Abstract
Anti-Tumor Necrosis Factor Alpha (TNF-α) therapy with infliximab has shown to be effective for patients with steroid-refractory acute graft-versus-host disease (aGVHD). An open-labeled, phase III trial was conducted to determine if the addition of infliximab to steroids could improve results for patients with newly diagnosed grade II-IV aGVHD. A total of 63 patients were randomized either to 2 mg/kg/day methylprednisolone (MP) or infliximab + MP. Average age was 47 years (range: 20–70 years); 64% were male. Fifty-three percent and 51% of patients received a matched-sibling and/or bone marrow (BM) graft. Sixty-seven percent had grade II, 33% grade III-IV aGVHD; 62% had skin, 53% gastrointestinal (GI), and 7% had liver involvement. At days 7 and 28, the response rate for infliximab + MP versus MP was 52% versus 78%, P=.03 and 62% versus 58%, P=.7, respectively. Cumulative incidences of GVHD-related mortality, nonrelapse mortality (NRM), and overall survival (OS) were not significantly different between the 2 groups (GVHD-related mortality: 38% versus 32%, P=.6; NRM: 52% versus 36%, P=.3; OS: 17% and 28%, P=.4 for infliximab + MP versus MP, respectively). Patients with newly diagnosed aGVHD derive no benefit from the addition of anti-TNF-α therapy with infliximab when compared to corticosteroids alone.
Keywords: Acute GVHD, Infliximab
INTRODUCTION
Graft-versus-host disease (GVHD) remains a major limitation of allogeneic hematopoietic stem cell transplantation (HSCT), with the acute form of it occurring in approximately 20% to 50% of patients [1–4]. Corticosteroids still remain the standard initial therapy [5]. Unfortunately, only 50% of patients with grade II or greater acute GVHD (aGVHD) will respond to this initial therapy, with the remaining patients being unresponsive or steroid resistant [6]. The outcome for those with steroid-refractory aGVHD is poor, with a mortality rate of about 70%, irrespective of the type of secondary therapy [7]. Strategies that included either higher doses of corticosteroids [8] or their combination with an additional agent, such as antithymocyte globulin (ATG) [9] or daclizumab [10], have not proved beneficial. Thus, better prophylaxis and upfront therapies are of vital importance to prevent progression to steroid refractory disease.
The pathophysiology of aGVHD has been divided in 3 phases [11]. The first phase occurs as a result of tissue damage resulting from toxicity associated with the conditioning chemo- or radiotherapy employed prior to transplant. Damaged tissues create an inflammatory milieu consisting of cytokines, including tumor necrosis factor-alpha (TNF-α), interleukin-1 (IL-1), and interferon gamma (IFN-γ). In the second phase, recipient and donor antigen-presenting cells (APC) along with inflammatory cytokines trigger the activation of donor-derived T cells, which expand and differentiate into effector cells. In the third phase, the effector phase, activated donor T cells mediate cytotoxicity against target host cells through Fas-Fas ligand interactions, perforin-granzyme B, and the further production of cytokines, such as TNF-α [12]. Thus TNF-α, which is mainly produced by monocytes and macrophages and secondarily by T lymphocytes and natural killer (NK) cells, has been implicated in the pathophysiology of GVHD at each of these steps in the process [13]. In support of the central role for TNF-α has been the correlation between high serum levels of TNF-α and increased incidence of GVHD in transplant recipients [14]. Therefore, therapies directed at reducing the amount of circulating TNF-α have been hypothesized as one method to treat aGVHD.
Infliximab, which is currently approved for the treatment of patients with rheumatoid arthritis and Crohn’s Disease, is a chimeric mouse/human IgG1 antibody that binds with high affinity to soluble and transmembrane forms of human TNF-α [15]. Binding of the antibody to soluble TNF-α results in neutralizing its activity, whereas binding to the transmembrane form results in cell lysis through complement mediated processes [16]. Results from retrospective studies evaluating the use of infliximab in the setting of steroid-refractory GVHD have shown response rates ranging from 59% to 67%, better in patients with intestinal GVHD [17,18].
Therefore, we decided to evaluate infliximab earlier in the treatment of aGVHD. This is a prospective, randomized phase III study that compares standard therapy with corticosteroids versus infliximab in addition to standard therapy for the initial treatment of grade II-IV aGVHD.
PATIENTS
Patients 18 years of age or older were eligible for inclusion if they developed grade II or greater aGVHD involving the skin, liver, or gastrointestinal (GI) tract or any organ combination following an allogeneic bone marrow (BM) or peripheral blood stem cell transplantation (PBSCT). The diagnoses are shown on Table 1, and these were considered to be at low risk of relapse if they were in complete remission at the time of transplant or in chronic phase in the case of chronic myelogenous leukemia (CML). Patients received conditioning regimens that were myeloablative (MA) or reduced intensity (RIC). Preparative regimens were considered myeloablative (MA) if they were expected to produce profound pancytopenia for more than 28 days without transplantation and if, after transplantation, hematopoietic recovery was completely donor derived. RIC regimens were defined as those in which hematopoietic recovery was expected to occur within 28 days without transplantation and, after transplantation, chimerism could be documented in most patients.
Table 1.
Patient Characteristics
| MP n=28 | (%) | Inflix +MP n=29 | (%) | P-Value | |
|---|---|---|---|---|---|
| Age (median, range) | 48 years (20–70 years) | 49 years (22–65 years) | .9 | ||
| Sex M/F | 21/7 | 17/12 | .3 | ||
| Disease status at TP (remission/chronic phase) | 10 | (36) | 7 | (24) | .4 |
| Diagnosis | |||||
| ALL | 3 | (11) | 2 | (7) | |
| AML | 9 | (32) | 14 | (48) | |
| CLL | 5 | (18) | 1 | (3) | |
| CML | 4 | (14) | 1 | (3) | |
| Lymphoma | 3 | (11) | 6 | (21) | |
| Hodgkin | 2 | (7) | 0 | (0) | |
| Myeloma | 2 | (7) | 2 | (7) | |
| Other | 0 | (0) | 3 | (10) | |
| Donor type | .9 | ||||
| Matched sibling | 15 | (54) | 15 | (52) | |
| Matched Unrelated | 10 | (36) | 10 | (34) | |
| 1 Ag MM rel/unrel | 2/1 | (11) | 3/1 | (13) | |
| Preparative regimen | .3 | ||||
| Reduced intensity | 8 | (29) | 3 | (10) | |
| Myeloablative | 20 | (71) | 26 | (90) | |
| Cell type | .9 | ||||
| BM | 14 | (50) | 15 | (52) | |
| PBPC | 14 | (50) | 14 | (48) | |
| GVHD prophylaxis | .6 | ||||
| Tacro/MTX | 24 | (86) | 26 | (90) | |
| Other | 4 | (14) | 3 | (10) | |
| Median days between TP and therapy | 35 (9–222) | 28 (13–207) | .09 | ||
| Grade aGVHD at study entry | .5 | ||||
| 2 | 19 | (68) | 19 | (65) | |
| 3 | 9 | (32) | 9 | (31) | |
| 4 | 0 | 1 | (03) | ||
| Skin stage at study entry | |||||
| 0 | 10 | (36) | 12 | (41) | |
| 1–2 | 5 | (18) | 4 | (14) | |
| 3 | 13 | (46) | 13 | (45) | |
| GI stage at study entry | |||||
| 0 | 13 | (46) | 14 | (48) | |
| 1–2 | 12 | (43) | 12 | (42) | |
| 3–4 | 3 | (11) | 3 | (10) | |
| UGI stage at study entry | |||||
| 0 | 25 | (89) | 25 | (86) | |
| 1 | 3 | (11) | 4 | (14) | |
| Liver stage at study entry | |||||
| 0 | 25 | (89) | 28 | (97) | |
| 1 | 2 | (07) | 1 | (03) | |
| 2 | 1 | (04) | 0 | (0) | |
1-Ag MM rel indicates 1-antigen mismatch-related donor; aGVHD; acute graft-versus-host disease; ALL, acute lymphoblastic leukemia; AML, acute myelogenous leukemia; BM, bone marrow; CML, chronic myelogenous leukemia; F, female; inflix, infliximab; M, male; MM, mismatch; MP, methylprednisolone; MTX, methotrexate; PBPC, peripheral blood progenitor cell; tacro, tacrolimus; TP, transplant; tmt, treatment; UGI, upper gastrointestinal; unrel, unrelated.
Patients were included on the study if they had evidence of engraftment defined as an absolute neutrophil count (ANC) >500, and received GVHD prophylaxis with a calcineurin inhibitor (i.e., tacrolimus or cyclosporine) plus methotrexate (MTX). Other agents that were allowed in the prophylaxis of aGVHD included ATG, pentostatin, and steroids. Patients could not have received more than 48 hours of MP at a dose of 2 mg/kg for the treatment of aGVHD or have received any additional agents for the treatment of aGVHD at the time of enrollment. Patients were excluded if they were receiving hemodialysis, had a concomitant diagnosis of congestive heart failure (even if medically controlled), history of a demyelinating disorder, infection with HIV, hepatitis B, or hepatitis C, and any uncontrolled infections. Patients with a history of tuberculosis (TB) (even latent/treated infections), a recent close contact with a person with active TB, or chest radiograph suggestive of prior TB were excluded from participation on the study.
METHODS
Study Design
The study was a single-center, open-labeled phase III trial with patients randomized between conventional treatment with MP or MP plus infliximab. Patients enrolled on the study were stratified based on donor status (matched-related versus matched unrelated/mismatched-related donor) and GVHD site (GI versus no GI GVHD involvement). All patients signed written, informed consent prior to receiving therapy on study. The protocol, amendments, and informed consents documents were approved by the institutional review board of the University of Texas, M.D. Anderson Cancer Center.
Diagnosis and Staging of aGVHD
The diagnosis of aGVHD was made on clinical grounds with biopsy of involved organ(s) encouraged but not required. Patients whose biopsy specimens confirmed a non-GVHD diagnosis were removed from the study. When biopsy specimens failed to confirm the diagnosis but there was strong clinical suspicion for aGVHD and other differential diagnoses were ruled out, patients were allowed to stay in the study. All 57 evaluable patients had their GVHD diagnosis confirmed by biopsy. Patients were assigned an aGVHD grade (I-IV) based on skin, liver, and GI stage (I-IV) [19].
Treatment of aGVHD
Patients randomized to the control arm received MP at a dose of 2 mg/kg/day, followed by a taper schedule that mandated patients receive a minimum of 1 mg/kg/day throughout the first 2 weeks of treatment followed by further tapering according to departmental guidelines at the discretion of the treating physician. Methylprednisolone was initiated in both arms at 2 mg/kg daily, and was kept at no less than 1 mg/kg daily for the first 2 weeks of therapy. Beyond the first 2 weeks, MP was tapered by 10% to 20% weekly. Patients in the study arm received infliximab at a dose of 10 mg/kg intravenously over 2 hours weekly for 4 weeks in addition to MP following the same regimen as the control group.
Follow-up and Assessment of Response
A physical exam, complete blood count (CBC), and chemistries were required once weekly throughout the first 30 days after enrollment on the study followed by every 2 weeks between days 30 to 60. A formal GVHD assessment was required twice weekly for the first 30 days followed by once weekly between days 30 to 60 after enrollment on the study. Patients were assigned an aGVHD grade (I-IV) based on skin, liver, and GI stage (I-IV).
Complete response (CR) to therapy required resolution of all manifestations of aGVHD. Partial response (PR) was a decrease in organ stage by 1 without worsening in any other organ. Progressive disease (PD) was worsening by 1 in organ stage after at least 48 hours of therapy for GI and liver GVHD or 72 hours for skin GVHD. No response (NR) was absence of response after a minimum of 7 days of therapy for skin GVHD or 3 days for GI or liver GVHD. A mixed response (MR) was an improvement of 1 stage in 1 affected organ with deterioration in another affected organ. The response rates were determined as the maximal response to treatment by 1 week, 2 weeks, and 1 month following initiation of treatment. Patients who could not have formal response determined for any reason were classified as treatment failures. All participants initiated therapy as inpatients, and were discharged after showing clinical improvement.
Safety Evaluation
Study drug toxicities were assessed continually with a directed physical exam and laboratory monitoring twice weekly for the first 30 days after initiation of therapy, followed biweekly between days 30 to 60, and then at 6 months. Adverse events (AEs) were evaluated according to National Cancer Center (NCI) CTC version 2.0.
Statistical Analysis
The primary objective of the trial was to determine the response rate and toxicity of infliximab when combined with MP versus steroids alone in untreated patients with aGVHD of the skin, GI, or liver. In addition, overall survival (OS) was compared between the 2 treatment arms at 180 days, 1 year, and 2 years following transplant were evaluated.
Sample size determination was based on the expectation that 45% of patients with newly diagnosed aGVHD would respond to standard therapy with MP alone based on historic data. The study was designed to provide 88% power to detect an increase in response rate to 75% associated with the addition of infliximab (Type I error=0.05, 2 sided). The sample size required to accept the alternative hypothesis was 70 patients. All patients who received a minimum of 1 dose of therapy were included in final analysis whether or not they completed the planned therapy unless they were removed from the study on account of their biopsy suggesting a non-GVHD diagnosis along with low clinical suspicion for aGVHD. Patients and response characteristics were compared using the chi-square and Fisher’s exact tests for categoric variables and the Mann-Whitney test for continuous variables. Actuarial OS was estimated by the Kaplan-Meier method. The cumulative incidence method was used to estimate the incidence of chronic GVHD (cGVHD), disease progression, nonrelapse mortality (NRM), and GVHD mortality accounting for competing risks. Outcomes were compared between the 2 arms using Cox’s proportional hazards model.
RESULTS
Patient Characteristics
A total of 63 patients were enrolled in this study between August 2000 and July 2003. Six of them were removed from the study after biopsies failed to identify GVHD in the setting of low clinical suspicion. The remainder 57 have their diagnosis of GVHD confirmed by biopsy. The average age was 47 years (range: 20–70 years). Forty of the 63 patients were male. Twenty-eight evaluable patients were randomized to treatment with MP and 29 patients received infliximab + MP. All patients were transplanted for hematologic malignancies, with a comparable risk of relapse for both groups. Patient characteristics did not statistically differ for patients on each study arm as represented in Table 1.
Response
Response was assessed at 1 week, 2 weeks, and 1 month following initiation of treatment for aGVHD. The response at different time points is summarized on Table 2. At 1 week the response in the infliximab group was lower than that in patients receiving MP alone (52% and 78%, respectively, P=.03). By 1 month, 62% of patients in the infliximab group had maintained a response versus 58% in the MP arm P=.7. The CR rates for aGVHD were not different between the 2 groups at any time point. There was no difference in response between the 2 arms based on GVHD severity or organ involvement (Table 2). The need and response to salvage therapy for aGVHD were similar in the 2 treatment arms. In the MP alone arm, 13 patients (46%) required salvage therapy, all of them with GI GVHD. The overall response rate was 54%, and the most common salvage treatments were infliximab (n=7) and ATG (n=5). Three of these 7 patients responded to infliximab when administered as salvage therapy, 2 of them with a CR. In the group randomized to infliximab + MP a total of 10 patients (34%) required salvage therapy and 30% responded (P=.2). The organ involvement in the 10 patients requiring salvage therapy included isolated GI GVHD (n=4), skin GVHD (n=4), liver GVHD (n=1), and GI plus liver GVHD (n=1). ATG and daclizumab were the most common salvage treatments (n=6 and 3, respectively).
Table 2.
Response to Therapy
| MP
|
Inflix + MP
|
P-Value | |||||
|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | ||||
| Resp at 7 days | CR | 13 | (46) | CR | 8 | (28) | .1 |
| PR/MR | 9 | (32) | PR/MR | 7 | (24) | .03 | |
| NR/PD | 6 | (22) | NR/PD | 14 | (48) | ||
| Resp at 2 weeks | CR | 13 | (46) | CR | 13 | (45) | CR=.9 |
| PR/MR | 8 | (29) | PR/MR | 6 | (21) | CR/PR/MR=.3 | |
| NR/PD | 7 | (25) | NR/PD | 10 | (34) | ||
| Resp at 1 month | CR | 15 | (54) | CR | 16 | (55) | CR=.9 |
| PR/MR | 1 | (04) | PR/MR | 2 | (07) | CR/PR/MR=.7 | |
| NR/PD | 12 | (43) | NR/PD | 11 | (38) | ||
| Resp by organ at 1 month: | |||||||
| Skin | CR/PR | 13/18 | (72) | CR/PR | 12/17 | (71) | Skin=.9 |
| GI | CR/PR | 9/14 | (64) | CR/PR | 10/15 | (67) | GI=.9 |
| Liver | CR/PR | 1/3 | CR/PR | 0/1 | |||
| Resp by severity at 1 month: | |||||||
| Grade II | CR/PR | 12/19 | (63) | CR/PR | 12/19 | (63) | Grade II=1.0 |
| Grade III/IV | CR/PR | 4/9 | (43) | CR/PR | 6/10 | (60) | Grade III/IV=.5 |
| Required salvage treatment | 14/28 (50) | 12/29 (41) | .5 | ||||
| Resp to salvage treatment | CR | 7 | (50) | CR | 2 | (17) | |
| PR | 1 | (7) | PR/MR | 3 | (25) | ||
| NR/PD | 6 | (43) | NR/PD | 7 | (58) | ||
CR indicates complete response; inflix, infliximab; MP, methylprednisolone; MR, mixed response; NR, no response; PD, progressive disease; PR, partial response; Resp, response; GI, gastrointestinal.
Therapy-Related Toxicity and Infections
Therapy-related toxicities and infections were followed for 6 months after initiation of treatment for GVHD. There were no infusion-related reactions or any other toxicity attributable to the administration of infliximab. The addition of infliximab to MP as initial therapy for aGVHD did not increase the risk for infection compared to MP alone (79% versus 86% MP versus MP + infliximab, respectively, P=.4). The frequency of bacterial, viral, or fungal infections were also similar in both arms as shown in Table 3. Serious infections, defined as those associated directly or indirectly with death, or any invasive fungal or myco-bacterial infections, occurred in 46% (n=13) and 51% (n=15) of patients (P=.7) in the MP and the MP + infliximab groups, respectively.
Table 3.
Infectious Complications
| MP (%) | Inflix+ MP (%) | P-Value | |
|---|---|---|---|
| 24 (86) | 23 (79) | .4 | |
| Infection type | |||
| Bacterial | 20 (83) | 18 (78) | .2 |
| Viral (all) | 13 (54) | 13 (57) | .6 |
| CMV | 8 (33) | 7 (30) | .5 |
| Fungal (all) | 8 (33) | 8 (35) | .6 |
| Aspergillus | 2 (08) | 2 (08) | .7 |
| Candida | 3 (12) | 4 (17) | .5 |
| Mycobacterium | 2 (08) | 1 (04) | .5 |
CMV indicates cytomegalovirus; Inflix, infliximab; MP, methylprednisolone.
Survival and Long-Term Complications
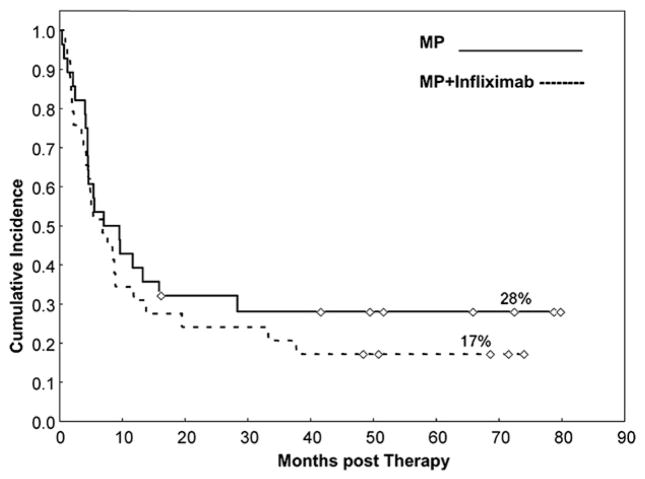
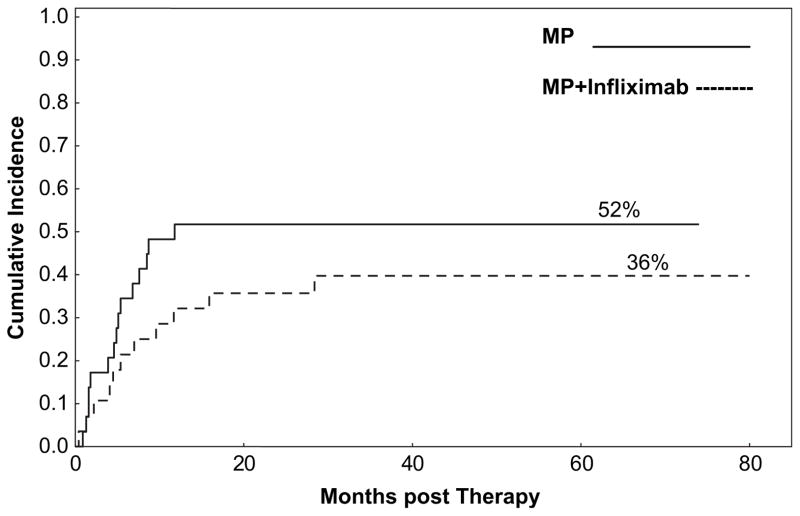
With a median follow-up among survivors of 59 months for the MP group and 68 months in the infliximab + MP arm, OS was 28% and 17%, respectively, P=.4 (Figure 1 and Table 4). Cumulative incidences (CIs) of GVHD-related mortality and NRM were not significantly different between the 2 groups (GVHD-related mortality: MP alone, 32% versus infliximab + MP, 38%, P=.6 and NRM: MP-alone, 36% versus infliximab + MP, 52%, P=.3) (Figure 2 and Table 4). The CI at 2 years for cGVHD was similar, with a CI of 48% in the MP arm versus 43% in the infliximab + MP arm, P=.9. Finally, the addition of infliximab as initial therapy did not increase the CI for tumor progression. Patients in the MP alone arm had a CI for tumor progression or recurrence of 28% compared with 25% in the infliximab + MP arm, P=.9. In both arms, either aGVHD or cGVHD was the most common cause of death, followed by death because of recurrence/progression of malignancy (Table 5).
Figure 1.
CI of survival for patients with aGVHD treated with MP or infliximab plus MP.
Table 4.
Patient Outcomes
| MP | Inflix + MP | p-Value (HR, 95% CI) | |
|---|---|---|---|
| Median Follow-up among survivors (range) | 59 (16–80) | 68 (48–74) | |
| 100-day survival (95% CI) | 82% (62–92) | 76% (56–88) | .6 |
| Overall survival (95% CI) | 28% (13–45) | 17% (6–33) | .4 |
| Nonrelapse mortality at 2 years | 36% (22–59) | 52% (36–73) | .3 (0.6, 0.3–1.4) |
| Cumulative incidence of chronic GVHD at (95% CI) | 48% (32–71) | 43% (28–66) | .9 (1.1, 0.5–2.3) |
| Cumulative incidence of progression at 2 years (95% CI) | 28% (15–52) | 25% (13–47) | .9 (1.0, 0.3–2.9) |
| Cumulative incidence of death from acute GVHD at 2 years (95% CI) | 11% (4–31) | 24% (13–46) | .2 (0.4, 0.1–1.7) |
| Cumulative incidence of death from acute or chronic GVHD at 2 years (95% CI) | 32% (19–56) | 38% (24–60) | .6 (1.3, 0.4–4.8) |
CI indicates confidence interval; GVHD, graft-versus-host disease; HR, hazard ratio; Inflix, infliximab; MP, methylprednisolone.
Figure 2.
CI of NRM for patients with aGVHD treated with MP or infliximab plus MP.
Table 5.
Causes of Death
| MP N (%) | Inflix + MP N (%) | P-Value (HR, 95% CI) | |
|---|---|---|---|
| Acute GVHD | 3 (15) | 7 (29) | .2 |
| Chronic GVHD | 6 (30) | 4 (17) | |
| Infection | 1 (05) | 1 (04) | |
| Relapse | 9 (45) | 9 (38) | |
| Organ failure | 0 (0) | 1 (04) | |
| Other/unknown | 1 (05) | 2 (08) |
GVHD indicates graft-versus-host disease; HR, hazard ratio; CI, confidence interval.
Study Termination
A futility analysis with the Cochran-Mantel-Haenszel [20] test was conducted after the accrual of the 63rd patient (data not shown). This showed that no significant difference would be detected between groups after completion of accrual, even with the best possible response to infliximab in the remainder of patients. Thus, the study was closed prior to reaching target accrual.
DISCUSSION
aGVHD is still the main complication following transplantation, and corticosteroids continue to be the initial standard approach when patients cannot be accrued into a clinical study. Infliximab, a monoclonal antibody (mAb) to the soluble and transmembrane forms of human TNF-α, has shown activity in the treatment of steroid refractory aGVHD, particularly in cases of GI involvement [18]. We designed a single-center, open-labeled, randomized study of infliximab for the initial treatment of aGVHD, with the hypothesis of a potentially higher efficacy earlier in the treatment of GVHD.
In this trial, the addition of infliximab to corticosteroids in the initial treatment of aGVHD did not provide any benefit, and response rates were similar in both arms. Infection rate and progression of malignancy were also comparable between groups. Similarly, survival was not influenced by the addition of infliximab, with only about a third of the patients surviving in each arm.
Previous randomized studies with a similar design included other agents in addition to corticosteroids for the initial treatment of aGVHD. These studies failed to show any benefit from the addition of the study drug, and in some instances outcomes were worse. Cragg et al. [9] randomized newly diagnosed patients with aGVHD to receive equine ATG in addition to prednisone versus prednisone alone. This study found that not only was the response rate for aGVHD not improved by the addition of ATG, but patients had aworse outcome as a result of a higher frequency of infections. More recently, Lee et al. [10] performed a randomized study combining upfront therapy with daclizumab, a humanized monoclonal antibody against the interleukin 2 receptor expressed on activated T lymphocytes. As in the ATG study, the daclizumab arm failed to improve response rates in patients with aGVHD and had a lower OS because of higher relapse and GVHD-related mortality. Unlike these previous studies, the addition of infliximab in our study did not appear to either increase the risk for infection or relapse, and survival was not inferior in the combination arm.
One hypothesis for the failure of infliximab to improve results in this study could be that therapy directed at 1 specific cytokine, TNF-α, does not sufficiently impact response rates, and overlaps with the broad anticytokine activity of corticosteroids. If this is true, similar results would be expected for etanercept, a similar anti-TNF-α antibody that has also been studied as a treatment for aGVHD. However, an initial report with etanercept demonstrated that this drug might be effective when combined with corticosteroids for patients with newly diagnosed aGVHD. In this single-center, phase II study, the combination of etanercept and corticosteroids resulted in a day 28 cumulative complete response rate of 69% in 65 patients with newly diagnosed aGVHD. When these results were then compared to the institution’s historic experience with steroids alone, a benefit was seen for patients enrolled onto the trial with combination therapy. More recently, the Blood and Marrow Clinical Trials Network (BMT CTN) conducted a multicenter, randomized, phase II trial comparing the combination of steroids with either etanercept, mycophenolate mofetil, denileukin diftitox, or pentostatin. The primary purpose of this trial was to find the most promising combination regimen to study in a definitive phase III trial [21]. In this trial, patients randomized to the MMF plus steroids arm had the highest CR rate with 60% of patients in complete response on day 28 and 73% on day 56. Despite similar design as the single institution, phase II trial with etanercept, dissimilar results were achieved for patients randomized to the etanercept arm with only 26% and 44% of the 46 patients achieving a complete response by day 28 and 56, respectively. The poor results for patients enrolled on the etanercept arm in this multicenter trial along with the results from our randomized trial with infliximab would suggest that anti-TNF-α therapy offers little advantage as initial therapy for patients with newly diagnosed aGVHD. The difference between infliximab and etanercept lies in the ability of infliximab to bind to both soluble and transmembrane bound TNF-α, whereas etanercept binds only to the transmembrane form. Although such a difference would seem modest, only a randomized phase III study with etanercept can conclude with certainty that the lack of benefit is not specific to infliximab alone.
The identification of patients at risk for failing initial therapy with MP alone and intensifying their therapy with additional immunomodulatory drugs remains a strategy for future studies. Unfortunately, in this study, we were not able to identify any risk factors or subset of patients that would suggest benefit from the combination of MP and infliximab. Neither patients with severe aGVHD (grade III/IV) nor did those patients with GI tract involvement appear to benefit from the addition of infliximab. Correlative studies measuring serum TNF-α levels might help to better define those patients who may benefit from TNF-α directed therapy. Thus, treatment could be directed toward patients whose aGVHD is associated with disproportionately high levels of TNF-α at diagnosis. In support of this hypothesis, Uberti et al. [22] demonstrated in their phase 2 study of etanercept that a reduction in TNF-α correlated with response to therapy. Whether this was simply a marker for response or a direct consequence of anti-TNF-α therapy remains untested.
The fact that now 3 randomized studies incorporating drugs that appeared effective in the steroid-refractory setting have proved ineffective when incorporated earlier in treatment questions such an approach. One possibility is that broadening immunosuppression in the prophylaxis setting or earlier in the course of aGVHD can impair T-regulatory cell reconstitution and immune tolerance as shown in preclinical models [23]. At this time, there is no justification for adding anti-TNF-α therapy or other forms of immunomodulation to the initial therapy for aGVHD with corticosteroids alone other than in the context of a clinical trial. However, the use of immunomodulators with a different mechanism of action and the availability of broader antimicrobial prophylaxis could result in better outcomes. Based on the encouraging phase II results with MMF in the BMT CTN trial, a phase III trial with MMF is due to open in mid-2009. It remains to be seen whether MMF can succeed where now 3 previous tested drugs have failed.
Footnotes
AUTHORSHIP
Rima Saliba analyzed data and performed statistical analysis. Sergio Giralt, Marcos de Lima, Borje Andersson, Issa Khouri, and Chitra Hosing participated in the designed of the study and performed the research. Elizabeth J. Shpall participated in the analysis of the data. Cindy Ippoliti coordinated investigational pharmacy issues and participated in the research. Richard Champlin participated in the design of the study, research, and data analysis. Amin Alousi participated in data analysis and writing the manuscript.
Financial disclosure: This article was supported by Centocor, Inc. Preliminary results of this study were presented in the American Society of Blood and Marrow Transplantation Annual Meeting in February 2004.
References
- 1.Ratanatharathorn V, Nash RA, Przepiorka D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92:2303–2314. [PubMed] [Google Scholar]
- 2.Mielcarek M, Martin PJ, Leisenring W, et al. Graft-versus-host disease after nonmyeloablative versus conventional hematopoietic stem cell transplantation. Blood. 2003;102:756–762. doi: 10.1182/blood-2002-08-2628. [DOI] [PubMed] [Google Scholar]
- 3.Chao NJ, Snyder DS, Jain M, et al. Equivalence of 2 effective graft-versus-host disease prophylaxis regimens: results of a prospective double-blind randomized trial. Biol Blood Marrow Transplant. 2000;6:254–261. doi: 10.1016/s1083-8791(00)70007-3. [DOI] [PubMed] [Google Scholar]
- 4.Chao NJ, Schmidt GM, Niland JC, et al. Cyclosporine, methotrexate, and prednisone compared with cyclosporine and prednisone for prophylaxis of acute graft-versus-host disease. N Engl J Med. 1993;329:1225–1230. doi: 10.1056/NEJM199310213291703. [DOI] [PubMed] [Google Scholar]
- 5.Martin PJ, Schoch G, Fisher L, et al. A retrospective analysis of therapy for acute graft-versus-host disease: initial treatment. Blood. 1990;76:1464–1472. [PubMed] [Google Scholar]
- 6.MacMillan ML, Weisdorf DJ, Wagner JE, et al. Response of 443 patients to steroids as primary therapy for acute graft-versus-host disease: comparison of grading systems. Biol Blood Marrow Transplant. 2002;8:387–394. doi: 10.1053/bbmt.2002.v8.pm12171485. [DOI] [PubMed] [Google Scholar]
- 7.Weisdorf D, Haake R, Blazar B, et al. Treatment of moderate/severe acute graft-versus-host disease after allogeneic bone marrow transplantation: an analysis of clinical risk features and outcome. Blood. 1990;75:1024–1030. [PubMed] [Google Scholar]
- 8.Van Lint MT, Uderzo C, Locasciulli A, et al. Early treatment of acute graft-versus-host disease with high- or low-dose 6-methyl-prednisolone: a multicenter randomized trial from the Italian Group for Bone Marrow Transplantation. Blood. 1998;92:2288–2293. [PubMed] [Google Scholar]
- 9.Cragg L, Blazar BR, Defor T, et al. A randomized trial comparing prednisone with antithymocyte globulin/prednisone as an initial systemic therapy for moderately severe acute graft-versus-host disease. Biol Blood Marrow Transplant. 2000;6:441–447. doi: 10.1016/s1083-8791(00)70036-x. [DOI] [PubMed] [Google Scholar]
- 10.Lee SJ, Zahrieh D, Agura E, et al. Effect of up-front daclizumab when combined with steroids for the treatment of acute graft-versus-host disease: results of a randomized trial. Blood. 2004;104:1559–1564. doi: 10.1182/blood-2004-03-0854. [DOI] [PubMed] [Google Scholar]
- 11.Ferrara JL, Cooke KR, Teshima T. The pathophysiology of acute graft-versus-host disease. Int J Hematol. 2003;78:181–187. doi: 10.1007/BF02983793. [DOI] [PubMed] [Google Scholar]
- 12.Schmaltz C, Alpdogan O, Horndasch KJ, et al. Differential use of Fas ligand and perforin cytotoxic pathways by donor T cells in graft-versus-host disease and graft-versus-leukemia effect. Blood. 2001;97:2886–2895. doi: 10.1182/blood.v97.9.2886. [DOI] [PubMed] [Google Scholar]
- 13.Schmaltz C, Alpdogan O, Muriglan SJ, et al. Donor T cell-derived TNF is required for graft-versus-host disease and graft-versus-tumor activity after bone marrow transplantation. Blood. 2003;101:2440–2445. doi: 10.1182/blood-2002-07-2109. [DOI] [PubMed] [Google Scholar]
- 14.Remberger M, Jaksch M, Uzunel M, Mattsson J. Serum levels of cytokines correlate to donor chimerism and acute graft-vs.-host disease after haematopoietic stem cell transplantation. Eur J Haematol. 2003;70:384–391. doi: 10.1034/j.1600-0609.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 15.Knight DM, Trinh H, Le J, et al. Construction and initial characterization of a mouse-human chimeric anti-TNF antibody. Mol Immunol. 1993;30:1443–1453. doi: 10.1016/0161-5890(93)90106-l. [DOI] [PubMed] [Google Scholar]
- 16.Nesbitt A, Fossati G, Bergin M, et al. Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis. 2007;13:1323–1332. doi: 10.1002/ibd.20225. [DOI] [PubMed] [Google Scholar]
- 17.Patriarca F, Sperotto A, Damiani D, et al. Infliximab treatment for steroid-refractory acute graft-versus-host disease. Haematologica. 2004;89:1352–1359. [PubMed] [Google Scholar]
- 18.Couriel D, Saliba R, Hicks K, et al. Tumor necrosis factor-alpha blockade for the treatment of acute GVHD. Blood. 2004;104:649–654. doi: 10.1182/blood-2003-12-4241. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. Consensus conference on acute GVHD grading. Bone Marrow Transplant 1995. 1994;15:825–828. [PubMed] [Google Scholar]
- 20.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 21.Alousi A, Weisdorf DJ, Logan BR, et al. BMT CTN 0302: a phase II randomized trial evaluating etanercept, mycophenolate mofetil (MMF), denileukin diftitox, and pentostatin in combination with corticosteroids in 180 patients (pts) with newly diagnosed acute graft vs. host disease (aGVHD) ASH Annu Meet Abstr. 2008;112:55. [Google Scholar]
- 22.Uberti JP, Ayash L, Ratanatharathorn V, et al. Pilot trial on the use of etanercept and methylprednisolone as primary treatment for acute graft-versus-host disease. Biol Blood Marrow Transplant. 2005;11:680–687. doi: 10.1016/j.bbmt.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Zeiser R, Nguyen VH, Beilhack A, et al. Inhibition of CD4+ CD25+ regulatory T-cell function by calcineurin-dependent interleukin-2 production. Blood. 2006;108:390–399. doi: 10.1182/blood-2006-01-0329. [DOI] [PMC free article] [PubMed] [Google Scholar]