Abstract
Throughout life, new neurons arise from the ventricular zone of the adult songbird brain and are recruited to the song control nucleus HVC, from which they extend projections to its target, nucleus robustus of the arcopallium (RA). This process of ongoing parenchymal neuronal addition and circuit integration is both triggered and modulated by seasonal surges in systemic testosterone. Brain aromatase converts circulating testosterone to estradiol, so that HVC is concurrently exposed to both androgenic and estrogenic stimulation. These two signals cooperate to trigger HVC endothelial cell division and angiogenesis, by inducing the regionally-restricted expression of vascular endothelial growth factor (VEGF), its matrix-releasing protease MMP9, and its endothelial receptor VEGFR2. The expanded HVC microvascular network then secretes the neurotrophic factor BDNF, which in turn supports the recruitment of newly generated neurons. This process is striking for its spatial restriction and hence functional specificity. While androgen receptors are broadly expressed by the nuclei of the vocal control system, estrogen receptor (ERα) expression is largely restricted to HVC and its adjacent mediocaudal neopallium. The geographic overlap of these receptor phenotypes in HVC provides the basis for a regionally-defined set of paracrine interactions between the vascular bed and neuronal progenitor pool, that both characterize and distinguish this nucleus. These interactions culminate in the focal attraction of new neurons to the adult HVC, the integration of those neurons into the extant vocal control circuits, and ultimately the acquisition and elaboration of song.
Keywords: androgen, estrogen, testosterone, angiogenesis, neurogenesis, adult neurogenesis, VEGF, BDNF, neuroethology, songbird
Introduction
In the adult songbird forebrain, testosterone induces extensive angiogenesis, gliogenesis, and neuronal addition within the neopallial Higher Vocal Center (HVC) (Goldman and Nottebohm, 1983). The HVC, which is situated in the dorsomedial aspect of the caudal forebrain, is the central vocal control nucleus of oscine songbirds; anatomically, it couples the two pathways that comprise the song system, the anterior forebrain pathway and the motor pathway (Figure 1). The anterior pathway is responsible for song learning; it connects HVC with area X, the dorsolateral anterior thalamic nucleus (DLM), the lateral magnocellular nucleus of the anterior nidopallium (LMAN), and the robust nucleus of the arcopallium (RA) (Bottjer et al., 1984, Bottjer et al., 1989, Sohrabji et al., 1990, Scharff and Nottebohm, 1991, Vates and Nottebohm, 1995, Vates et al., 1997, Brainard and Doupe, 2000, Andalman and Fee, 2009). In contrast, the motor pathway, controls song production; it connects the nucleus interfacialis (NIf) to the HVC, and thence to RA, followed by the tracheosyringeal portion of the XIIth cranial nerve (nXIIts), and its target vocal organ, the syrinx (Nottebohm et al., 1982, Okuhata and Saito, 1987, Vu et al., 1994, Nottebohm, 2005).
Figure 1. Schematic diagram of the song system and distribution of steroid hormone receptors and aromatase in adult songbird brain.
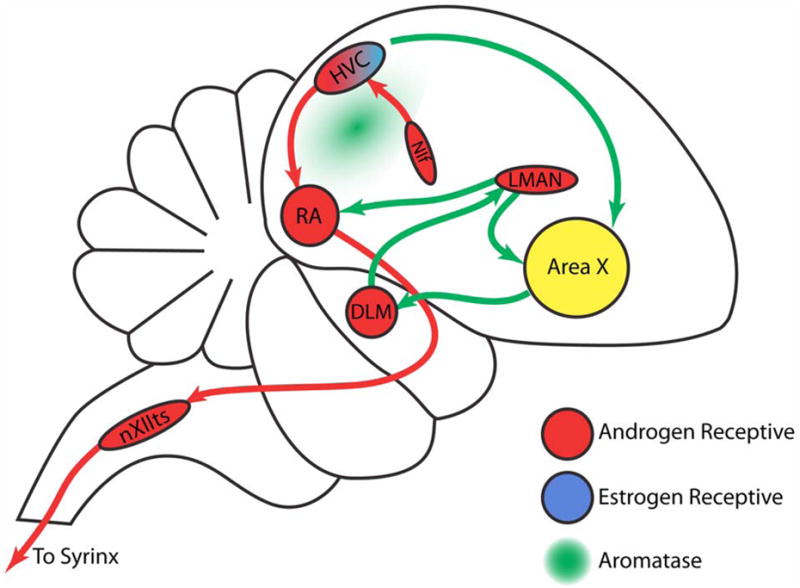
Red arrows depict projections within the motor pathway; green arrows illustrate connections of the anterior forebrain pathway. The nuclei are colored to indicate their expression of aromatase (green) or gonadal hormone receptors (red, androgen receptor; blue, estrogen receptor).
Abbreviations: DLM, medial dorsolateral nucleus of the thalamus; HVC, higher vocal center; LMAN, lateral magnocellular nucleus of the anterior nidopallium; NIF, nucleus interfacialis of the nidopallium; nXIIts, tracheosyringealis portion of the twelfth cranial nerve nucleus; RA, robust nucleus of the arcopallium.
Figure adapted from (Gil and Gahr, 2002).
Singing is a male-specific behavior in many oscine songbirds, including both the canary (Serinus canaria) and zebra finch (Taeniopygia guttata). During the breeding season, males sing to attract females, which sing rarely if at all. This marked difference in singing behavior reflects the profound sexual dimorphism of the song control nuclei, which are substantially larger in males than females (Nottebohm and Arnold, 1976). Yet as long ago as the 1930s, it was observed that female canaries, once given testosterone, could produce male-like songs (Leonard, 1939, Herrick and Harris, 1957). Nottebohm and colleagues investigated the neuroanatomic basis of this observation, and found that the forebrain nuclei HVC and RA were roughly 90% and 50% larger, respectively, in testosterone-treated female canaries than in their untreated controls (Nottebohm, 1980). This gross morphological transformation reflected profound changes at the cellular level. New dendritic growth and synapse formation resulted in a substantial expansion of both the HVC and RA neuropil (DeVoogd and Nottebohm, 1981a, DeVoogd and Nottebohm, 1981b, DeVoogd et al., 1985, Canady et al., 1988), while new vascular endothelial cells and glia supplemented their cellular complements (Goldman and Nottebohm, 1983).
Besides the testosterone-stimulated addition of new vascular and glial cells to HVC, new neurons were also added, in a process of ongoing, gonadal steroid-modulated adult neurogenesis (Goldman and Nottebohm, 1983, Goldman, 1998). In brief, new neurons are derived from radial cells residing in the ventricular ependyma lining the lateral ventricle(Goldman et al., 1996b); these cells divide to generate new neurons in several geographically discrete regions of the lateral ventricular wall (Alvarez-Buylla et al., 1990, Alvarez-Buylla and Kirn, 1997). The young neuroblasts leave the ventricular wall and migrate along fibers extending from the radial cells to their destinations, which include not only HVC, but a number of other forebrain targets (Alvarez-Buylla and Nottebohm, 1988, Alvarez-Buylla et al., 1990). Though a variable fraction of these newborn cells die, large numbers nonetheless survive to maturity. In HVC, these new neurons receive synaptic inputs (Goldman and Nottebohm, 1983, Burd and Nottebohm, 1985), and become both anatomically and functionally incorporated into the song control system (Paton and Nottebohm, 1984). The newly integrated neurons send axonal projections to their distant target RA, resulting in a seasonal reconstruction of the HVC to RA efferent pathway (Kirn et al., 1991). Although the mitogenesis of new neurons is not affected by testosterone, the survival and incorporation of these newly generated neurons is mediated by testosterone and its metabolites, in particular 17β-estradiol (Rasika et al., 1994, Hidalgo et al., 1995). This process of androgenic modulation of neuronal recruitment is enabled by the paracrine interactions of neuronal, glial and vascular cells in HVC, which result in the local production of vascular endothelial growth factor (VEGF). The resultant burst of VEGF-mediated angiogenesis is followed by the HVC microvascular production of brain-derived neurotrophic factor (BDNF), which in turn directs neuronal recruitment and survival (Rasika et al., 1999, Louissaint et al., 2002, Goldman and Chen, 2011) (Figure 2)
Figure 2. Schematic overview of gonadal hormone-associated angiogenesis and neuronal recruitment in the adult songbird HVC.
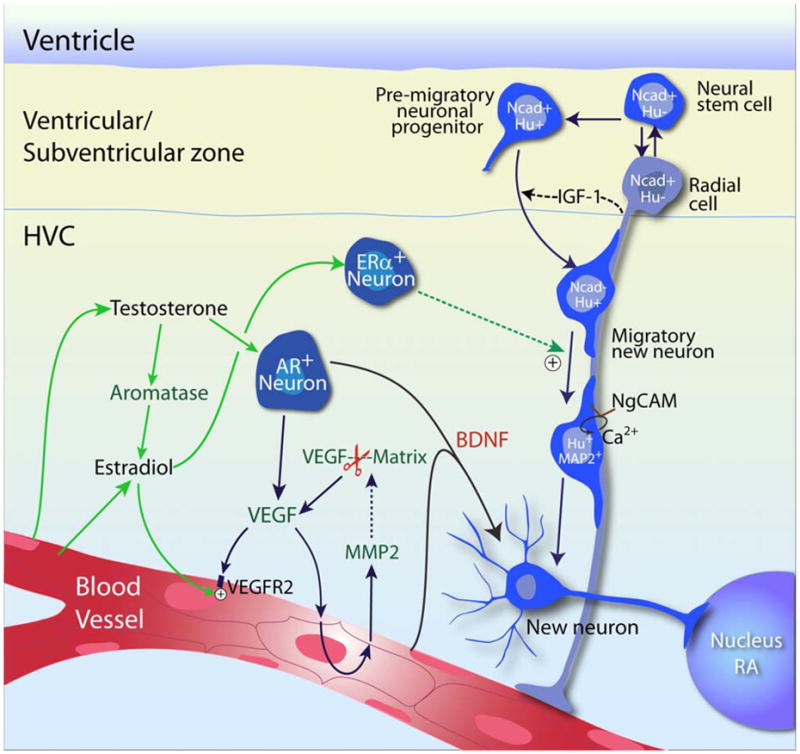
Testosterone-induced neuronal recruitment to the adult songbird vocal control center, HVC, requires the androgenic induction of VEGF, followed by VEGF-triggered MMP2 expression and activation, which further augments VEGF concentration by releasing matrix-bound VEGF and initiates angiogenesis. The expanded microvascular bed acts as a source of BDNF, which supports the immigration of new neurons derived from neuronal progenitors in the overlying ventricular zone. This figure is derived and modified from (Goldman and Chen, 2011). AR, androgen receptor; ER, estrogen receptor; Ncad, N-cadherin; Hu, Hu protein; IGF-1, insulin growth factor 1.
Gonadal steroid receptivity of the songbird brain
Canonical signaling via gonadal steroids occurs via their specific binding to cognate cytosolic receptors, which subsequently translocate to the nucleus and activate steroid-dependent transcription. As such, the testosterone sensitivity of the songbird brain reflects the distribution not only of androgen receptors (AR), but also of the testosterone-aromatizing enzyme aromatase and its product 17ß-estradiol, as well as that of estrogen receptors (ERα and ERβ) (Gahr and Metzdorf, 1997). Accordingly, soon after the discovery of sexual dimorphism in adult songbirds (Nottebohm and Arnold, 1976), the vocal control nuclei were found to express receptors for androgens or estrogens, or in the case of HVC, both (Arnold et al., 1976, Arnold and Saltiel, 1979, Brenowitz and Arnold, 1989, 1992). In addition, brain aromatase was identified and mapped, and its role in estrogen production and receptor activation elucidated, highlighting the importance of estrogen receptors to the masculinizing effects of androgens (Walters and Harding, 1988, Gahr et al., 1993, Dittrich et al., 1999, Fusani et al., 2003, Fusani and Gahr, 2006).
Androgen receptor expression has been intensively investigated in the songbird brain, including in zebra finches (Balthazart et al., 1992, Gahr and Metzdorf, 1997), canaries (Balthazart et al., 1992, Nastiuk and Clayton, 1995, Gahr et al., 1996, Gahr and Metzdorf, 1997), sparrows (Smith et al., 1996, Soma et al., 1999), and starlings (Bernard et al., 1999). These studies revealed that a number of telencephalic song control nuclei are highly androgen-sensitive; these include HVC, LMAN and RA, among others (Gahr and Metzdorf, 1997, Metzdorf et al., 1999, Gahr, 2004) (Figure 1).
In contrast to the widespread forebrain expression of androgen receptors, ERα is expressed primarily in the HVC and its adjacent mediocaudal neopallium (MCN), within which ERα expression extends medially from HVC along the ventricular border of the caudal neostriatum (Gahr et al., 1993, Hidalgo et al., 1995). The distribution of ERβ has been less well-studied, but it does not appear to be selectively expressed by any of the vocal areas (Bernard et al., 1999). Of note, the distribution of ERα varies among oscine songbirds: whereas ERα is expressed throughout HVC in the canary, it appears only in the ventromedial HVC in zebra finches (Gahr et al., 1993, Gahr and Metzdorf, 1997), an observation that may contribute to the marked differences between these species in their patterns of adult neuronal recruitment and song learning.
Importantly, ERα and AR do not colocalize to individual neurons in the canary HVC (Gahr, 1990). Thus, distinct pools of androgen receptive and estrogen receptive neurons overlap and intermingle in the adult HVC, almost uniquely so within the songbird neopallium. Indeed, neither AR nor ER are expressed in nonlimbic forebrain regions of quails and doves, representative non-passerine species (Balthazart et al., 1998, Metzdorf et al., 1999, Voigt et al., 2009). The adult songbird HVC, and that of the canary in particular, is characterized then by a co-association of androgen and estrogen receptive neurons unusual in the neopallium, that allows the close apposition and hence cooperative interaction of these distinct neuronal pools.
Aromatase as modulator of estradiol availability in the songbird brain
In light of the androgen dependence of both song behavior and its neuroanatomic substrate, the high concentration of estrogen receptors in the male songbird brain once seemed counterintuitive. Schlinger and Arnold (1991) addressed this issue by asking whether brain aromatase might generate sufficient estradiol to engage ERα̃ by locally-generated estradiol. Studying male zebra finches, they found that forebrain aromatase activity was indeed so high that the male brain appeared to be the principal source of systemic estradiol (Schlinger and Arnold, 1991). In these finches, a number of regions were found to contain active aromatase, including the hypothalamic nuclei, limbic areas, and the major serially connected nuclei of the song system (X, LMAN, HVC and RA) (Vockel et al., 1990). These results contrasted to studies in non-songbirds, including doves (Hutchison and Steimer, 1986), Japanese quail (Schlinger and Callard, 1987, Schumacher and Balthazart, 1987), and Wilson's phalarope (Schlinger et al., 1989), in which aromatase activity was high in diencephalic and limbic brain regions, but otherwise low or absent. Thus, high levels of neopallial aromatase activity appear to be a characteristic feature of oscine songbirds, with enriched expression in both vocal and auditory neural circuits (Shen et al., 1994, Shen et al., 1995, Schlinger, 1997, Metzdorf et al., 1999, Saldanha et al., 2000, Silverin et al., 2000). Of note though, HVC itself expresses little aromatase, which is instead more abundant in the adjacent parenchyma (Metzdorf et al., 1999, Saldanha et al., 2000).
The effects of testosterone on the songbird brain may be both modulated and delimited by its aromatization to estradiol, but testosterone may also regulate steroid sensitivity. Although there are no qualitative differences between the vocal control areas of males and females in their respective distributions of either the sex hormone receptors or testosterone-metabolizing enzymes (Perlman et al., 2003, Gahr, 2007), seasonal changes in both androgen and estrogen receptor expression, as well as in aromatase expression, have been observed in HVC (Gahr and Metzdorf, 1997, Soma et al., 1999, Fusani et al., 2000). These changes appear to serve as permissive checkpoints that define the extent to which seasonal fluctuations in gonadal steroids may effect seasonally-modulated changes in cellular composition and architecture (Nottebohm et al., 1986, Nottebohm et al., 1987, Smith et al., 1997). These changes in steroid responsiveness may be subject to both positive and negative feedback based on gonadal steroid levels; both androgen receptor and aromatase levels may themselves be modulated by ambient gonadal steroids (Nastiuk and Clayton, 1995, Fusani et al., 2001, Kim et al., 2004).
Estrogenic modulation of neuronal addition to the adult HVC
The most actively neurogenic regions of the canary neopallium, the ventricular wall abutting HVC and its adjacent mediocaudal neopallium (MCN), overlay a layer of estrogen receptive cells lying directly subjacent to the subependyma (Hidalgo et al., 1995). Intrigued by this subventricular layer of ERα+ cells in the HVC – which essentially comprise a gatekeeper through which newly generated cells must pass to enter the brain parenchyma of HVC and MCN – as well as by the finding that aromatase can locally convert testosterone into estradiol, Hidalgo and colleagues (1995) asked whether estradiol signaling was necessary for neuronal recruitment to HVC. To that end, they assessed the effect of ovariectomy on HVC neuronal production in adult female canaries. They found that the addition of new neurons to the adult songbird HVC was indeed inhibited by ovariectomy, and rescued by estradiol replacement. Importantly though, the new neurons themselves never expressed estrogen receptor immunoreactivity; the ERα+ cell population instead comprised a stable pool of resident subventricular neurons. These observations suggested the possibility that neuronal addition to HVC might be potentiated by the estrogen-stimulated release of neurotrophic agents by the ERα subventricular neurons (Hidalgo et al., 1995). Subsequent studies identified IGF1 as a powerful survival signal for the newly generated neurons, and established that IGF1 was released by ventricular zone radial cells in response to estradiol (Jiang et al., 1998). These findings suggested the presence of an intercellular interaction, by which the engagement of ERα+ neurons by aromatase-generated estradiol results in the production of a paracrine signal triggering radial cell IGF1 production and release, followed by the IGF1-dependent support of newly generated neuronal migrants. Follow-up studies determined that this process was associated with an estrogen-triggered initiation of NgCAM-dependent calcium signaling by the newly generated neurons (Williams et al., 1999), and that the acquisition of this signal, and its associated increase in cytosolic calcium during migration, were necessary conditions for neuronal migration and post-migration survival (Barami et al., 1994, Goldman et al., 1996a).
Testosterone-triggered, VEGF-induced angiogenesis in the adult HVC
The initial discovery of neuronal production in the adult songbird brain was accompanied by the observation that testosterone-triggered neuronal addition to HVC was preceded by a burst of endothelial cell division and angiogenesis in HVC. To better understand the cellular basis for androgen-induced angiogenesis, Louissaint et al. (2002) assessed the relationship between testosterone treatment and microvascular expansion in the adult canary HVC, with the goal of identifying any causal relationship of angiogenesis with neuronal addition. To that end, they focused on the androgenic modulation of the endothelial mitogen VEGF, which had previously been found to be induced by both androgen and estrogen in carcinoma cells, as well as in the uterine endometrium (Charnock-Jones et al., 1993, Cullinan-Bove and Koos, 1993, Jain et al., 1998). Louissaint and colleagues observed that cultured canary HVC endothelial cells were unresponsive to testosterone, but dramatically increased their rate of division in response to VEGF, suggesting the possibility that testosterone acted by inducing local VEGF. This proved to be the case, in that in vivo, HVC VEGF mRNA and protein were both found to rise sharply in response to testosterone, several days before the onset of testosterone-induced endothelial proliferation. These findings suggested that androgen-induced angiogenesis might require VEGF signaling; accordingly, pharmacological inhibition of VEGFR2 tyrosine kinase activity indeed suppressed testosterone-induced HVC angiogenesis and microvascular expansion, and substantially so.
These observations indicated that VEGF serves as a paracrine mediator of gonadal hormone-stimulated angiogenesis. Subsequent in situ hybridization and immunolabeling established that VEGF was produced locally in response to testosterone, by androgen-responsive HVC neurons; its highest expression occurred during the first week of androgen exposure, preceding angiogenesis by 2-3 days (Louissaint et al., 2002). Importantly, besides this surge in VEGF, the HVC expression of its receptor VEGFR2 was up-regulated as well, not by testosterone, but rather by its aromatase-generated metabolite estradiol. Interestingly, this finding would seem to explain prior data that estradiol, like testosterone, can potentiate HVC endothelial mitogenesis (Hidalgo et al., 1995). Together, these observations indicated that androgen-induced angiogenesis in the adult HVC requires the coordinated induction of VEGF by testosterone, and that of its receptor VEGFR2 by aromatase-generated estradiol.
Gonadal steroid modulation of VEGF-dependent MMP activity potentiates angiogenesis
Since testosterone-induced, VEGF-mediated angiogenesis was found to precede neuronal addition to HVC, the time course and cellular mechanisms of testosterone-triggered VEGF production became a focus on interest. Paradoxically though, Louissaint and colleagues had observed that testosterone exposure induced endothelial cell proliferation, in a VEGF-dependent manner, even before significant increases in VEGF mRNA were noted. On that basis, Kim et al. (2008) asked whether VEGF might be released in HVC in a transcriptionally-independent manner, through matrix release of bound or sequestered stores of interstitial VEGF (Kim et al., 2008). To address this issue, these investigators asked whether testosterone's early actions on angiogenesis might be dependent upon androgen-stimulated matrix metalloproteinase (MMP) release. The MMPs, and specifically the gelatinases MMP2 and MMP9, have been found to potentiate angiogenesis during both development and oncogenesis, in part through mediating the release of sequestered angiogenic factors from the extracellular matrix (Bergers et al., 2000). Using in situ zymography, Kim and colleagues asked whether testosterone treatment induced gelatinase activity in HVC, and if so, by what cells and according to what time course/ They determined that testosterone stimulated MMP2 enzymatic activity in HVC, in a regionally-restricted pattern limited to HVC endothelial cells. Interestingly, when canary HVC endothelial cells were directly challenged in vitro, MMP2 secretion could be triggered by VEGF, but not by testosterone (Kim et al., 2008). These observations strongly suggested that androgen-stimulated HVC endothelial MMP2 was induced by VEGF, acting as a paracrine mediator of androgen signaling.
Testosterone-triggered, VEGF-induced gelatinase activity may promote the breakdown of the HVC interstitial matrix, and hence facilitate migration of new neurons, as well as the may ration and remodeling of the HVC neuropil. In addition though, MMP gelatinase activity act to liberate sequestered growth factors, including VEGF, from bound matrix stores. Indeed, tumor-derived MMP gelatinase activity has been reported to release matrix-bound VEGF, and to thereby initiate angiogenesis by tumor vasculature (Bergers et al., 2000, Egeblad and Werb, 2002). These observations suggested that MMP2 activity might then be a critical contributor to testosterone-associated microvascular expansion. To test this postulate, testosterone-treated canaries were injected with the potent gelatinase inhibitor SB-3CT, which indeed suppressed testosterone-induced HVC endothelial proliferation, and ultimately impaired neuronal recruitment as well (Kim et al., 2008). Taken together, these observations indicated that androgen-induced VEGF and its signaling through endothelial VEGFR2, followed by the downstream activation of endothelial MMP2 transcription and consequent proteolytic release of VEGF from the local matrix, act in a feed-forward manner to sustain angiogenesis and permit neuronal recruitment in the testosterone-treated HVC.
Endothelial BDNF regulates testosterone-associated neuronal recruitment
A transient burst of angiogenesis in the canary HVC is observed within the first week to 10 days after testosterone treatment, appearing at least two weeks before concurrently-generated new neurons are incorporated (Goldman and Nottebohm, 1983, Barami et al., 1995). On this basis, Louissaint and colleagues asked whether this androgenic induction of angiogenesis was required for neuronal addition to HVC (Louissaint et al., 2002). To that end, they delivered a systemic VEGFR2 inhibitor to suppress angiogenesis in testosterone-treated canaries, and then assessed the incidence of neuronal addition to HVC in the treated canaries. The treated brains manifested substantially diminished HVC neuronal recruitment, supporting the hypothesis that angiogenesis is a necessary prerequisite for testosterone-associated neuronal addition.
This causal relationship between angiogenesis and neuronal recruitment triggered a search for angiogenesis-associated agents able to promote neuronal recruitment. The neurotrophic factor BDNF was a strong candidate, since earlier reports had demonstrated that BDNF supported the survival and recruitment of newly generated neurons arising from both the subependymal zones of both rodents and humans (Kirschenbaum et al., 1994, Kirschenbaum and Goldman, 1995, Pincus et al., 1998), and that human brain-derived endothelial cells were able to produce BDNF at levels sufficient to support this process (Leventhal et al., 1999). Moreover, Rasika and colleagues had demonstrated that BDNF infusion substantially increased neuronal recruitment to the canary HVC, while infusion with BDNF antibodies suppressed testosterone-induced neuronal addition; these data strongly suggested that BDNF is required for androgen-induced neuronal addition to HVC (Rasika et al., 1999).
Interestingly, both BDNF and its receptor TrkB are expressed in a sexually dimorphic manner in the songbird HVC — both are expressed at higher levels in males than females (Dittrich et al., 1999, Rasika et al., 1999). TrkB's sexually dimorphic expression may simply be a product of its allelic dosage, since its gene is located on the avian Z sex chromosome, which is doubly represented in male songbirds (ZZ) relative to females (ZW) (Chen et al., 2005). In contrast, BDNF is not a Z-linked gene; its sexually dimorphic expression is more likely ascribed to gonadal hormone regulation, as its expression can be stimulated by both testosterone and estradiol (Dittrich et al., 1999, Rasika et al., 1999). Accordingly, whereas BDNF expression is decreased by castration (Alvarez-Borda et al., 2004), TrkB expression is unaffected (Wissman and Brenowitz, 2009).
On the basis of these observations, Louissaint et al (2002) asked whether BDNF was specifically generated by microvascular endothelial cells derived from the adult canary HVC. They found that BDNF production was indeed stimulated by both testosterone and estradiol in cultured HVC endothelial cells, and furthermore, that BDNF mRNA was expressed by HVC capillary endothelial cells in vivo, and was sharply upregulated in response to testosterone (Louissaint et al., 2002). Interestingly, both androgen and estrogen receptor activation appear necessary for BDNF production. Gahr and colleagues first noted that the blockade of estradiol production from testosterone, using the aromatase inhibitor fadrozole, considerably reduced HVC BDNF (Fusani et al., 2003). This observation suggested that the co-dependence of angiogenesis upon androgen-induced VEGF and estrogen-induced VEGFR2 expression, coupled with the dependence of BDNF secretion upon antecedent microvascular expansion, renders HVC BDNF production dependent upon both androgenic and estrogenic stimulation. As such, BDNF's endothelial origin, its regionally-restricted regulation by both androgens and estrogens, and its pivotal role in HVC neuronal recruitment, all suggest its central role in mediating gonadal steroid-mediated neuronal addition to the adult HVC.
Neuronal recruitment to the adult HVC requires antecedent angiogenesis
When HVC VEGF and BDNF transcription and protein levels were assessed in adult female canaries as a function of time after testosterone implantation, it was noted that whereas both VEGF and BDNF were up-regulated in response to androgen, VEGF expression significantly preceded that of BDNF: Whereas VEGF peaked one week after testosterone implant, BDNF expression was scarcely apparent at a week, and did not peak until 3 weeks (Louissaint et al., 2002). In this regard, new neurons appear maximally responsive to BDNF during a restricted time window at 14-20 days after birth, when they migrate and integrate into the HVC (Alvarez-Borda et al., 2004), highlighting the importance of timely release of BDNF from newly expanded microvasculature to HVC neuronal recruitment. Together, these findings suggested that testosterone-stimulated angiogenesis might be a necessary antecedent to BDNF-dependent neuronal addition. This postulate was subsequently confirmed by the suppression of HVC neuronal addition by administration of a VEGFR tyrosine kinase inhibitor, which not only inhibited HVC angiogenesis, but also substantially abrogated its recruitment of new neurons (Louissaint et al., 2002).
BDNF rescues VEGFR2 inhibitor-suppressed testosterone-induced song
While endothelial BDNF was found to mediate androgen-induced HVC neuronal recruitment, the contribution of that process to androgen-stimulated song was unclear. The extent of BDNF expression predicts the amount of singing in adult songbirds (Li et al., 2000), while diminished BDNF production in aromatase-inhibited birds altered their song pattern (Fusani et al., 2003). In addition, the recruitment of new neurons to HVC is positively correlated with the number of songs (Alvarez-Borda and Nottebohm, 2002), and is promoted by BDNF (Alvarez-Borda et al., 2004). Together, these studies suggested the dependence of song acquisition and performance on androgen exposure and its associated elevations in BDNF; nonetheless, these studies did not establish a causal relationship between HVC BDNF availability and song learning.
To better understand the causal relationship of neuronal addition to song development in adults, Hartog and colleagues treated testosterone-stimulated female canaries with a VEGFR2 inhibitor to suppress angiogenesis, and by so doing to suppress neuronal addition (Hartog et al., 2009). They found that the blockade of neuronal addition indeed inhibited the development of male-like song behavior in the androgen-treated females. Furthermore, they found that the local overexpression of BDNF in HVC was sufficient to rescue VEGFR2 inhibitor-suppressed song development. In particular, BDNF-expressing plasmids delivered to HVC were sufficient to trigger song by 10 days after transfection, despite sustained VEGFR2 inhibition; birds whose HVCs were transfected with control plasmids failed to develop song. Thus, the exposure of HVC to BDNF appeared sufficient to permit song development, downstream of testosterone-triggered VEGF signaling. Interestingly though, the recruitment of new neurons appeared to follow BDNF-rescued song, so that while BDNF supported both the incorporation of new neurons and the development of song, the dependence of song upon neuronal recruitment remained unclear in this study; to the contrary, neuronal addition appeared to be a consequence rather than a cause of BDNF-induced song initiation, suggesting a role for neuronal addition in song maintenance and elaboration rather than acquisition (Hartog et al., 2009).
Androgen-induced HVC BDNF acts distantly upon HVC target nuclei
Besides its local secretion by the HVC microvasculature, BDNF may also act distantly, following its anterograde transport to RA within HVCRA projection neurons (Dittrich et al., 1999, Li et al., 2000). Both the high-affinity TrkB and low-affinity p75 receptors for BDNF are expressed by RA magnocellular neurons, suggesting the functional dependence of these cells upon BDNF production by HVCRA neurons (Johnson et al., 1997, Dittrich et al., 1999). Intraparenchymal infusions of BDNF into RA induces the hypertrophy of RA magnocellular neurons, an effect that was blocked by TrkB antibody infusion (Wissman and Brenowitz, 2009). Furthermore, when BDNF-coated beads were injected into RA of adult zebra finches, juvenile-like song plasticity was elicited, and RA's synaptic density increased (Kittelberger and Mooney, 2005). This observation recalled the testosterone-stimulated dendritic growth and synaptogenesis reported in the RA of testosterone-treated female canaries (DeVoogd and Nottebohm, 1981a, DeVoogd et al., 1985, Canady et al., 1988). Together, these studies suggest that the androgen-elicited production of BDNF by HVC projection neurons, and its transport to RA and effects therein on RA magnocellular neurons, may contribute significantly to song development in adult oscines. Importantly, regardless of the extent to which testosterone modulates BDNF expression by HVCRA projection neurons, such neuronal BDNF would likely be released in RA rather than in HVC, and would thus be unlikely to influence HVC neuronal recruitment. Rather, our observations suggest that endothelial cells are the major contributors to available HVC BDNF, and likely the dominant source of testosterone-stimulated BDNF accessible to newly generated neurons.
Enriching this story yet further, BDNF-secreting HVCRA projection neurons comprise the adult-generated population of HVC neurons, at least in adult canaries. One may then postulate that androgen-induced endothelial BDNF in HVC might permit the addition of new neurons to HVC in one season, that mature thereafter as HVCRA projection neurons able to provide androgen-stimulated BDNF to RA in the following season. As such, BDNF appears to function at multiple timepoints in the life of HVC projection neurons, as well as at multiple loci within the song control circuit, to enable development of the neural infrastructure for song acquisition and elaboration.
Conclusion
These studies of the hormonal control of neuronal production and recruitment to neurogenic regions of the adult songbird brain have yielded considerable insight into tehn cellular mechanisms and interactions by which new neurons may be added to functional circuits within the adult brain. Importantly, the implications of these studies extend beyond the natural history of bird song, to the direction of structural neural repair. The centrality of BDNF-dependent signaling to neuronal addition in neurogenic regions of both the avian and mammalian brain suggested that its overexpression in regions with competent neural stem and neuronal progenitor cells, that were otherwise not associated with neuronal addition, might be sufficient to elicit heterotopic neuronal recruitment to those regions. Accordingly, a number of studies have now demonstrated that ectopic BDNF overexpression can induce neuronal recruitment to the adult mammalian neostriatum, from resident neural stem and progenitor cells in the striatal ventricular zone (Benraiss et al., 2001, Pencea et al., 2001, Bedard et al., 2006). The new medium spiny neurons thereby recruited are competent to extend axons to their appropriate targets in the globus pallidus, thereby recapitulating the normal development of this circuit (Chmielnicki et al., 2004), in a manner analogous to the seasonal regrowth of the HVC to RA circuit in canaries. In rodents just as in songbirds, these BDNF-induced striopallidal neurons become functionally competent as well as anatomically integrated. Indeed, the BDNF-induced recruitment of new medium spiny neurons - when potentiated by co-treatment with noggin to suppress gliogenesis – appears to be sufficient to slow disease progression in a mouse model of Huntington's Disease, by compensating for the loss of medium spiny neurons with newly generated replacements (Cho et al., 2007, Benraiss and Goldman, 2011). The clinical implications of this capability for induced neuronal recruitment and circuit reconstruction from endogenous neural stem cells are potentially profound, and may be germane to a broad range of neurodegenerative and traumatic-ischemic disorders characterized by neuronal loss (Goldman, 2005). As such, the songbird brain, by teaching us the rules by which new neurons may be added to existing neural circuits, may provide us fundamental insights into both the latent cellular and structural plasticity of the adult brain, and the means by which that plasticity may be evoked to achieve cellular replacement and structural repair.
Highlights.
Testosterone mediates the recruitment of new neurons into the vocal control nucleus HVC of the adult songbird brain
HVC includes both androgen and estrogen receptive cells, whose paracrine interactions are critical to neuronal addition
Testosterone and estradiol induce angiogenesis by enabling VEGF signaling
VEGF stimulated vascular expansion triggers BDNF production by HVC endothelium
BDNF expression is sufficient to promote singing and neuronal recruitment
The inhibition of angiogenesis blocks both neuronal recruitment and singing
Acknowledgments
This work was funded by NIH/NINDS grant R37/R01NS29813 to S.G., by the G. Harold and Leila Y. Mathers Foundation, and by a predoctoral fellowship to Z.C. from the NY State Stem Cell Research Program (NYSTEM). We thank Max H. Sims for editorial assistance.
Abbreviations
- HVC
high vocal center
- RA
robust nucleus of the arcopallium
- DLM
dorsolateral anterior thalamic nucleus
- LMAN
lateral magnocellular nucleus of the anterior nidopallium
- NIf
the nucleus interfacialis
- nXIIts
nucleus XII tracheosyringelis
- HVCRA
RA-projecting HVC neurons
- AR
androgen receptor
- ER
estrogen receptor
- VEGF
vascular endothelial growth factor
- VEGFR2
VEGF receptor 2
- BDNF
brain-derived neurotrophic factor
- MMP
matrix metalloproteinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Borda B, Haripal B, Nottebohm F. Timing of brain-derived neurotrophic factor exposure affects life expectancy of new neurons. Proc Natl Acad Sci U S A. 2004;101:3957–3961. doi: 10.1073/pnas.0308118101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Borda B, Nottebohm F. Gonads and singing play separate, additive roles in new neuron recruitment in adult canary brain. J Neurosci. 2002;22:8684–8690. doi: 10.1523/JNEUROSCI.22-19-08684.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Kirn JR. Birth, migration, incorporation, and death of vocal control neurons in adult songbirds. J Neurobiol. 1997;33:585–601. [PubMed] [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–354. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Proliferation “hot spots” in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–109. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Andalman AS, Fee MS. A basal ganglia-forebrain circuit in the songbird biases motor output to avoid vocal errors. Proc Natl Acad Sci U S A. 2009;106:12518–12523. doi: 10.1073/pnas.0903214106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold AP, Nottebohm F, Pfaff DW. Hormone concentrating cells in vocal control and other areas of the brain of the zebra finch (Poephila guttata) J Comp Neurol. 1976;165:487–511. doi: 10.1002/cne.901650406. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Saltiel A. Sexual difference in pattern of hormone accumulation in the brain of a songbird. Science. 1979;205:702–705. doi: 10.1126/science.205.4407.702. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Houbart M, Prins GS, Ball GF. Distribution of androgen receptor-immunoreactive cells in the quail forebrain and their relationship with aromatase immunoreactivity. J Neurobiol. 1998;35:323–340. [PubMed] [Google Scholar]
- Balthazart J, Foidart A, Wilson EM, Ball GF. Immunocytochemical localization of androgen receptors in the male songbird and quail brain. J Comp Neurol. 1992;317:407–420. doi: 10.1002/cne.903170407. [DOI] [PubMed] [Google Scholar]
- Barami K, Iversen K, Furneaux H, Goldman SA. Hu protein as an early marker of neuronal phenotypic differentiation by subependymal zone cells of the adult songbird forebrain. J Neurobiol. 1995;28:82–101. doi: 10.1002/neu.480280108. [DOI] [PubMed] [Google Scholar]
- Barami K, Kirschenbaum B, Lemmon V, Goldman SA. N-cadherin and Ng-CAM/8D9 are involved serially in the migration of newly generated neurons into the adult songbird brain. Neuron. 1994;13:567–582. doi: 10.1016/0896-6273(94)90026-4. [DOI] [PubMed] [Google Scholar]
- Bedard A, Gravel C, Parent A. Chemical characterization of newly generated neurons in the striatum of adult primates. Exp Brain Res. 2006;170:501–512. doi: 10.1007/s00221-005-0233-5. [DOI] [PubMed] [Google Scholar]
- Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA. Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cells in the adult forebrain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001;21:6718–6731. doi: 10.1523/JNEUROSCI.21-17-06718.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benraiss A, Goldman SA. Cellular therapy and induced neuronal replacement for Huntington's disease. Neurotherapeutics. 2011;8:577–590. doi: 10.1007/s13311-011-0075-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergers G, Brekken R, McMahon G, Vu TH, Itoh T, Tamaki K, Tanzawa K, Thorpe P, Itohara S, Werb Z, Hanahan D. Matrix metalloproteinase-9 triggers the angiogenic switch during carcinogenesis. Nat Cell Biol. 2000;2:737–744. doi: 10.1038/35036374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Bentley GE, Balthazart J, Turek FW, Ball GF. Androgen receptor, estrogen receptor alpha, and estrogen receptor beta show distinct patterns of expression in forebrain song control nuclei of European starlings. Endocrinology. 1999;140:4633–4643. doi: 10.1210/endo.140.10.7024. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Interruption of a basal ganglia-forebrain circuit prevents plasticity of learned vocalizations. Nature. 2000;404:762–766. doi: 10.1038/35008083. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Arnold AP. Accumulation of estrogen in a vocal control brain region of a duetting song bird. Brain Res. 1989;480:119–125. doi: 10.1016/0006-8993(89)91574-6. [DOI] [PubMed] [Google Scholar]
- Brenowitz EA, Arnold AP. Hormone accumulation in song regions of the canary brain. JNeurobiol. 1992;23:871–880. doi: 10.1002/neu.480230708. [DOI] [PubMed] [Google Scholar]
- Burd GD, Nottebohm F. Ultrastructural characterization of synaptic terminals formed on newly generated neurons in a song control nucleus of the adult canary forebrain. J Comp Neurol. 1985;240:143–152. doi: 10.1002/cne.902400204. [DOI] [PubMed] [Google Scholar]
- Canady RA, Burd GD, DeVoogd TJ, Nottebohm F. Effect of testosterone on input received by an identified neuron type of the canary song system: a Golgi/electron microscopy/degeneration study. J Neurosci. 1988;8:3770–3784. doi: 10.1523/JNEUROSCI.08-10-03770.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnock-Jones DS, Sharkey AM, Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA, Smith SK. Identification and localization of alternately spliced mRNAs for vascular endothelial growth factor in human uterus and estrogen regulation in endometrial carcinoma cell lines. Biol Reprod. 1993;48:1120–1128. doi: 10.1095/biolreprod48.5.1120. [DOI] [PubMed] [Google Scholar]
- Chmielnicki E, Benraiss A, Economides AN, Goldman SA. Adenovirally expressed noggin and brain-derived neurotrophic factor cooperate to induce new medium spiny neurons from resident progenitor cells in the adult striatal ventricular zone. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2004;24:2133–2142. doi: 10.1523/JNEUROSCI.1554-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho SR, Benraiss A, Chmielnicki E, Samdani A, Economides A, Goldman SA. Induction of neostriatal neurogenesis slows disease progression in a transgenic murine model of Huntington disease. Journal of Clinical investigation. 2007;117:2889–2902. doi: 10.1172/JCI31778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullinan-Bove K, Koos RD. Vascular endothelial growth factor/vascular permeability factor expression in the rat uterus: rapid stimulation by estrogen correlates with estrogen-induced increases in uterine capillary permeability and growth. Endocrinology. 1993;133:829–837. doi: 10.1210/endo.133.2.8344219. [DOI] [PubMed] [Google Scholar]
- DeVoogd T, Nottebohm F. Gonadal hormones induce dendritic growth in the adult avian brain. Science. 1981a;214:202–204. doi: 10.1126/science.7280692. [DOI] [PubMed] [Google Scholar]
- DeVoogd TJ, Nixdorf B, Nottebohm F. Synaptogenesis and changes in synaptic morphology related to acquisition of a new behavior. Brain Res. 1985;329:304–308. doi: 10.1016/0006-8993(85)90539-6. [DOI] [PubMed] [Google Scholar]
- DeVoogd TJ, Nottebohm F. Sex differences in dendritic morphology of a song control nucleus in the canary: a quantitative Golgi study. J Comp Neurol. 1981b;196:309–316. doi: 10.1002/cne.901960209. [DOI] [PubMed] [Google Scholar]
- Dittrich F, Feng Y, Metzdorf R, Gahr M. Estrogen-inducible, sex-specific expression of brain-derived neurotrophic factor mRNA in a forebrain song control nucleus of the juvenile zebra finch. Proc Natl Acad Sci U S A. 1999;96:8241–8246. doi: 10.1073/pnas.96.14.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- Fusani L, Gahr M. Hormonal influence on song structure and organization: the role of estrogen. Neuroscience. 2006;138:939–946. doi: 10.1016/j.neuroscience.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Fusani L, Hutchison JB, Gahr M. Testosterone regulates the activity and expression of aromatase in the canary neostriatum. J Neurobiol. 2001;49:1–8. doi: 10.1002/neu.1061. [DOI] [PubMed] [Google Scholar]
- Fusani L, Metzdorf R, Hutchison JB, Gahr M. Aromatase inhibition affects testosterone-induced masculinization of song and the neural song system in female canaries. J Neurobiol. 2003;54:370–379. doi: 10.1002/neu.10141. [DOI] [PubMed] [Google Scholar]
- Fusani L, Van't Hof T, Hutchison JB, Gahr M. Seasonal expression of androgen receptors, estrogen receptors, and aromatase in the canary brain in relation to circulating androgens and estrogens. J Neurobiol. 2000;43:254–268. [PubMed] [Google Scholar]
- Gahr M. Localization of androgen receptors and estrogen receptors in the same cells of the songbird brain. Proc Natl Acad Sci U S A. 1990;87:9445–9448. doi: 10.1073/pnas.87.23.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahr M. Hormone-dependent neural plasticity in the juvenile and adult song system: what makes a successful male? Ann N Y Acad Sci. 2004;1016:684–703. doi: 10.1196/annals.1298.025. [DOI] [PubMed] [Google Scholar]
- Gahr M. Sexual differentiation of the vocal control system of birds. Adv Genet. 2007;59:67–105. doi: 10.1016/S0065-2660(07)59003-6. [DOI] [PubMed] [Google Scholar]
- Gahr M, Guttinger HR, Kroodsma DE. Estrogen receptors in the avian brain: survey reveals general distribution and forebrain areas unique to songbirds. J Comp Neurol. 1993;327:112–122. doi: 10.1002/cne.903270109. [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R. Distribution and dynamics in the expression of androgen and estrogen receptors in vocal control systems of songbirds. Brain Res Bull. 1997;44:509–517. doi: 10.1016/s0361-9230(97)00233-5. [DOI] [PubMed] [Google Scholar]
- Gahr M, Metzdorf R, Aschenbrenner S. The ontogeny of the canary HVC revealed by the expression of androgen and oestrogen receptors. Neuroreport. 1996;8:311–315. doi: 10.1097/00001756-199612200-00062. [DOI] [PubMed] [Google Scholar]
- Gil D, Gahr M. The honesty of bird song: multiple constraints for multiple traits. Trends Ecol Evol. 2002;17:133–141. [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36:267–286. [PubMed] [Google Scholar]
- Goldman SA. Stem and progenitor cell-based therapy of the human central nervous sytem. Nature Biotech. 2005;23:862–871. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Chen Z. Perivascular instruction of cell genesis and fate in the adult brain. Nat Neurosci. 2011;14:1382–1389. doi: 10.1038/nn.2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Nottebohm F. Neuronal production, migration, and differentiation in a vocal control nucleus of the adult female canary brain. Proc Natl Acad Sci U S A. 1983;80:2390–2394. doi: 10.1073/pnas.80.8.2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman SA, Williams S, Barami K, Lemmon V, Nedergaard M. Transient coupling of Ng-CAM expression to NgCAM-dependent calcium signaling during migration of new neurons in the adult songbird brain. Mol Cell Neurosci. 1996a;7:29–45. doi: 10.1006/mcne.1996.0003. [DOI] [PubMed] [Google Scholar]
- Goldman SA, Zukhar A, Barami K, Mikawa T, Niedzwiecki D. Ependymal/subependymal zone cells of postnatal and adult songbird brain generate both neurons and nonneuronal siblings in vitro and in vivo. J Neurobiol. 1996b;30:505–520. doi: 10.1002/(SICI)1097-4695(199608)30:4<505::AID-NEU6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hartog TE, Dittrich F, Pieneman AW, Jansen RF, Frankl-Vilches C, Lessmann V, Lilliehook C, Goldman SA, Gahr M. Brain-derived neurotrophic factor signaling in the HVC is required for testosterone-induced song of female canaries. J Neurosci. 2009;29:15511–15519. doi: 10.1523/JNEUROSCI.2564-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick EH, Harris JO. Singing Female Canaries. Science. 1957;125:1299–1300. doi: 10.1126/science.125.3261.1299. [DOI] [PubMed] [Google Scholar]
- Hidalgo A, Barami K, Iversen K, Goldman SA. Estrogens and non-estrogenic ovarian influences combine to promote the recruitment and decrease the turnover of new neurons in the adult female canary brain. J Neurobiol. 1995;27:470–487. doi: 10.1002/neu.480270404. [DOI] [PubMed] [Google Scholar]
- Hutchison JB, Steimer T. Formation of behaviorally effective 17 beta-estradiol in the dove brain: steroid control of preoptic aromatase. Endocrinology. 1986;118:2180–2187. doi: 10.1210/endo-118-6-2180. [DOI] [PubMed] [Google Scholar]
- Jain RK, Safabakhsh N, Sckell A, Chen Y, Jiang P, Benjamin L, Yuan F, Keshet E. Endothelial cell death, angiogenesis, and microvascular function after castration in anandrogen-dependent tumor: role of vascular endothelial growth factor. Proc Natl AcadSci U S A. 1998;95:10820–10825. doi: 10.1073/pnas.95.18.10820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, McMurtry J, Niedzwiecki D, Goldman SA. Insulin-like growth factor-1 is a radial cell-associated neurotrophin that promotes neuronal recruitment from the adult songbird edpendyma/subependyma. J Neurobiol. 1998;36:1–15. doi: 10.1002/(sici)1097-4695(199807)36:1<1::aid-neu1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Johnson F, Hohmann SE, DiStefano PS, Bottjer SW. Neurotrophins suppress apoptosis induced by deafferentation of an avian motor-cortical region. J Neurosci. 1997;17:2101–2111. doi: 10.1523/JNEUROSCI.17-06-02101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Lilliehook C, Roides B, Chen Z, Chang M, Mobashery S, Goldman SA. Testosterone-induced matrix metalloproteinase activation is a checkpoint for neuronaladdition to the adult songbird brain. J Neurosci. 2008;28:208–216. doi: 10.1523/JNEUROSCI.3674-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Perlman WR, Arnold AP. Expression of androgen receptor mRNA in zebra finch song system: developmental regulation by estrogen. J Comp Neurol. 2004;469:535–547. doi: 10.1002/cne.11033. [DOI] [PubMed] [Google Scholar]
- Kirn JR, Alvarez-Buylla A, Nottebohm F. Production and survival of projection neurons in a forebrain vocal center of adult male canaries. J Neurosci. 1991;11:1756–1762. doi: 10.1523/JNEUROSCI.11-06-01756.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Goldman SA. Brain-derived neurotrophic factor promotes the survival of neurons arising from the adult rat forebrain subependymal zone. Proceedings of the National Academy of Sciences. 1995;92:210–214. doi: 10.1073/pnas.92.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum B, Nedergaard M, Preuss A, Barami K, Fraser RA, Goldman SA. In vitro neuronal production and differentiation by precursor cells derived from the adult human forebrain. Cerebral Cortex. 1994;4:576–589. doi: 10.1093/cercor/4.6.576. [DOI] [PubMed] [Google Scholar]
- Kittelberger JM, Mooney R. Acute injections of brain-derived neurotrophic factor in a vocal premotor nucleus reversibly disrupt adult birdsong stability and trigger syllable deletion. J Neurobiol. 2005;62:406–424. doi: 10.1002/neu.20109. [DOI] [PubMed] [Google Scholar]
- Leonard SL. Induction of Singing in Female Canaries by Injections of Male Hormone. Proc Soc Exp Biol Med. 1939;41:229–230. [Google Scholar]
- Leventhal C, Rafii S, Rafii D, Shahar A, Goldman SA. Endothelial trophic support of neuronal production and recruitment from the adult mammalian subependyma. Mol Cell Neurosci. 1999;13:450–464. doi: 10.1006/mcne.1999.0762. [DOI] [PubMed] [Google Scholar]
- Li XC, Jarvis ED, Alvarez-Borda B, Lim DA, Nottebohm F. A relationship between behavior, neurotrophin expression, and new neuron survival. Proc Natl Acad Sci U S A. 2000;97:8584–8589. doi: 10.1073/pnas.140222497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Metzdorf R, Gahr M, Fusani L. Distribution of aromatase, estrogen receptor, and androgen receptor mRNA in the forebrain of songbirds and nonsongbirds. J Comp Neurol. 1999;407:115–129. [PubMed] [Google Scholar]
- Nastiuk KL, Clayton DF. The canary androgen receptor mRNA is localized in the song control nuclei of the brain and is rapidly regulated by testosterone. J Neurobiol. 1995;26:213–224. doi: 10.1002/neu.480260206. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Testosterone triggers growth of brain vocal control nuclei in adult female canaries. Brain Res. 1980;189:429–436. doi: 10.1016/0006-8993(80)90102-x. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. The neural basis of birdsong. PLoS Biol. 2005;3:e164. doi: 10.1371/journal.pbio.0030164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottebohm F, Arnold AP. Sexual dimorphism in vocal control areas of the songbird brain. Science. 1976;194:211–213. doi: 10.1126/science.959852. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Kelley DB, Paton JA. Connections of vocal control nuclei in the canary telencephalon. J Comp Neurol. 1982;207:344–357. doi: 10.1002/cne.902070406. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane L. Developmental and seasonal changes in canary song and their relation to changes in the anatomy of song-control nuclei. Behav Neural Biol. 1986;46:445–471. doi: 10.1016/s0163-1047(86)90485-1. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Nottebohm ME, Crane LA, Wingfield JC. Seasonal changes in gonadal hormone levels of adult male canaries and their relation to song. Behav Neural Biol. 1987;47:197–211. doi: 10.1016/s0163-1047(87)90327-x. [DOI] [PubMed] [Google Scholar]
- Okuhata S, Saito N. Synaptic connections of thalamo-cerebral vocal nuclei of the canary. Brain Res Bull. 1987;18:35–44. doi: 10.1016/0361-9230(87)90031-1. [DOI] [PubMed] [Google Scholar]
- Paton JA, Nottebohm FN. Neurons generated in the adult brain are recruited into functional circuits. Science. 1984;225:1046–1048. doi: 10.1126/science.6474166. [DOI] [PubMed] [Google Scholar]
- Pencea V, Bingaman KD, Wiegand SJ, Luskin MB. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J Neurosci. 2001;21:6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman WR, Ramachandran B, Arnold AP. Expression of androgen receptor mRNA in the late embryonic and early posthatch zebra finch brain. J Comp Neurol. 2003;455:513–530. doi: 10.1002/cne.10510. [DOI] [PubMed] [Google Scholar]
- Pincus DW, Keyoung HM, Harrison-Restelli C, Goodman RR, Fraser RA, Edgar M, Sakakibara S, Okano H, Nedergaard M, Goldman SA. Fibroblast growth factor-2/brain-derived neurotrophic factor-associated maturation of new neurons generated from adult human subependymal cells. Ann Neurol. 1998;43:576–585. doi: 10.1002/ana.410430505. [DOI] [PubMed] [Google Scholar]
- Rasika S, Alvarez-Buylla A, Nottebohm F. BDNF mediates the effects of testosterone on the survival of new neurons in an adult brain. Neuron. 1999;22:53–62. doi: 10.1016/s0896-6273(00)80678-9. [DOI] [PubMed] [Google Scholar]
- Rasika S, Nottebohm F, Alvarez-Buylla A. Testosterone increases the recruitment and/or survival of new high vocal center neurons in adult female canaries. Proc Natl Acad Sci U S A. 1994;91:7854–7858. doi: 10.1073/pnas.91.17.7854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha CJ, Tuerk MJ, Kim YH, Fernandes AO, Arnold AP, Schlinger BA. Distribution and regulation of telencephalic aromatase expression in the zebra finch revealed with a specific antibody. J Comp Neurol. 2000;423:619–630. doi: 10.1002/1096-9861(20000807)423:4<619::aid-cne7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA. The activity and expression of aromatase in songbirds. Brain Res Bull. 1997;44:359–364. doi: 10.1016/s0361-9230(97)00215-3. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci U S A. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlinger BA, Callard GV. A comparison of aromatase, 5 alpha-, and 5 beta- reductase activities in the brain and pituitary of male and female quail (C. c. japonica) J Exp Zool. 1987;242:171–180. doi: 10.1002/jez.1402420208. [DOI] [PubMed] [Google Scholar]
- Schlinger BA, Fivizzani AJ, Callard GV. Aromatase, 5 alpha- and 5 beta-reductase in brain, pituitary and skin of the sex-role reversed Wilson's phalarope. J Endocrinol. 1989;122:573–581. doi: 10.1677/joe.0.1220573. [DOI] [PubMed] [Google Scholar]
- Schumacher M, Balthazart J. Neuroanatomical distribution of testosterone-metabolizing enzymes in the Japanese quail. Brain Res. 1987;422:137–148. doi: 10.1016/0006-8993(87)90548-8. [DOI] [PubMed] [Google Scholar]
- Shen P, Campagnoni CW, Kampf K, Schlinger BA, Arnold AP, Campagnoni AT. Isolation and characterization of a zebra finch aromatase cDNA: in situ hybridization reveals high aromatase expression in brain. Brain Res Mol Brain Res. 1994;24:227–237. doi: 10.1016/0169-328x(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Shen P, Schlinger BA, Campagnoni AT, Arnold AP. An atlas of aromatase mRNA expression in the zebra finch brain. J Comp Neurol. 1995;360:172–184. doi: 10.1002/cne.903600113. [DOI] [PubMed] [Google Scholar]
- Silverin B, Baillien M, Foidart A, Balthazart J. Distribution of aromatase activity in the brain and peripheral tissues of passerine and nonpasserine avian species. Gen Comp Endocrinol. 2000;117:34–53. doi: 10.1006/gcen.1999.7383. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Prins GS. Use of PG-21 immunocytochemistry to detect androgen receptors in the songbird brain. J Histochem Cytochem. 1996;44:1075–1080. doi: 10.1177/44.9.8773574. [DOI] [PubMed] [Google Scholar]
- Sohrabji F, Nordeen EJ, Nordeen KW. Selective impairment of song learning following lesions of a forebrain nucleus in the juvenile zebra finch. Behav Neural Biol. 1990;53:51–63. doi: 10.1016/0163-1047(90)90797-a. [DOI] [PubMed] [Google Scholar]
- Soma KK, Hartman VN, Wingfield JC, Brenowitz EA. Seasonal changes in androgen receptor immunoreactivity in the song nucleus HVc of a wild bird. J Comp Neurol. 1999;409:224–236. [PubMed] [Google Scholar]
- Vates GE, Nottebohm F. Feedback circuitry within a song-learning pathway. Proc Natl Acad Sci U S A. 1995;92:5139–5143. doi: 10.1073/pnas.92.11.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vates GE, Vicario DS, Nottebohm F. Reafferent thalamo- “cortical” loops in the song system of oscine songbirds. J Comp Neurol. 1997;380:275–290. [PubMed] [Google Scholar]
- Vockel A, Prove E, Balthazart J. Sex- and age-related differences in the activity of testosterone-metabolizing enzymes in microdissected nuclei of the zebra finch brain. Brain Res. 1990;511:291–302. doi: 10.1016/0006-8993(90)90174-a. [DOI] [PubMed] [Google Scholar]
- Voigt C, Ball GF, Balthazart J. Sex differences in the expression of sex steroid receptor mRNA in the quail brain. J Neuroendocrinol. 2009;21:1045–1062. doi: 10.1111/j.1365-2826.2009.01929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu ET, Mazurek ME, Kuo YC. Identification of a forebrain motor programming network for the learned song of zebra finches. J Neurosci. 1994;14:6924–6934. doi: 10.1523/JNEUROSCI.14-11-06924.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walters MJ, Harding CF. The effects of an aromatization inhibitor on the reproductive behavior of male zebra finches. Horm Behav. 1988;22:207–218. doi: 10.1016/0018-506x(88)90067-0. [DOI] [PubMed] [Google Scholar]
- Williams S, Leventhal C, Lemmon V, Nedergaard M, Goldman SA. Estrogen promotes the initial migration and inception of NgCAM- dependent calcium-signaling by new neurons of the adult songbird brain. Mol Cell Neurosci. 1999;13:41–55. doi: 10.1006/mcne.1998.0729. [DOI] [PubMed] [Google Scholar]
- Wissman AM, Brenowitz EA. The role of neurotrophins in the seasonal-like growth of the avian song control system. J Neurosci. 2009;29:6461–6471. doi: 10.1523/JNEUROSCI.0638-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]


