Abstract
Background:
Neonatal conjunctivitis leads to several ocular consequences in the affected neonates such as blindness. Currently available therapeutic options include NaNO3, Gentamicin, Neomycin and so on, in which each of them has their own limitations. Regarding the immunologic content of colostrum and its safety and easy accessibility, we aimed to evaluate its preventive effects against neonatal conjunctivitis.
Materials and Methods:
In this clinical trial, conducted from November 2011 to July 2012, 300 preterm neonates, with culture negative eye swab, were enrolled and randomly assigned into three groups. The intervention group received two drops of colostrum. Control group received no treatment and other neonates were treated with topical Erythromycin ointment (0.5%). All neonates were followed for occurrence of clinical conjunctivitis for 28 days. Data analysis were performed by Chi-square test.
Results:
Our data demonstrate the beneficial preventive effects of Colostrum against neonatal conjunctivitis (P = 0.036).
Conclusion:
Colostrum is suggested as an alternative prophylactic option for antibiotics against neonatal conjunctivitis. As colostrum is easily accessible without cost, potential hazards and side effects, public education about its topical favorable effects is worthwhile.
Keywords: Antibiotic prophylaxis, colostrum, neonatal conjunctivitis, ophthalmia neonatorum
INTRODUCTION
Neonatal conjunctivitis produces various complications for the neonate in the infancy period or further life stages.[1] Some of the common complications of the neonatal conjunctivitis include pseudofollicular formation in tarsal conjunctiva, nasolacrimal obstruction and blindness. The main pathologic organism responsible for the incidence of neonatal conjunctivitis is staphylococcus aureus.[2,3]
Nowadays, different chemical prophylaxis is used worldwide for prevention of neonatal conjunctivitis like AgNO3, Erythromycin, Gentamicin, Neomycin, Chloramphenicol, Tetracycline and Povidone–Iodine.[4,5,6,7] Each of them has their own limitations and side effects. AgNO3 has no coverage on the Chlamydia Trachomatis, thyroid disorders due to the application of Povidone–Iodine and so on.[8] The great failure rate of these prophylactic strategies draw attentions toward novel methods with enhanced safety. In studies performed by Ibhanesebhor, it has been demonstrated that sensitivity of Staphylococcus aureus and Escherichia coli to Gentamicin were 100% and 42%, respectively.[9] The sensitivity rate of E. coli to Colostrum and mature milk were 57% and 28%, respectively.[9] Staphylococcus aureus organisms were sensitive to Colostrum and mature milk in about 0.50% and 0%, respectively.[9] Growth inhibition zone for S. aureus and E. coli were between 3 and 5 mm, while marginal or none prevention for mature milk and Colostrum were seen with E. coli and S. aureus, respectively.[9] Mean preventive period for Colostrum and mature milk were 6 and 3 h, respectively.[9] Mature milk has been shown to exert 28% inhibitor effect on E. coli organisms for about 3 h in vitro and no effect on S. aureus organisms. Using Colostrum, this effect was seen to be ≥50% against S. aureus and E. coli organisms for about 6 h, which is much more than mature milk.[10]
These data favors the use of Colostrum as a cheap, safe and effective agent in the prevention of blindness induced by Neonatorum Ophthalmia. Thus the aim of this study was to investigate the possible protective role of the Colostrum droplets in neonates rather than conventional currently available agents used for the routine pediatric care.
MATERIALS AND METHODS
This randomized clinical trial was approved by the Ethics committee of Isfahan University of Medical Sciences, Isfahan, Iran. Written informed consent was obtained from parents. It was conducted from November 2011 to July 2012.
Prior to the study, an analysis was performed to estimate the necessary patient number in each group required to detect a between-group difference of one SD or less, with a 90% power and an error of less than 0.05. Based on the biological variability described in previous studies, a minimum of 80 patients was calculated for each group.[9]
Overall, 300 preterm neonates, with culture negative eye swab, were enrolled and then by using the table of random numbers, they were randomly assigned into three groups. We selected preterm neonates only because the maternal milk of the premature neonates contain more antibodies and immunologic components; obtaining more valuable data seems possible using their milk. The intervention group received two drops of colostrum in each eye. For applying the Colostrum, we disinfected the nipple first and then we cut the head of one 10 cc sterile syringe with a sterile bistoury and putted the open head around the nipple and pulled back the piston; negative pressure make the colostrum come to syringe. Control group received no treatment and other neonates were treated with topical Erythromycin ointment (0.5%). All neonates were followed for occurrence of clinical conjunctivitis for 28 days. Using swab, ocular culture from each participant was examined and neonates with positive eye cultures were excluded from the study. All treatments were used once and immediately after birth. Parents of all of the neonates signed the assigned contest form. Premature neonates were followed up for a period of 28 days after birth for the incidence of neonatal conjunctivitis, weekly or at the time of the occurrence of symptoms of neonatal conjunctivitis. The study was double-masked, the parents and the person who followed up the patients were not aware about the treatment used. Data were gathered in pre-prepared forms and were analyzed using Chi-square test by SPSS version 17.0.
RESULTS
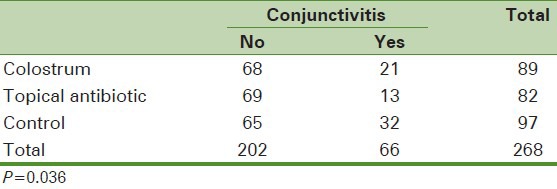
Data of 268 neonates were complete and were included to the analysis. As presented in Table 1, the frequency of conjunctivitis was higher in the control group, followed by the group receiving topical colostrum and the group receiving topical antibiotic (P = 0.03).
Table 1.
Frequency of conjunctivitis in the three groups of neonates
The results of eye swab culture of all patients reported Staphylococcus aureus.
DISCUSSION
Regarding the main goal of this investigation, our results demonstrate the effective role of colostrum in the prevention of neonatal conjunctivitis. This is in accordance with the results of the Ibhahesbler et al., who demonstrated the positive effects of colostrum on the organisms involved in the pathogenesis of neonatal conjunctivitis.[9] Despite of the well-recognized role of the breast feeding on the prevention of some infectious diseases as Giardiasis and acute Otitis media in neonates, there are limited data regarding the focal effectiveness of the maternal milk and especially Colostrum.[11,12] Local application of maternal milk on the human umbilical vein has been shown to be associated with accelerated umbilical separation and decreased focal infection.[13]
The immunologic efficacy of maternal milk has been investigated by several authors. It has been demonstrated that maternal milk especially Colostrum contain leukocytes and various kinds of immunoglobulins.[14] Thus the preventive effect of the Colostrum in the case of neonatal conjunctivitis might be associated with the immunological properties of the Colostrum. Although the effect of Colostrum in our study was not similar to the antibiotic effect, Colostrum can be suggested as a second choice for antibiotics application. The absence of side effects in the case of Colostrum, in contrast with antibiotics, is also noteworthy.
Due to the limited available data in this regard, further confirmatory investigations seems necessary. Since the maternal milk of the premature neonates contain more antibodies and immunologic components, obtaining more valuable data seems possible using their milk. Finally, maternal education and exclusive breast feeding are the most fundamental measures decreasing the incidence of various kinds of infections as neonatal conjunctivitis.
CONCLUSION
Colostrum is suggested as an alternative prophylactic option for antibiotics against neonatal conjunctivitis. Colostrum is easily accessible without potential hazards and side effects. Public education about such favorable effects of topical colostrum would be worthwhile.
ACKNOWLEDGMENTS
The authors are thankful to Shima Rahimirad, Mohammad Karimian, and Mohammad Kazemi who helped in conducting this study.
Footnotes
Source of Support: The research is funded by Medical School, Isfahan University of Medical Sciences, Isfahan, Iran
Conflict of Interest: None declared
References
- 1.Krasny J, Tomasova-Borovanska J, Hruba D. The Relationship between chlamydia trachomatis and chlamydia pneumoniae as the cause of neonatal conjunctivitis (ophthalmia neonatorum) Ophthalmologica. 2005;219:232–6. doi: 10.1159/000085733. [DOI] [PubMed] [Google Scholar]
- 2.Hick JF, Block DJ, Ilstrup DM. A controlled study of silver nitrate prophylaxis and the incidence of nasolacrimal duct obstruction. J Pediatr Ophthalmol Strabismus. 1985;22:92–3. doi: 10.3928/0191-3913-19850501-04. [DOI] [PubMed] [Google Scholar]
- 3.Sandstrom I. Treatment of neonatal conjunctivitis. Arch Ophthalmol. 1987;105:925–8. doi: 10.1001/archopht.1987.01060070061029. [DOI] [PubMed] [Google Scholar]
- 4.Zar HJ. Neonatal chlamydial infections: Prevention and treatment. Paediatr Drugs. 2005;7:103–10. doi: 10.2165/00148581-200507020-00003. [DOI] [PubMed] [Google Scholar]
- 5.Assadian O, Assadian A, Aspock C, Hahn D, Koller W. Prophylaxis of ophthalmia neonatorum-a nationwide survey of the current practice in Austria. Wien Klin Wochenschr. 2002;114:194–9. [PubMed] [Google Scholar]
- 6.Olatunji FO. A case control study of ophthalmia Neonatorum in Kaduna II: Causative agents and their antibiotic sensitivity. West Afr J Med. 2004;23:215–20. doi: 10.4314/wajm.v23i3.28124. [DOI] [PubMed] [Google Scholar]
- 7.Richter R, Below H, Kadow I, Kramer A, Muller C, Fusch C. Effect of topical 1.25% povidone-iodine eyedrops used for prophylaxis of ophthalmia neonatorum on renal iodine excretion and thyroid-stimulating hormone level. J Pediatr. 2006;148:401–3. doi: 10.1016/j.jpeds.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Radtsig MA, Koksharova OA, Khmel IA. Antibacterial effects of silver ions: Effect on gram-negative bacteria growth and biofilm formation. Mol Gen Mikrobiol Virusol. 2009;4:27–31. [PubMed] [Google Scholar]
- 9.Ibhanesebhor SE, Otobo ES. In vitro activity of human milk against the causative organisms of ophthalmia neonatorum in Benin City, Nigeria. J Trop Pediatr. 1996;42:327–9. doi: 10.1093/tropej/42.6.327. [DOI] [PubMed] [Google Scholar]
- 10.Marinelli KA. Philadelphia, Pa: Lippincott Williams and Wilkins; 2005. Breast feeding and the use of human milk in the NICU Avery›s neonatology: Pathophysiology and management of the newborn. [Google Scholar]
- 11.Ghorbani R, Sadat-Hashemi SM, Pazooki R. Does breast-feeding protect the child from Giardia lamblia infection? , Tehran Univ Med J. 2008;66:425–31. [Google Scholar]
- 12.Vogazianos E, Vogazianos P, Fiala J, Janecek D, Slapak I. The effect of breastfeeding and its duration on acute otitis media in children in brno, czech republic. Cent Eur J Public Health. 2007;15:143–6. doi: 10.21101/cejph.a3427. [DOI] [PubMed] [Google Scholar]
- 13.Ahmadpour-Kacho M, Zahedpasha Y, Hajian K, Javadi G, Talebian H. The effect of topical application of human milk, ethyl alcohol 96%, and silver sulfadiazine on umbilical cord separation time in newborn infants. Arch Iran Med. 2006;9:33–8. [PubMed] [Google Scholar]
- 14.Hurley WL, Theil PK. Perspectives on Immunoglobulins in Colostrum and Milk. Nutrients. 2011;3:442–74. doi: 10.3390/nu3040442. [DOI] [PMC free article] [PubMed] [Google Scholar]


