Abstract
Diverse 4-aryl-2-quinolinones are prepared from propionamides in one pot via ligand-promoted triple sequential C–H activation reactions and a stereospecific Heck reaction. In these cascade reactions, three new C–C bonds and one C–N bond are formed to rapidly build molecular complexity from propionic acid
Keywords: C–H activation, arylation, dehydrogenation, amidation, quinolinone
Ligand-controlled or -accelerated C(sp3)–H activation with Pd(II) catalysts has recently emerged as a promising strategy for developing new catalytic transformations.[1–3] Notable examples include the cross-coupling of γ-C(sp3)–H bonds of protected amines with arylboron reagents enabled by mono-protected amino acid ligands[1] as well as β-arylation of primary and secondary C(sp3)–H bonds promoted by pyridine and quinoline ligands.[2] The compatibility of these ligands with C(sp3)–H activation and subsequent functionalization steps offers unprecedented opportunities to discover new catalytic reaction pathways by influencing the reactivity of various potential organopalladium intermediates. In particular, if a common ligand can be identified to promote cascade C–H activation reactions, molecular complexity and diversity can be readily generated from simple starting materials via sequential and diverse C–H functionalizations.[4] Indeed cascade reactions involving a Heck reaction and a subsequent C–H activation step provide an elegant route for the synthesis of spirodihydroquinolin-2-ones.[4f] However, cascade C–H activation reactions of simple aliphatic substrates leading to complexity have not been demonstrated.
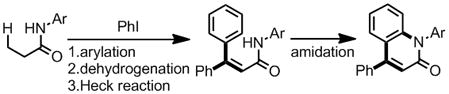
Inspired by our studies of ligand-controlled β-arylation reactions of primary and secondary C(sp3)–H bonds,[2] we envisioned that sequential arylation of the β-C(sp3)–H bonds of propionamide followed by seven-membered cyclopalladation/amidation would result in a one-pot procedure for the preparation of 4-aryl-3,4-dihydro-2-quinolinones (eq 1). Herein we report the unexpected
 |
(1) |
 |
(2) |
discovery of a novel procedure that combines three sequential C–H activation reactions of propionamide and a stereospecific Heck reaction in one pot to afford a diverse range of 4-aryl-2-quinolinones (eq 2). 4-Aryl-2-quinolinone derivatives constitute a valuable class of biologically active compounds, including natural products[5] and medicinal compounds[6] (Scheme 1). Moreover, they may serve as valuable synthetic intermediates to 2-(pseudo)-haloquinolines as well as 2-alkoxyquinolines for use as ligands in C–H activation reactions.[2] Considering the broad interest in 4-aryl-2-quinolinones,[7] this newly developed one-pot procedure could prove useful in synthetic and medicinal chemistry.
Scheme 1.

Biologically active 4-aryl-2-quinolinones.
Our initial efforts were largely prompted by the prospect that simple and abundant starting materials (e.g. propionic acid) can be potentially transformed into complex molecules via multiple C–H functionalizations. Thus, amide 1a, derived from propionic acid, was subjected to various arylation conditions previously developed in our laboratory in order to achieve diarylation and subsequent ligand promoted C–H lactamization (eq 1). We found, through extensive screening of palladium catalysts and solvents, that a combination of PdCl2 and 2-alkoxylquinoline ligand L1[2b] in t-AmylOH catalyzed the sequential C–H activations/oxidative carbon-carbon bond forming reaction of 1a to afford the unexpected 4-phenyl-2-quinolinone 3a in 58% yield, along with a 17% yield of β-phenylated propionamide product 2a (Table 1, for detailed screening, see Supporting Information). The control experiment in the absence of ligand showed that 2a was formed in 11% yield as the only product. The observed dramatic impact of ligand L1 on this reaction prompted us to further test a variety of pyridine- or quinoline-based ligands. Surprisingly, the use of simple pyridine L3 as the ligand provided 3a in 68% yield and 2a in 23% yield. 2-Picoline (L6) and 3-picoline (L7) also gave comparable yields. 2,6-Dimethoxypyridine (L9) is highly selective for monoarylation, albeit in a relatively low yield (42% yield). Further investigation reveals that lutidines L10, L11, and L12 are more effective than other pyridine ligands, affording the cyclized product 3a in 80–84% yields.
Table 1.

|
ArF = (4-CF3)C6F4.
1a (0.2 mmol), PdCl2 (0.02 mmol), Ag2CO3 (0.6 mmol), PhI (0.8 mmol), ligand (0.04 mmol), t-AmylOH (0.5 mL), 140 °C, 24 h.
Yield determined by NMR spectroscopy with CH2Br2 as the internal standard.
With these conditions in hand, we examined the scope of aryl iodides with 1a to test the feasibility of preparing a variety of 4-aryl-2-quinolinones 3 (Table 2). Reactions with mono- or dimethyl substituted-phenyl iodides yield 4-aryl-2-quinolinones 3b–3e in 68–75% yields, with intramolecular amidation occurring at the less hindered position of the aryl group. para-Methoxyphenyl iodide was also reactive, affording the desired product 3f in 60% yield. Electron-withdrawing fluoro, chloro and trifluoromethyl groups are also well tolerated (3g–3i), while the presence of a para-ester group decreased the yield to 44% (3j).
Table 2.

|
ArF = (4-CF3)C6F4.
1a (0.2 mmol), PdCl2 (0.02 mmol), Ag2CO3 (0.6 mmol), ArI (0.8 mmol), 2,5-lutidine (0.04 mmol), t-AmylOH (0.5 mL), 140 °C, 24 h.
Isolated yield.
While one-pot synthesis of 4-aryl-2-quinolinones from a simple propionamide demonstrates excellent step economy, the incorporation of two identical aryl groups into the products results in limited structural diversity. In order to address this issue, we envisioned the installation of two distinct arenes through the mono-selective arylation of 1a to yield various hydrocinnamic acid derivatives, followed by subsequent secondary arylation with a different aryl iodide to furnish heterodiaryl 2-quinolinones. Thus, we established moderately effective ligandless conditions for mono-arylation of 1a and prepared hydrocinnamic acid amides 2a–2e on gram scale (see Supporting Information). Hydrocinnamide 2a was then further reacted with different aryl iodides (Ar2I) under ligand-mediated conditions to afford a set of diverse 4-aryl-2-quinolinones 3k–3q in 62–86% yield, with the Ar2 group introduced regioselectively at the 4-position of the 2-quinolinone moiety (Table 3). While the use of other less hindered ligands L3 and L7 afforded the same regioselectivity, the yields decreased significantly (see Supporting Information). Hydrocinnamides 2b–2e were also reacted with PhI under the standard conditions to afford various 4-aryl-2-quinolinones 3r–3u in 60–81% yield. Notably, previous syntheses of 4-aryl-2-quinolinones via sequential Heck reaction/C-H lactamization use acrylamide as the starting material and proceed through three distinct steps catalyzed by three different catalysts.[7c] In addition, this method gave a mixture of regioisomers when two different aryls are incorporated into the products.
Table 3.

|
ArF = (4-CF3)C6F4.
2 (0.2 mmol), PdCl2 (0.02 mmol), Ag2CO3 (0.4 mmol), Ar2I (0.6 mmol), 2,5-lutidine (0.04 mmol), t-AmylOH (0.5 mL), 140 °C, 24 h.
Isolated yield.
Considering that various 4-aryl-2-quinolinones contain different aryl groups (Ar) on the nitrogen, we made efforts to broaden the reaction scope by using differentially substituted propionamide substrates 1, prepared from different anilines and propionyl chloride (Table 4). Thus, cascade products 3v–3z were prepared in 62–75% yield with 2,6-difluoro or dichloro substitutions on the aryl ring (Ar). Further studies show that the 2,6-disubstitutions are required for sufficient reactivity. The amides derived from simple anilines also proceeded to afford 3a1–3i1 in 40–54% yield. Methoxy (3e1), OTs (3f1), chloro (3h1), and bromo (3i1) groups on the aryl ring are also amenable to further synthetic elaborations. Although the yields remain to be improved, this one-pot procedure utilizing inexpensive propionic acid as the starting material could prove broadly useful for medicinal chemistry.
Table 4.

|
1 (0.2 mmol), PdCl2 (0.02 mmol), Ag2CO3 (0.6 mmol), PhI (0.8 mmol), 2,5-lutidine (0.04 mmol), t-AmylOH (0.5 mL), 140 °C, 24 h.
Isolated yield.
The use of para-methoxyaniline[8] also allowed us to establish a new route for the deprotection of N-substituted lactams.[9] Thus, 4-phenyl-2-quinolinone 3e1 (prepared using our newly-developed one-pot procedure) was deprotected to yield lactam 4, which is a known precursor for the synthesis of drug molecules such as 5[10] and 6[11] (Scheme 2).
Scheme 2.
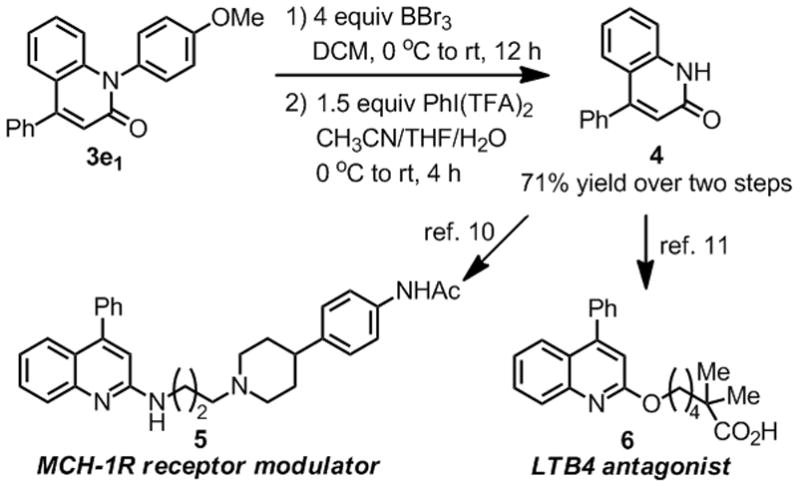
Synthetic applications.
Initially, it appeared plausible that the reaction pathway involves sequential β-arylations followed by dehydrogenation via Pd-insertion, and finally intramolecular C(sp2)–H amidation (eq 2). However, as illustrated in Table 3, incorporation of the second aryl is exclusively selective for the 4-position of the 2-quinolinone ring. This result reveals that the second aryl is consistently in a trans relationship with the amide directing group prior to the C–H lactamization. These observed results are inconsistent with a mechanism involving consecutive β-arylations followed by Pd-mediated dehydrogenation. In order to account for this selectivity, we instead propose participation of a stereospecific Heck reaction of a cinnamide intermediate with the second aryl iodide coupling partner.[12]
As such, we present the following mechanism: β-arylation of the primary C(sp3)–H bond affords hydrocinnamide 2. Pd-insertion into the secondary C(sp3)–H bond followed by β-hydride elimination would then yield cinnamide intermediate 7,[13] which allows for subsequent Heck coupling with a second aryl iodide. Intramolecular C–H amidation[14] would then provide the 4-aryl-2-quinolinone product 3 (Scheme 3). The reaction of independently prepared 7 with ArI also gave the desired quinolinones, thus verifying the viability of 7 as an intermediate (see Supporting Information).
Scheme 3.

Proposed catalytic pathway.
In conclusion, we have developed an unprecedented pyridine ligand-promoted cascade C–H activation of propionamides under oxidative palladium catalysis, which provides a one-pot procedure for the preparation of diverse 4-aryl-2-quinolinones from propionic acid. This cascade reaction involves the cleavage of five C–H bonds, two C–I bonds, and one N–H bond, and the formation of three C–C bonds and one C–N bond via four different types of palladium catalytic cycles. Further studies on the scope, mechanism, and synthetic application of this reaction are underway in our laboratory.
Supplementary Material
Footnotes
We gratefully acknowledge The Scripps Research Institute and the NIH (NIGMS, 2R01GM084019) for financial support.
Dedicated to Professor Björn Åkermark on the occasion of his 80th birthday
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201xxxxxx.
References
- 1.Chan KSL, Wasa M, Chu L, Laforteza BN, Miura M, Yu J-Q. Nat Chem. 2014;6:146. doi: 10.1038/nchem.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) He J, Li S, Deng Y, Fu H, Laforteza BN, Spangler JE, Homs A, Yu JQ. Science. 2014;343:1216. doi: 10.1126/science.1249198. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Wasa M, Chan KSL, Zhang XG, He J, Miura M, Yu JQ. J Am Chem Soc. 2012;134:18570. doi: 10.1021/ja309325e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.a) He J, Wasa M, Chan KSL, Yu JQ. J Am Chem Soc. 2013;135:3387. doi: 10.1021/ja400648w. [DOI] [PubMed] [Google Scholar]; b) Wasa M, Engle KM, Yu JQ. J Am Chem Soc. 2009;131:9886. doi: 10.1021/ja903573p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu P-F, Wang W, editors. Catalytic Cascade Reactions. Wiley-VCH; Weinheim: 2013. for selected examples of cascade reactions involving palladium-catalyzed C-H activations, see: Wang GW, Yuan TT, Li DD. Angew Chem. 2011;123:1416. doi: 10.1002/anie.201005874.Angew Chem Int Ed. 2011;50:1380.Karthikeyan J, Cheng CH. Angew Chem. 2011;123:10054. doi: 10.1002/anie.201104311.Angew Chem Int Ed. 2011;50:9880.Piou T, Neuville L, Zhu J. Angew Chem. 2012;124:11729. doi: 10.1002/anie.201206267.Angew Chem Int Ed. 2012;51:11561.Piou T, Bunescu A, Wang Q, Neuville L, Zhu J. Angew Chem. 2013;125:12611. doi: 10.1002/anie.201306532.Angew Chem Int Ed. 2013;52:12385.Piou T, Neuville L, Zhu J. Org Lett. 2012;14:3760. doi: 10.1021/ol301616w.
- 5.For natural products, see: Kitahara Y, Shimizu M, Kubo A. Heterocycles. 1990;31:2085.Kobayashi Y, Harayama T. Org Lett. 2009;11:1063. doi: 10.1021/ol900255g. and references cited therein.
- 6.For selected important medicinal products, see: Angibaud PR, Venet MG, Filliers W, Broeckx R, Ligny YA, Muller P, Poncelet VS, End DW. Eur J Org Chem. 2004:479.Andresen BM, Couturier M, Cronin B, D’Occhio M, Ewing MD, Guinn M, Hawkins JM, Jasys VJ, LaGreca SD, Lyssikatos JP, Moraski G, Ng K, Raggon JW, Stewart AM, Tickner DL, Tucker JL, Urban FJ, Vazquez E, Wei L. Org Process Res Dev. 2004;8:643.van Cutsem E, van de Velde H, Karasek P, Oettle H, Vervenne WL, Szawlowski A, Schoffski P, Post S, Verslype C, Neumann H, Safran H, Humblet Y, Ruixo JP, Ma Y, von Hoff D. J Clin Oncol. 2004;22:1430. doi: 10.1200/JCO.2004.10.112.Cheng P, Zhang Q, Ma YB, Jiang ZY, Zhang XM, Zhang FX, Chen JJ. Bioorg Med Chem Lett. 2008;18:3787. doi: 10.1016/j.bmcl.2008.05.065.Chen MH, Fitzgerald P, Singh SB, O’Neill EA, Schwartz CD, Thompson CM, O’Keefe SJ, Zallerb DM, Doherty JB. Bioorg Med Chem Lett. 2008;18:2222. doi: 10.1016/j.bmcl.2006.10.097.Wall MJ, Chen J, Meegalla S, Ballentine SK, Wilson KJ, DesJarlais RL, Schubert C, Chaikin MA, Crysler C, Petrounia IP, Donatelli RR, Yurkow EJ, Boczon L, Mazzulla M, Player MR, Patch RJ, Manthey CL, Molloy C, Tomczuk B, Illig CR. Bioorg Med Chem Lett. 2008;18:2097. doi: 10.1016/j.bmcl.2008.01.088.Kraus JM, Verlinde CLMJ, Karimi M, Lepesheva GI, Gelb MH, Buckner FS. J Med Chem. 2009;52:1639. doi: 10.1021/jm801313t.Kraus JM, Tatipaka HB, McGuffin SA, Chennamaneni NK, Karimi M, Arif J, Verlinde CLMJ, Buckner FS, Gelb MH. J Med Chem. 2010;53:3887. doi: 10.1021/jm9013136.
- 7.For selected examples of synthesis of 4-aryl-2-quinolinones, see: Ferguson J, Zeng F, Alwis N, Alper H. Org Lett. 2013;15:1998. doi: 10.1021/ol4006739.Inamoto K, Kawasaki J, Hiroya K, Kondo Y, Doi T. Chem Commun. 2012;48:4332. doi: 10.1039/c2cc30600j.Berrino R, Cacchi S, Fabrizi G, Goggiamani A. J Org Chem. 2012;77:2537. doi: 10.1021/jo202427m.Shibuya T, Shibata Y, Noguchi K, Tanaka K. Angew Chem. 2011;123:4049. doi: 10.1002/anie.201100152.Angew Chem Int Ed. 2011;50:3963.Inamoto K, Saito T, Hiroya K, Doi T. J Org Chem. 2010;75:3900. doi: 10.1021/jo100557s.Wasa M, Yu JQ. J Am Chem Soc. 2008;130:14058. doi: 10.1021/ja807129e.Battistuzzi G, Bernini R, Cacchi S, De Salve I, Fabrizi G. Adv Synth Catal. 2007;349:297.Cortese NA, Ziegler CB, Jr, Hrnjez BJ, Heck RF. J Org Chem. 1978;43:2952.
- 8.The N-deprotection of product 3x derived from 2,6-difluoro-4-methoxyaniline was also extensively tried under different conditions, but has not been realized so far.
- 9.Nakazaki A, Mori A, Kobayashi S, Nishikawa T. Tetrahedron Lett. 2012;53:7131. [Google Scholar]
- 10.Hino K, Furukawa K, Nagai Y, Uno H. Chem Pharm Bull. 1980;28:2618. doi: 10.1248/cpb.28.2618. [DOI] [PubMed] [Google Scholar]
- 11.Labaudinière R, Hendel W, Terlain B, Cavy F, Marquis O, Dereu N. J Med Chem. 1992;35:4306. doi: 10.1021/jm00101a007. [DOI] [PubMed] [Google Scholar]
- 12.Bernini R, Cacchi S, Salve ID, Fabrizi G. Synlett. 2006:2947. [Google Scholar]
- 13.For selected examples involving palladium-catalyzed dehydrogenations, see: Gao W, He Z, Qian Y, Zhao J, Huang Y. Chem Sci. 2012;3:883.Diao T, Stahl SS. J Am Chem Soc. 2011;133:14566. doi: 10.1021/ja206575j.Diao T, Wadzinski TJ, Stahl SS. Chem Sci. 2012;3:887. doi: 10.1039/C1SC00724F.Diao T, Pun D, Stahl SS. J Am Chem Soc. 2013;135:8205. doi: 10.1021/ja4031648.Huang Z, Dong G. J Am Chem Soc. 2013;135:17747. doi: 10.1021/ja410389a.Gigant N, Bäckvall JE. Chem Eur J. 2014;20 doi: 10.1002/chem.201402063.
- 14.Louillat M-L, Patureau FW. Chem Soc Rev. 2014;43:901. doi: 10.1039/c3cs60318k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


