Abstract
Purpose
The tree shrew model of refractive development is particularly useful because, like humans, tree shrews have a fibrous sclera. Selective changes in some candidate extracellular matrix proteins and mRNAs have been found in the sclera during the development of, and recovery from, induced myopia. We undertook a more neutral proteomic analysis using two-dimensional gel electrophoresis and mass spectrometry to identify scleral proteins that are differentially expressed during the development of, and recovery from, lens-induced myopia.
Methods
Five tree shrews (Tupaia glis belangeri) wore a monocular –5 D lens for 4 days, starting 24 days after natural eye opening. At the end of this time, all treated eyes had partially compensated for the lens and were –3.5±0.7 D (mean ± SEM) myopic relative to the untreated fellow control eyes. An additional five animals wore a –5 D lens for 11–13 days, followed by 4 days of recovery without the –5 D lens. The amount of recovery was 1.6±0.4 D. Scleral proteins from both groups were then isolated and resolved by two-dimensional gel electrophoresis and spots that were differentially expressed were identified by mass spectrometry.
Results
The scleral protein profile typically displayed ~700 distinct protein spots within the pH 5–8 range. Comparison of the treated-eye and control-eye scleras of the lens-compensation animals revealed five spots that were significantly differentially expressed in all five pairs of eyes; all were downregulated 1.2 to 1.7 fold in the treated eye. These proteins were identified as: pigment epithelium-derived factor (PEDF), procollagen I α1, procollagen I α2, and thrombospondin I (two spots). In the recovering eyes, the two thrombospondin I spots remained lower in abundance while PEDF and the procollagens were no longer downregulated. In addition, 78 kDa glucose-regulated protein (GRP 78), a member of the heat shock protein 70 family, was slightly upregulated 1.3 fold.
Conclusions
We found consistent results across animals that were of a magnitude consistent with the physiologically small changes to the focal plane of these eyes. Changes in collagen confirm previous findings, but downregulation of thrombospondin I adds detail to our understanding of the chain of signals that regulates scleral creep rate. The differential changes in PEDF and GRP 78 were not expected, based on previous studies, and demonstrate the utility of the proteomic approach in tree shrew sclera.
Introduction
Tree shrews are small mammals that are closely related to the primate line [1,2] and have excellent vision for their size [3]. During normal postnatal development, tree shrew eyes demonstrate visually-guided emmetropization: visual signals regulate the axial elongation rate of the growing eye so that the retina comes to reside at the focal plane, producing an eye that is in good focus [4-6]. When a concave (negative-power) lens is placed before one eye of a juvenile tree shrew and held in a goggle frame [7], the eye is made artificially hyperopic. In response, the eye rapidly elongates until, after 10 – 15 days, it has compensated for the lens so that the retina again lies at the focal plane and the eye is again emmetropic while wearing the lens [6,8-10]. If the lens is then removed, the eye is myopic. In juvenile animals, ‘recovery’ from the induced myopia occurs [11]: the axial elongation rate of the eye slows dramatically while the optics of the eye continue to mature, until the retina is once again located at the focal plane. Recovery occurs in most, but not all, juvenile tree shrews.
The sclera is the outer coating of the eye that, in addition to protecting the retina and allowing the attachment of the extraocular muscles, controls the size of the eye and the location of the retina, relative to the focal plane. Tree shrews, like primates, have a fibrous sclera comprised largely of type I collagen along with lower amounts of type III and type V collagen, elastin, proteoglycans and other structural proteins [12-18]. This extracellular matrix (ECM) is produced by the scleral fibroblasts and is arranged in interwoven layers, or lamellae. Both negative-lens compensation and recovery from induced myopia involve changes in a biomechanical property of the sclera, viscoelasticity, which is measured as creep rate: the rate of increase in the length of a strip of sclera while under constant tension. The creep rate increases during the early phase of negative-lens compensation, reaching a peak after 4 days of lens wear, and then declines toward normal as the eye completes its compensation [8]. During recovery, creep rate falls rapidly (within 2 days) to below normal values [8]. Underlying the biomechanical changes is selective tissue remodeling that involves alteration to both the synthesis and degradation of ECM components such as collagen, proteoglycans, and glycosaminoglycans [12,19,20]. It appears that these changes allow normal intraocular pressure to expand the globe during negative-lens compensation, perhaps by increasing the ease with which the scleral lamellae slip across each other.
Several studies have examined mRNA levels in sclera during negative-lens compensation and recovery and have found selective regulation for ‘candidate’ proteins in tree shrews and chicks [21-24], with a few of these changes also being detected at the protein level – collagen I, MMP-2, and TGF-β being examples [12,19,20]. However, it is still largely unknown how most of the observed changes in mRNA levels correlate to changes in protein levels – protein levels that more accurately represent the state of a biologic system.
To complement our previous ‘candidate’ approach that examined levels of mRNA for specific targets, we have now undertaken a more neutral proteomic analysis using two-dimensional gel electrophoresis (2DGE) and mass spectrometry which has the potential to identify any additional scleral proteins that are differentially expressed during myopia development and recovery. In 2DGE, proteins extracted from the sclera are first separated by pH using isoelectric focusing and are then separated by molecular weight using a standard SDS–PAGE gel, producing a unique pattern of protein spots. Comparison of gels from treated and control eyes reveals changes in abundance of individual proteins that are subsequently collected from the gel and identified using mass spectrometry.
Methods
Experimental groups
Juvenile tree shrews (Tupaia glis belangeri) were produced in our breeding colony and raised by their mothers on a 14 h on/10 h off light/dark cycle. All procedures complied with the ARVO Statement for the Use of Animals in Ophthalmic and Visual Research and were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham.
Groups of tree shrews (n=5 per group), balanced to include both males and females and avoiding pups from the same parents within a group, were divided into two conditions: a negative-lens compensation group and a recovery group. As in previous studies [8,23] the negative-lens compensation group wore a monocular –5 D lens (spherical power) for 4 days starting at 24±1 days after natural eye opening (days of visual experience [VE]) to induce axial elongation and myopia. The recovery group experienced –5 D lens wear for 11–13 days, also starting at 24±1 days of VE, to induce negative-lens compensation. Then the lens was removed and the now-myopic treated eye was allowed to recover for 4 days. These lens-wear periods were chosen because mRNA studies had previously shown significant expression changes at these time points [21,23].
Pedestal attachment
To attach the goggle containing the –5 D lens firmly to the head during lens treatment, at 21±1 days of VE, all animals were anesthetized (17.5 mg ketamine, 1.2 mg xylazine; supplemented with 0.5−2.0% isoflurane as needed) and received a dental acrylic pedestal following procedures described by Siegwart and Norton [7]. Three days later, the goggle frame was clipped to the pedestal. Animals in both groups wore a monocular –5 D lens in front of a randomly-selected treated eye. The control eye had unrestricted vision through an open (no lens) goggle frame. The goggle was removed for approximately 3 min in dim illumination twice daily (~9:00 AM and ~4:30 PM) while the lens was cleaned. During lens cleaning, the animals were kept in a darkened nest box to minimize exposure to visual stimuli. Badly scratched lenses were replaced as needed while the animal was kept in darkness (<30 min).
Axial and refractive measures
At the time the pedestal was attached, and at the end of the treatment or recovery period, ocular component dimensions were measured under anesthesia with A-scan ultrasound as described by Norton and McBrien [5]. At the start and end of the treatment or recovery period, non-cycloplegic measures of refractive state were taken on the animals while they were awake using a Nidek ARK 700-A infrared autorefractor [25]. This allowed us to assess the amount of myopia that developed during the lens compensation period and, in the recovery group, the amount of recovery that occurred in each animal. Prior studies have found that non-cycloplegic awake autorefractor measures provide a valid estimate of the amount of induced myopia in tree shrews. Actual values for each eye differ from the cycloplegic measures by less than 1 D, and the treated-eye versus control-eye differences are nearly identical between non-cycloplegic and cycloplegic measures [6].
2D gel electrophoresis
The animals were euthanized, approximately 2 h into the light phase, with an overdose of sodium pentobarbital at the end of the treatment or recovery period. Enucleated eyes were dissected in ice-cold 10 mM tris, 250 mM sucrose (pH 7). Scleral tissue was immediately frozen in liquid nitrogen, pulverized to a fine powder in a Teflon freezer mill (B. Braun Biotech, Allentown, PA) while still frozen, and suspended in 500 μl extraction buffer (7 M urea, 2 M thiourea, 2% Pharmalyte 3–10, 4% CHAPS). Samples were then simultaneously reduced and alkylated at room temperature for 90 min with 5 mM tributylphosphine (TBP) and 20 mM 4-vinyl pyridine (VP) respectively, before quenching the alkylation with 20 mM DTT for 20 min. Cellular debris was pelleted at 21,000 g for 20 min at 4 °C. Supernatants (~400 µl) were collected and the extraction buffer exchanged using Ultrafree 0.5 ml centrifugal ultrafiltration devices (Millipore, Billerica, MA) with a 10 kDa MW cutoff [26]. Samples were centrifuged at 12,000 g until a retentate volume of ~50 µl was obtained, which was then diluted with an additional 500 µl extraction buffer. In total, the extraction buffer was exchanged three times to ensure removal of any residual TBP or VP. Protein yields were estimated using the 2-D Quant Kit (GE Healthcare, Piscataway, NJ).
For the first dimension, 100 μg scleral protein was diluted in rehydration buffer (7 M urea, 2 M thiourea, 2% Pharmalyte 3–10) to give a final CHAPS concentration of 0.5%. Samples were loaded onto 17 cm immobilized pH gradient (IPG) pH 5–8 strips (Bio-Rad, Hercules, CA) by active rehydration for 16 h at 20 °C using the PROTEAN IEF cell (Bio-Rad, Hercules, CA). Electrode wicks were emplaced before isoelectric focusing (IEF) for 4 h at 300 V followed by 3500V until a total of 68,000 Vh had been reached. After IEF, IPG strips were equilibrated (6 M urea, 50 mM tris pH 8.8, 30% glycerol, 2% SDS) for 30 min. The IPG strips were immediately affixed to the top of 12% SDS–PAGE second dimension gels (20×25×0.1 cm). Assembled gels were electrophoresed using the DALTsix system (GE Healthcare, Piscataway, NJ): 1W per gel for 1 h, followed by 13 W per gel for 6 h, at 20 °C.
Following electrophoresis, the 2D gels were stained with Deep Purple according to the manufacturer’s instructions (GE Healthcare, Piscataway, NJ), with minor modifications. Gels were rinsed extensively in H2O to prevent them drying during scanning; gels were imaged at high resolution (100μm pixel size) using a Typhoon 8600 with 532 nm excitation and the 610BP emission filter. The resulting protein profiles from the treated and control eyes were compared for all animals in each group at the UAB Proteomics & Mass Spectrometry Shared Facility using single stain analysis with intelligent noise correction algorithm (INCA) processing by Progenesis 2D analysis software (Nonlinear Dynamics, Newcastle upon Tyne, UK) to identify protein spots that were shown to be differentially represented. Spots were considered to be differentially expressed if there was a significant (p<0.05; unpaired t test) difference in normalized total spot volume between fellow treated and control eyes in the same direction in at least four of the five animals in a group.
Mass spectrometry
For spot picking, gels were prepared as above except loading 800 µg scleral protein per gel. Spots of interest were excised from the gel and the plugs destained with three consecutive washes of 50 mM ammonium bicarbonate / acetonitrile (1:1, v/v) for 30 min. Gel plugs were then washed with 50 mM ammonium bicarbonate for 10 min, before digestion with freshly prepared trypsin (Trypsin Gold; Promega, Madison, WI) at 37 °C overnight. Peptides were recovered from the gel plug with two 100μl washes in 5% formic acid / acetonitrile (1:1, v/v) for 30 min; the washes were combined and the solvent evaporated in a vacuum centrifuge. Samples were resuspended in 10 μl 0.1% formic acid then desalted and concentrated using ZipTipsC18 (Millipore, Billerica, MA) in preparation for MALDI-TOF and LC-MSMS by the UAB Proteomics & Mass Spectrometry Shared Facility.
Desalted peptide samples were diluted 1/10 with a saturated solution of α-Cyano-4-hydroxycinnamic acid (CHCA) matrix and were applied to an Applied Biosystems MALDI-TOF 96-well target plate and dried. MALDI-TOF analyses were performed with a Voyager-DE PRO (Applied Biosystems, Foster City, CA) in positive mode. Spectra were analyzed using Voyager Data Explorer software; the resulting peptide mass fingerprints were submitted via MASCOT (Matrix Science) to the Mass Spectrometry protein sequence DataBase (MSDB) for protein identification.
Tandem mass spectral analyses were performed with a Q-TOF2 mass spectrometer (Micromass, Milford, MA) using electrospray ionization. Liquid chromatography was performed using an Ultimate LC, Switchos micro-column switching unit, and FAMOS autosampler (LC Packings, Bannockburn, IL). The samples were concentrated on a 300 μm i.d. C18 pre-column at a flow rate of 10 μl/min with 0.1% formic acid and then flushed onto a 75 μm i.d. C18 column at 200nl/min with a gradient of 5%–100% acetonitrile (0.1% formic acid) over 30 min. A nano-LC interface was used to transfer the LC eluent into the Q-TOF which was operated in automatic switching mode, whereby multiply-charged ions were subjected to MSMS if their intensities rose above 6 counts. Tandem mass spectra were processed with MassLynx MaxEnt 3 software (Micromass, Milford, MA) and submitted via ProteinLynx to the SwissProt database for protein identification. All identities were manually checked for accuracy.
Results
Changes during negative-lens compensation
At the end of the four-day period of lens wear, all treated eyes had partially compensated for the –5 D lens and were myopic relative to the untreated fellow control eyes. Figure 1 shows the refractive values for the control and treated eyes of each animal, corrected for the small eye artifact [25,27] by subtracting 4 D. As a group, the treated eyes were –3.5±0.7 D (mean ± SEM) myopic relative to the untreated fellow control eyes. A-scan measures (not shown) confirmed the vitreous chamber was elongated in the treated eyes relative to the control eyes.
Figure 1.
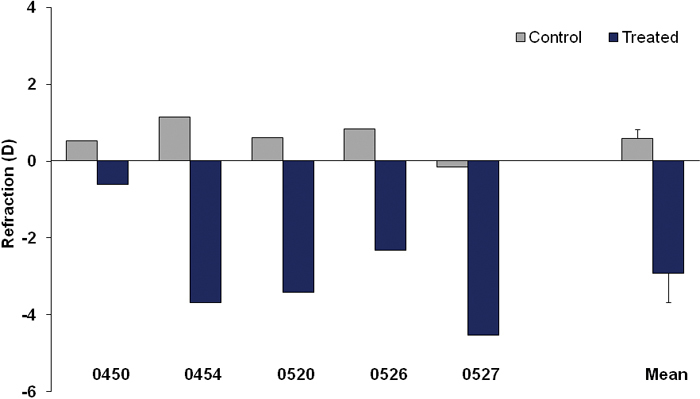
Refractive measures for the treated and control eyes after 4 days of –5 D lens wear with the group mean (± SEM). Values are corrected for the small-eye artifact by subtracting 4 D [25,27].
Five protein spots were significantly differentially expressed (downregulated) in the five pairs of eyes during myopia development. Figure 2 shows the relative expression values of each differentially expressed spot for treated and control eyes for each animal. All of the differentially expressed spots were slightly downregulated in the treated eye by 1.2 to 1.7 fold. Interestingly, the amount of downregulation appeared to be related to the amount of myopia that developed; the animal (0450) that developed the least myopia also had the smallest differences in expression. However, there was not a significant correlation. Figure 3 shows a representative 2D gel indicating the location of the five downregulated protein spots.
Figure 2.
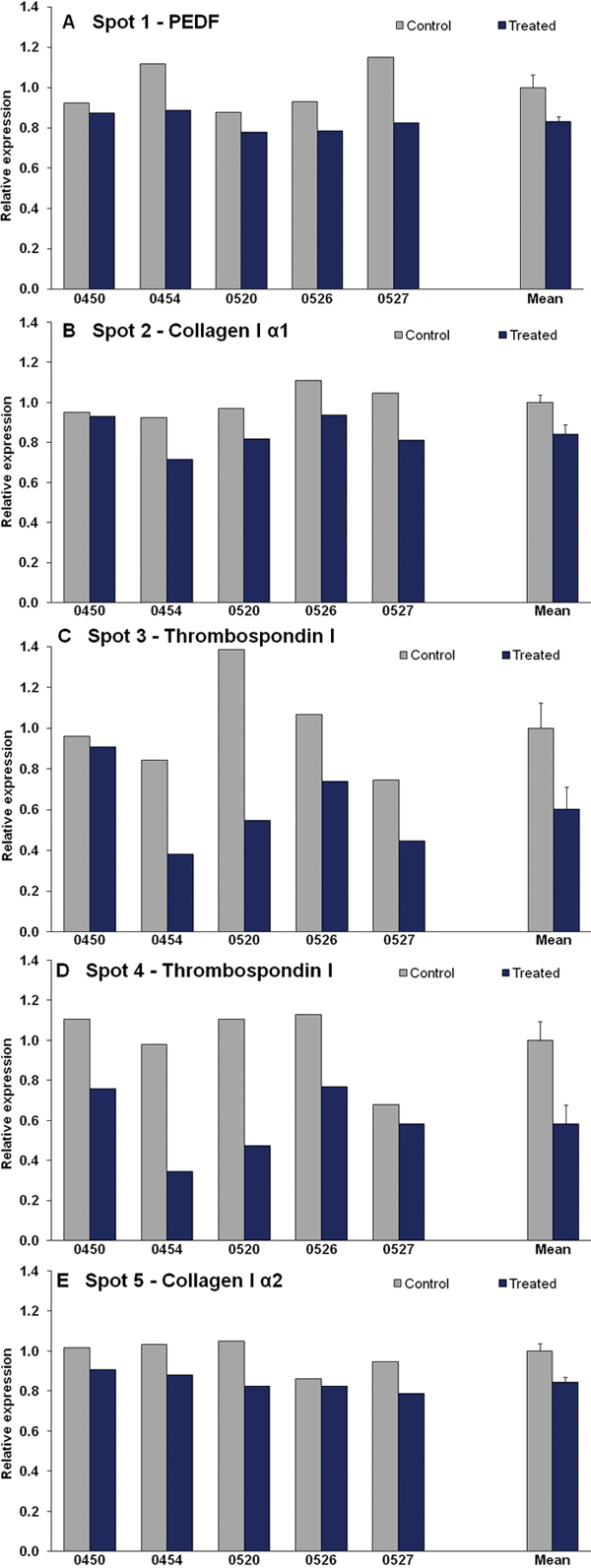
Relative expression values of each differentially expressed spot for treated and control eyes for each animal and the group mean (± SEM), normalized to the mean control expression level.
Figure 3.
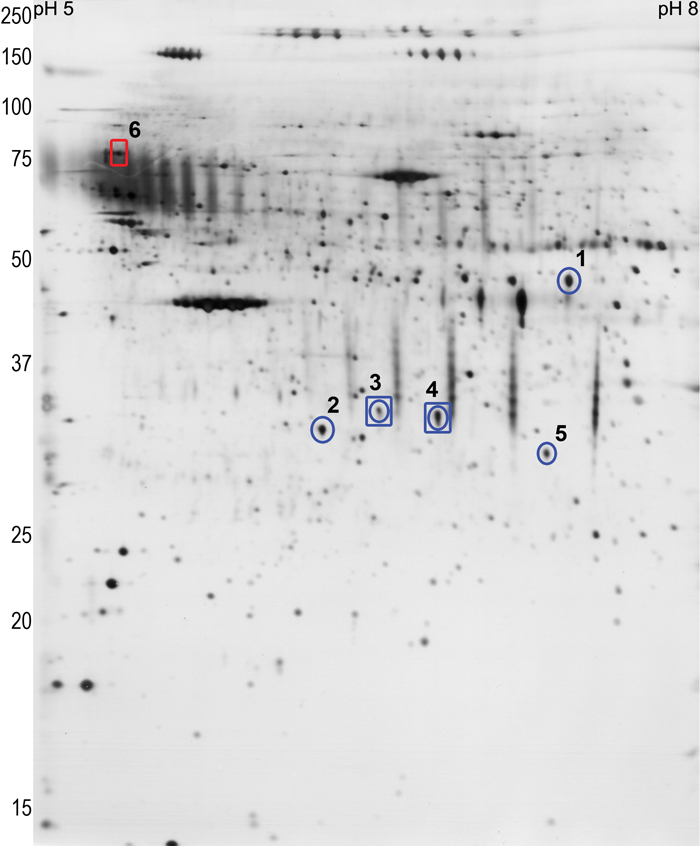
A representative silver stained scleral protein profile (2D gel) within the pH 5–8 (pI) and 15–250 kDa (MW) range to show the location of the spots found to change. Blue circles indicate the five proteins that were significantly downregulated in the treated eye of all five pairs of eyes. During recovery, two of these spots were still downregulated (blue squares) and an additional spot was upregulated (red square).
All five of the protein spots were definitively identified by mass spectrometry: spot 1 is pigment epithelium-derived factor (PEDF); spot 2 is procollagen I α1; spots 3 and 4 are thrombospondin I; spot 5 is procollagen I α2. Table 1 shows the relative change in expression level and peptide coverage for each of the proteins identified. Figure 4 is an example of an LC-MSMS spectrograph for one of the peptides from spot 1.
Table 1. Differentially expressed proteins identified from the scleral 2D gels.
| Spot # |
Protein identity |
Accession # |
Expression level (as % of control) |
Peptides matched |
|
|---|---|---|---|---|---|
| –5D lens | Recovery | ||||
| 1 |
PEDF |
P36955 |
83±2 (p=0.035) |
LAAAVSNFGYDLYR
IKSSFVAPLEK
*TVQAVLTIPK
LQSLFDSPDFSK
DTDTGALLFIGK |
|
| 2 |
Procollagen Iα1 |
P02452 |
84±5 (p=0.020) |
SLSQQIENIR
ALLLKGSNEIEIR |
|
| 3 |
Thrombospondin I |
P07996 |
60±11 (p=0.027) |
51±13 (p=0.015) |
SITLFVQEDR
FVFGTTPEDILR
TIVTTLQDSIR |
| 4 |
Thrombospondin I |
P07996 |
58±9 (p=0.008) |
55±9 (p=0.006) |
SITLFVQEDR
FVFGTTPEDILR
TIVTTLQDSIR |
| 5 |
Procollagen Iα2 |
P08123 |
84±2 (p=0.018) |
GETGPSGPVGPAGAVGPR
SLNNQIETLLTPEGSRK
EMATQLAFMR
AVILQGSNDVELVAEGNSR
TIIEYKTNKPSR |
|
| 6 | GRP 78 | P11021 | 129±7 (p=0.019) | ITPSYVAFTPEGER NQLTSNPENTVFDAK TWNDPSVQQDIK DAGTIAGLNVMR LTPEEIER VTHAVVTVPAYFNDAQR IINEPTAAAIAYGLDKR IEIESFYEGEDFSETLT AKFEELNMDLFR DNHLLGTFDLTGIPPAPR | |
Figure 4.
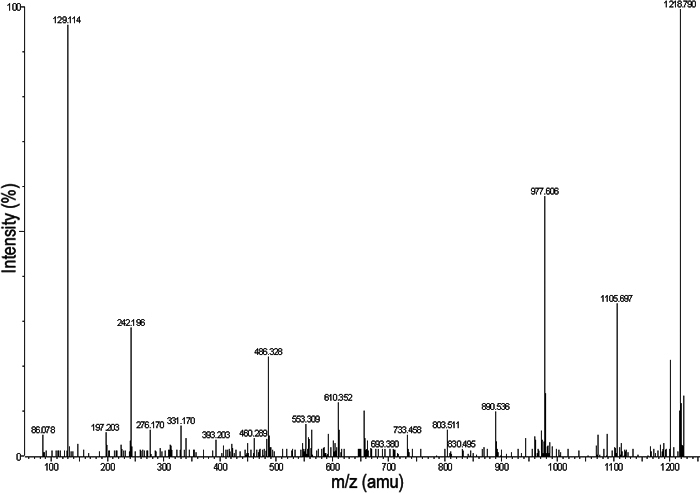
Representative spectrum from LC-MSMS of the peptide identified as SLSQQIENIR. The spectrum shows that data are of sufficiently high quality for definitive identification of the corresponding peptides.
Differences during recovery
After 11–13 days of lens treatment, four of the five animals in the recovery group had fully compensated for the –5 D lens. As shown in Figure 5, one animal (0570) developed less myopia. After 4 days of recovery, varying amounts of refractive recovery had occurred, as in previous studies [8,10,23].
Figure 5.
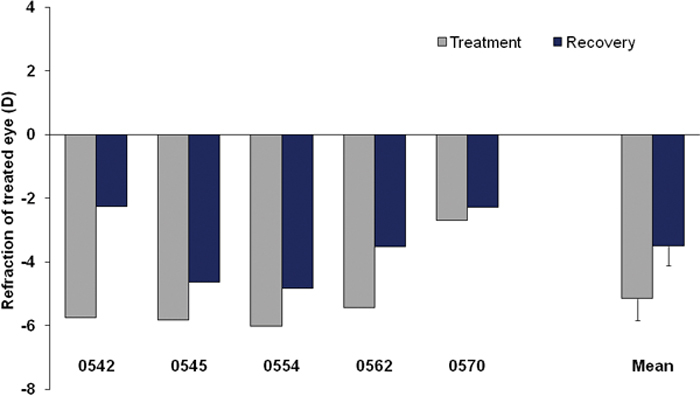
Refractive values for the treated eyes after 11–13 days of –5 D lens wear and after 4 days of recovery for each of the animals and the group mean (± SEM). Values are corrected for the small-eye artifact [25,27]. Control eyes (not shown) appeared unaffected by the treatment.
Comparison of the recovering and control eye gels from the five animals showed that three of the protein spots (the two procollagen I spots and PEDF) that were downregulated during lens compensation were not significantly different in the recovering versus control eyes. The two thrombospondin I spots remained lower in abundance in the recovering eyes (indicated by the blue squares in Figure 3). Levels of a sixth spot (red square in Figure 3), were upregulated in the recovering eyes. Figure 6 shows the relative protein levels in the recovering and control eyes for thrombospondin I (spots 3 & 4) and for the upregulated spot 6. As illustrated in Table 1, this protein spot has been identified as 78 kDa glucose-regulated protein (GRP 78; a member of the heat shock protein 70 family). Note that the animal (0570) that showed the least recovery did not show an upregulation in spot 6. This was the only case when differential expression was not seen in all five animals.
Figure 6.
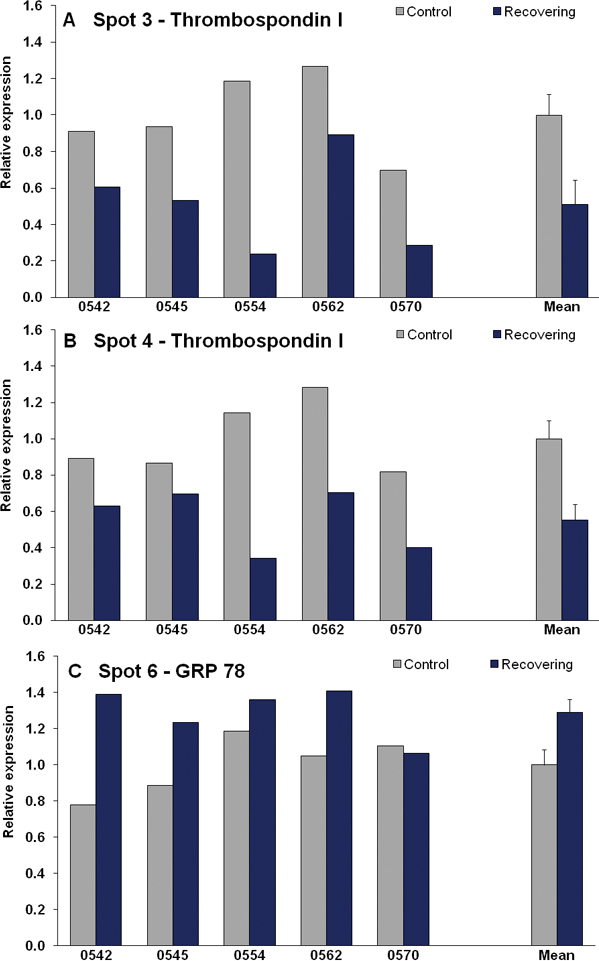
Relative expression values of each differentially expressed spot for recovering and control eyes for each animal and the group mean (± SEM), normalized to the mean control expression level.
Discussion
The results have allowed us to make several conclusions about both the proteomic methodology and its utility in the “neutral” approach to detecting protein changes in tree shrew fibrous sclera. First, as expected, the changes in protein levels were small in magnitude. This was expected because a –5 D lens is a relatively mild stimulus, well within the physiologically normal range of the eye, which merely shifts the focal plane in the hyperopic direction. It is not a disruptive stimulus that would be expected to cause on/off or massive up- or downregulation of proteins, and has previously been found to produce similar (<2 fold) changes in mRNA levels [23].
Second, the results are consistent across animals. During lens compensation specific proteins were downregulated in all five animals, and there was less downregulation in the animal that developed the least myopia. A similar parallel was found during recovery. This raises confidence in the precision of the method. Third, we have been able to identify the tree shrew proteins whose levels changed. This initially was a concern because there are currently few tree shrew entries in sequence databases. However, the tree shrew proteins have sufficient homology that we have been able to identify, unambiguously, all the proteins that we have submitted to the UAB Mass Spectrometry and Proteomics Core Facility. Fourth, we confirmed that we can find changes in proteins that were found to change in previous studies, such as type I collagen, which studies have found to be less abundant during myopia development in tree shrews [12,22]. Fifth, we have also found downregulation of a protein, thrombospondin I, that was not previously known to change in the sclera and that adds detail to our understanding of the chain of signals that regulates scleral creep rate. A function of thrombospondin I is to activate TGF-β [28]. The downregulation of thrombospondin I is consistent with the reports of lowered levels of TGF-β in tree shrew sclera [29] and the role of TGF-β which stimulates, via the SMAD signal transduction pathway, production of type I collagen [30]. Thus, the lower thrombospondin I level may reduce the levels of TGF-β which in turn may account for the lower levels of type I procollagen that we found here and that have been reported previously [14].
Finally, we have found differential changes in additional proteins, PEDF and GRP 78 that, with thrombospondin I, affirm the importance of using this proteomic approach – we would not have suspected that these proteins would change in the sclera and would not have examined them if we had restricted ourselves to using the “candidate protein” approach. GRP 78 is a chaperone, facilitating multimeric protein assembly in the endoplasmic reticulum that recognizes and binds to malfolded or denatured proteins, such as type I procollagen [31,32]. PEDF is known to have important signaling characteristics. It has high affinity to various extracellular matrix components such as glycosaminoglycans and collagen, and is a possible modulator of the integrin-collagen interaction [33,34]. Its expression is increased by all-trans-retinoic acid [35] and is a substrate for MMP-2 and MMP-9 [36], and thus could be involved in the control of scleral extensibility.
It is of interest that the number of proteins found to change is perhaps smaller than might have been expected, and that the proteins we have identified differ from the ones identified with 2DGE in the chick sclera [37]. For instance, it could be expected to see the previously described MMP and TIMP changes reproduced in this study; however, there are some limitations to the methodology. For example, the active form of MMP-2 has an isoelectric point (pI) of 5.0 and so cannot be resolved by the employed pH 5–8 IPG gradient. The latent form of MMP-2 does have a slightly higher pI and so should be present, but with a molecular weight of ~74 kDa it would be located just below spot 6 within the saturated region with its signal inundated (Figure 3). TIMP-3 would also not be displayed as both active and latent forms of the protein have isoelectric points greater than 9.0 and so neither can be resolved by this IPG gradient. A final example is MT1-MMP which should have both active (MW ~54 kDa; pI 5.8) and latent (MW ~66 kDa; pI 7.6) forms displayed on the gel. However, the 2DGE methodology cannot generally resolve membrane-bound proteins, such as MT1-MMP.
Further, in this study the proteins from the treated and control eyes were in separate gels, which were aligned and compared using software. The inherent variability across the gels may have limited the ability to detect biologic variation. Hence the results of the present study most likely provide a conservative survey of the number of proteins whose levels change during negative-lens compensation and recovery. Use of the Difference Gel Electrophoresis (DIGE) method in which proteins from a treated eye, control eye and a standard are each labeled with a different spectrally resolvable, co-localizing fluorescent dye and are all displayed on the same gel may, by reducing inter-gel variability, provide improved ability to detect biologic variation. Preliminary data using DIGE suggests that a larger group of subtly differentially expressed proteins await discovery.
Acknowledgments
Supported by the EyeSight Foundation of Alabama and by National Eye Institute Grants RO1 EY05922 and P30 EY03039 (CORE). The authors have no commercial interest in the subject matter of the manuscript. We thank Dr. John T. Siegwart, Jr. and Mr. Joel Robertson for assistance in the preparation of the animals used in this study. Data in this paper were presented at the Association for Research in Vision and Ophthalmology (2006) E-abstract 1148, and at the 11th International Myopia Conference, Ophthalmic and Physiologic Optics (2006) 26 (suppl 1), 52.
References
- 1.Cartmill M. Rethinking primate origins. Science. 1974;184:436–43. doi: 10.1126/science.184.4135.436. [DOI] [PubMed] [Google Scholar]
- 2.Luckett WP. Comparative biology and evolutionary relationships of tree shrews. New York: Plenum Press; 1980. [Google Scholar]
- 3.Petry HM, Fox R, Casagrande VA. Spatial contrast sensitivity of the tree shrew. Vision Res. 1984;24:1037–42. doi: 10.1016/0042-6989(84)90080-4. [DOI] [PubMed] [Google Scholar]
- 4.McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri). Vision Res. 1992;32:843–52. doi: 10.1016/0042-6989(92)90027-g. [DOI] [PubMed] [Google Scholar]
- 5.Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Res. 1992;32:833–42. doi: 10.1016/0042-6989(92)90026-f. [DOI] [PubMed] [Google Scholar]
- 6.Norton TT, Siegwart JT, Jr, Amedo AO. Effectiveness of hyperopic defocus, minimal defocus, or myopic defocus in competition with a myopiagenic stimulus in tree shrew eyes. Invest Ophthalmol Vis Sci. 2006;47:4687–99. doi: 10.1167/iovs.05-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegwart JT, Jr, Norton TT. Goggles for controlling the visual environment of small animals. Lab Anim Sci. 1994;44:292–4. [PubMed] [Google Scholar]
- 8.Siegwart JT, Jr, Norton TT. Regulation of the mechanical properties of tree shrew sclera by the visual environment. Vision Res. 1999;39:387–407. doi: 10.1016/s0042-6989(98)00150-3. [DOI] [PubMed] [Google Scholar]
- 9.Norton TT, Siegwart JT., Jr Animal models of emmetropization: Matching axial length to the focal plane. J Am Optom Assoc. 1995;66:405–14. [PubMed] [Google Scholar]
- 10.Moring AG, Baker JR, Norton TT. Modulation of glycosaminoglycan levels in tree shrew sclera during lens-induced myopia development and recovery. Invest Ophthalmol Vis Sci. 2007;48:2947–56. doi: 10.1167/iovs.06-0906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amedo AO, Siegwart JT Jr, Norton TT. The effect of age on compensation to a minus lens and recovery in tree shrews. ARVO Annual Meeting; 2007 May 6–10; Fort Lauderdale (FL). [Google Scholar]
- 12.Norton TT, Rada JA. Reduced extracellular matrix in mammalian sclera with induced myopia. Vision Res. 1995;35:1271–81. doi: 10.1016/0042-6989(94)00243-f. [DOI] [PubMed] [Google Scholar]
- 13.Gentle A, McBrien NA. Modulation of scleral DNA synthesis in development of and recovery from induced axial myopia in the tree shrew. Exp Eye Res. 1999;68:155–63. doi: 10.1006/exer.1998.0587. [DOI] [PubMed] [Google Scholar]
- 14.McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000;41:3713–9. [PubMed] [Google Scholar]
- 15.Gentle A, McBrien NA. Retinoscleral control of scleral remodelling in refractive development: A role for endogenous FGF-2? Cytokine. 2002;18:344–8. doi: 10.1006/cyto.2002.1046. [DOI] [PubMed] [Google Scholar]
- 16.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res. 2003;22:307–38. doi: 10.1016/s1350-9462(02)00063-0. [DOI] [PubMed] [Google Scholar]
- 17.Rada JA, Shelton S, Norton TT. The sclera and myopia. Exp Eye Res. 2006;82:185–200. doi: 10.1016/j.exer.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Norton TT, Metlapally R, Young TL. Myopia. In: Garner A, Klintworth GK, editors. The pathobiology of ocular disease. New York: Taylor & Francis; 2007; in press [Google Scholar]
- 19.Guggenheim JA, McBrien NA. Form-deprivation myopia induces activation of scleral matrix metalloproteinase-2 in tree shrew. Invest Ophthalmol Vis Sci. 1996;37:1380–95. [PubMed] [Google Scholar]
- 20.Gentle A, Martin JE, McBrien NA. Differential expression of collagen types I, III and V in the sclera of myopic eyes: A precursor to fibril diameter changes? ARVO Annual Meeting; 2002 May 5–10; Fort Lauderdale (FL). [Google Scholar]
- 21.Siegwart JT, Jr, Norton TT. The time course of changes in mRNA levels in tree shrew sclera during induced myopia and recovery. Invest Ophthalmol Vis Sci. 2002;43:2067–75. [PMC free article] [PubMed] [Google Scholar]
- 22.Gentle A, Liu Y, Martin JE, Conti GL, McBrien NA. Collagen gene expression and the altered accumulation of scleral collagen during the development of high myopia. J Biol Chem. 2003;278:16587–94. doi: 10.1074/jbc.M300970200. [DOI] [PubMed] [Google Scholar]
- 23.Siegwart JT, Jr, Norton TT. Selective regulation of MMP and TIMP mRNA levels in tree shrew sclera during minus lens compensation and recovery. Invest Ophthalmol Vis Sci. 2005;46:3484–92. doi: 10.1167/iovs.05-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schippert R, Brand C, Schaeffel F, Feldkaemper MP. Changes in scleral MMP-2, TIMP-2 and TGFβ-2 mRNA expression after imposed myopic and hyperopic defocus in chickens. Exp Eye Res. 2006;82:710–9. doi: 10.1016/j.exer.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Norton TT, Wu WW, Siegwart JT., Jr Refractive state of tree shrew eyes measured with cortical visual evoked potentials. Optom Vis Sci. 2003;80:623–31. doi: 10.1097/00006324-200309000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smejkal GB, Li C, Robinson MH, Lazarev AV, Lawrence NP, Chernokalskaya E. Simultaneous reduction and alkylation of protein disulfides in a centrifugal ultrafiltration device prior to two-dimensional gel electrophoresis. J Proteome Res. 2006;5:983–7. doi: 10.1021/pr050439w. [DOI] [PubMed] [Google Scholar]
- 27.Glickstein M, Millodot M. Retinoscopy and eye size. Science. 1970;168:605–6. doi: 10.1126/science.168.3931.605. [DOI] [PubMed] [Google Scholar]
- 28.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-β by thrombospondin-1: Mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 29.Jobling AI, Nguyen M, Gentle A, McBrien NA. Isoform-specific changes in scleral Transforming Growth Factor-β expression and the regulation of collagen synthesis during myopia progression. J Biol Chem. 2004;279:18121–6. doi: 10.1074/jbc.M400381200. [DOI] [PubMed] [Google Scholar]
- 30.Overall CM, Wrana JL, Sodek J. Independent regulation of collagenase, 72kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor β. J Biol Chem. 1989;264:1860–9. [PubMed] [Google Scholar]
- 31.Lee AS. Mammalian stress response: Induction of the glucose-regulated protein family. Curr Opin Cell Biol. 1992;4:267–73. doi: 10.1016/0955-0674(92)90042-b. [DOI] [PubMed] [Google Scholar]
- 32.Chessler SD, Byers PH. BiP binds type I procollagen pro α chains with mutations in the carboxyl-terminal propeptide synthesized by cells from patients with osteogenesis imperfecta. J Biol Chem. 1993;268:18226–33. [PubMed] [Google Scholar]
- 33.Becerra SP. Focus on molecules: Pigment epithelium-derived factor (PEDF). Exp Eye Res. 2006;82:739–40. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Ek ETH, Dass CR, Choong PFM. PEDF: A potential molecular therapeutic target with multiple anti-cancer activities. Trends Mol Med. 2006;12:497–502. doi: 10.1016/j.molmed.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 35.Tombran-Tink J, Lara N, Apricio SE, Potluri P, Gee S, Ma JX, Chader G, Barnstable CJ. Retinoic acid and dexamethasone regulate the expression of PEDF in retinal and endothelial cells. Exp Eye Res. 2004;78:945–55. doi: 10.1016/j.exer.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 36.Notari L, Miller A, Martínez A, Amaral J, Ju M, Robinson G, Smith LE, Becerra SP. Pigment Epithelium-Derived Factor is a substrate for matrix metalloproteinase type 2 and type 9: Implications for downregulation in hypoxia. Invest Ophthalmol Vis Sci. 2005;46:2736–47. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 37.Bertrand E, Fritsch C, Diether S, Lambrou G, Müller D, Schaeffel F, Schindler P, Schmid KL, van Oostrum J, Voshol H. Identification of Apolipoprotein A-I as a “STOP” signal for myopia. Mol Cell Proteomics. 2006;5:2158–66. doi: 10.1074/mcp.M600073-MCP200. [DOI] [PubMed] [Google Scholar]


