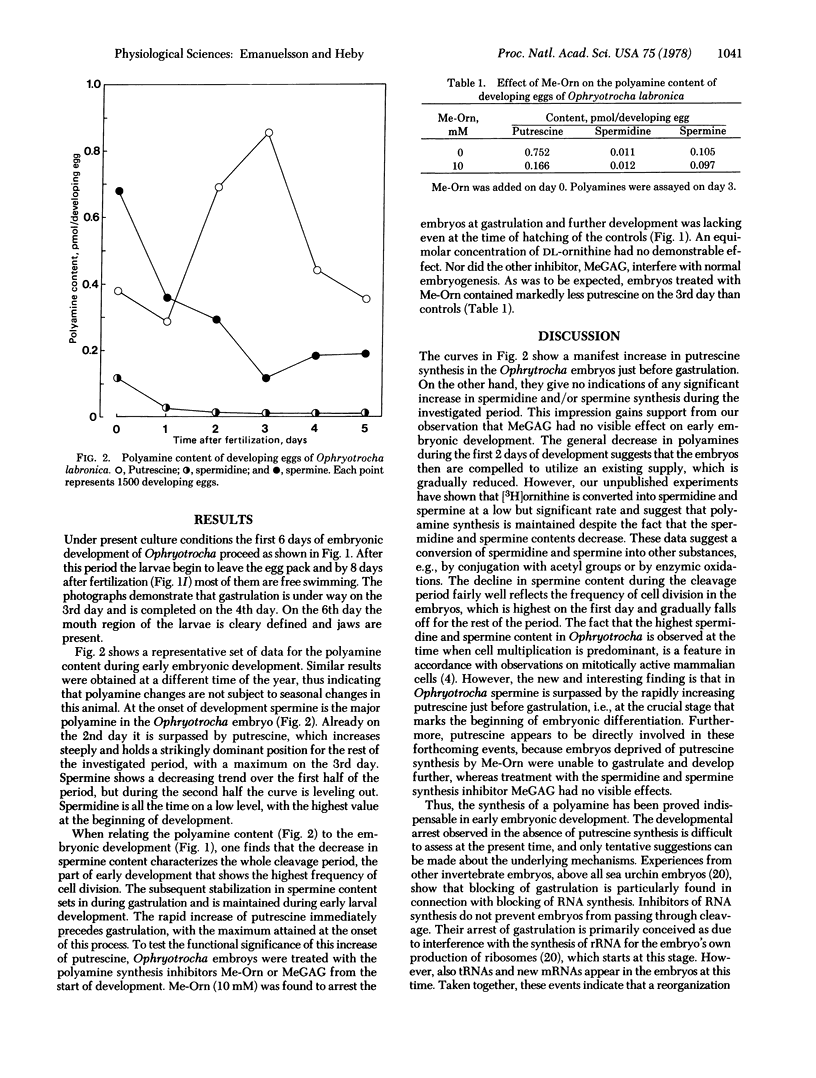
Abstract
Development eggs of the polychete Ophryotrocha labronica were analyzed for polyamines during the first 6 days after fertilization. The spermine content dominated initially, but gradually decreased. It was surpassed by putrescine, which rapidly increased to a maximum on the 3rd day, i.e., at the inception of grastrulation. The spermidine content was low during the entire period. Treatment of eggs with the putrescine synthesis inhibitor alpha-methylornithine from the onset of development led to developmental arrest at gastrulation and to an abnormally low content of putrescine in the treated embryos. Methylglyoxal bis(guanylhydrazone), an inhibitor of spermine and spermidine synthesis, had no visible effect of development. Our observations strongly suggest that putrescine synthesis is indispensable in early embryonic development of Ophryotrocha.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson G., Heby O. Kinetics of cell proliferation and polyamine synthesis during Ehrlich ascites tumor growth. Cancer Res. 1977 Dec;37(12):4361–4366. [PubMed] [Google Scholar]
- Andersson G., Heby O. Population kinetics of an Ehrlich ascites tumor following treatment with methylglyoxal-bis(guanylhydrazone), a polyamine synthesis inhibitor. Cancer Lett. 1977 Jul;3(1-2):59–63. doi: 10.1016/s0304-3835(77)94150-7. [DOI] [PubMed] [Google Scholar]
- Barros C., Giudice G. Effect of polyamines on ribosomal RNA synthesis during sea urchin development. Exp Cell Res. 1968 Jun;50(3):671–674. doi: 10.1016/0014-4827(68)90434-5. [DOI] [PubMed] [Google Scholar]
- Dion A. S., Cohen S. S. Polyamine stimulation of nucleic acid synthesis in an uninfected and phage-infected polyamine auxotroph of Escherichia coli K12 (arginine-agmatine ureohydrolase-putrescine-spermidine-lysine-cadaverine). Proc Natl Acad Sci U S A. 1972 Jan;69(1):213–217. doi: 10.1073/pnas.69.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dion A. S., Herbst E. J. The localization of spermidine in salivary gland cells of Drosophila melanogaster and its effect on H3-uridine incorporation. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2367–2371. doi: 10.1073/pnas.58.6.2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H., Jorstad C. M., Morris D. R. Increased cellular levels of spermidine or spermine are required for optimal DNA synthesis in lymphocytes activated by concanavalin A. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4042–4045. doi: 10.1073/pnas.72.10.4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H. A., Ahrens P., HAHLE K., Thiele H. Polyamine als Stimulatoren von Genaktivitäten. Eine autoradiographische Studie an Riesenchromosomen von Chironomus thummi. Histochemie. 1974 Mar 6;38(3):223–228. [PubMed] [Google Scholar]
- Heby O., Gray J. W., Lindl P. A., Marton L. J., Wilson C. B. Changes in L-ornithine decarboxylase activity during the cell cycle. Biochem Biophys Res Commun. 1976 Jul 12;71(1):99–105. doi: 10.1016/0006-291x(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Wilson C. B., Gray J. W. Effect of methylglyoxal-bis (guanylhydrazone), an inhibitor of spermidine and spermine synthesis, on cell cycle traverse. Eur J Cancer. 1977 Sep;13(9):1009–1017. doi: 10.1016/0014-2964(77)90180-3. [DOI] [PubMed] [Google Scholar]
- Heby O., Marton L. J., Wilson C. B., Martinez H. M. Polyamine metabolism in a rat brain tumor cell line: its relationship to the growth rate. J Cell Physiol. 1975 Dec;86(3 Pt 1):511–521. doi: 10.1002/jcp.1040860308. [DOI] [PubMed] [Google Scholar]
- Heby O., Sarna G. P., Marton L. J., Omine M., Perry S., Russell D. H. Polyamine content of AKR leukemic cells in relation to the cell cycle. Cancer Res. 1973 Nov;33(11):2959–2964. [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manen C. A., Russell D. H. Spermine is major polyamine in sea urchins: studies of polyamines and their synthesis in developing sea urchins. J Embryol Exp Morphol. 1973 Apr;29(2):331–345. [PubMed] [Google Scholar]
- Morris D. R., Fillingame R. H. Regulation of amino acid decarboxylation. Annu Rev Biochem. 1974;43(0):303–325. doi: 10.1146/annurev.bi.43.070174.001511. [DOI] [PubMed] [Google Scholar]
- Raina A., Jänne J. Physiology of the natural polyamines putrescine, spermidine and spermine. Med Biol. 1975 Jun;53(3):121–147. [PubMed] [Google Scholar]
- Russell D. H. Putrescine and spermidine biosynthesis in the development of normal and anucleolate mutants of Xenopus laevis. Proc Natl Acad Sci U S A. 1971 Mar;68(3):523–527. doi: 10.1073/pnas.68.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N. Use of the dansyl reaction in biochemical analysis. Methods Biochem Anal. 1970;18:259–337. doi: 10.1002/9780470110362.ch5. [DOI] [PubMed] [Google Scholar]
- Srinivason P. R., Young D. V., Maas W. Stable ribonucleic acid synthesis in stringent (rel+) and relaxed (rel-) polyamine auxotrophs of Escherichia coli K-12. J Bacteriol. 1973 Nov;116(2):648–655. doi: 10.1128/jb.116.2.648-655.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Ashman H. G., Corti A., Tadolini B. On the development of specific inhibitors of animal polyamine biosynthetic enzymes. Ital J Biochem. 1976 Jan-Feb;25(1):5–32. [PubMed] [Google Scholar]