Abstract
γδ T cells are resident in cerebrospinal fluid and central nervous system (CNS) lesions of multiple sclerosis (MS) patients, but as multifaceted cells exhibiting innate and adaptive characteristics, their function remains unknown. Previous studies in experimental autoimmune encephalomyelitis (EAE) are contradictory and identified these cells as either promoting or suppressing disease pathogenesis. This study examines distinct γδ T cell subsets during EAE and indicates they mediate differential functions in CNS inflammation and demyelination resulting in pathogenesis or protection. We identified two γδ subsets in the CNS, Vγ1+ and Vγ4+, with distinct cytokine profiles and tissue specificity. Anti-γδ T cell receptor (TCR) monoclonal antibody (mAb) administration results in activation and downregulation of surface TCR, rendering the cells undetectable, but with opposing effects: anti-Vγ4 treatment exacerbates disease whereas anti-Vγ1 treatment is protective. The Vγ4+ subset produces multiple proinflammatory cytokines including high levels of IL-17, and accounts for 15-20% of the interleukin-17 (IL-17) producing cells in the CNS, but utilize a variant transcriptional program than CD4+ Th17 cells. In contrast, the Vγ1 subset produces CCR5 ligands, which may promote regulatory T cell differentiation. γδ T cell subsets thus play distinct and opposing roles during EAE, providing an explanation for previous reports and suggesting selective targeting to optimize regulation as a potential therapy for MS.
Keywords: γδ T cells, experimental autoimmune encephalomyelitis, autoimmunity, T cells, multiple sclerosis, innate immunity, adaptive immunity
1. Introduction
MS and its murine model, EAE, are characterized by perivascular T cell and mononuclear cell infiltration in the central nervous system (CNS) with subsequent primary demyelination of axonal tracts leading to progressive paralysis. Autoreactive CD4+ T cells in MS patients and in EAE respond to a variety of myelin membrane constituents including myelin basic protein, myelin proteolipid protein (PLP), and/or myelin-oligodendrocyte glycoprotein, [1; 2] which induce CNS inflammation and demyelination. With the recent revelation that IL-17-mediated inflammation, rather than IFN-γ responses, are most critical during autoimmunity, the research focus has centered on understanding the differentiation and effector functions of CD4+ Th17 cells in EAE and MS [3; 4; 5]. However, conventional Th17 cells are not the sole producers of IL-17. γδ T cells secrete large amounts of IL-17, perhaps even without the clonal expansion or additional TCR stimulation required for the adaptive response [6; 7] [8]. Interestingly, IL-17 producing γδ T cells have been shown to be pathogenic in models of autoimmunity including collagen induced arthritis and protective for airway hyper-reactivity, indicating a pleiotropic role for γδ T cells in immune-mediated pathology [9; 10] [11].
Significant numbers of γδ T cells have been identified in the cerebral spinal fluid and the CNS demyelinating lesions of MS patients. In addition to clonally expanded αβ T cell populations, which use a restricted set of gene segments, γδ T cells also display a restricted repertoire that is over-expressed in MS plaques [12; 13]. Junctional sequence analysis of these expanded cells suggests they are oligoclonal in nature, perhaps indicating specific antigen stimulation. It has been proposed that γδ T cells respond to heat shock proteins, which could be released in response to inflammatory CNS tissue damage [14]. Although the antigen specificity and regulation of these cells is not well understood, it is clear γδ T cells are involved in the autoimmune CNS inflammation in MS.
Past attempts utilizing murine models of MS to study the role of γδ T cells in the pathogenesis of autoimmune demyelination have been contradictory [reviewed in [15]]. On the one hand, γδ T cells have been shown to play a protective role or no role at all during disease. It has been proposed that γδ T cells regulate autoimmune inflammation via Fas-FasL mediated killing of CNS antigen-specific T cells based on the observation that γδ T cell-deficient mice on the B10.PL background develop a chronic disease compared to the monophasic acute disease course seen in the control animals [16; 17]. However, an additional study concluded that regulation of autoreactive inflammation in EAE is specifically the role of T regulatory cells and not γδ T cells [18]. To further complicate the situation γδ T cells have been reported to enhance autoimmunity by restraining Treg responses [19]. Similarly, adoptive transfer of autoreactive CD4+ T cells into γδ-deficient recipient mice on the C57BL/6 (B6) background elicited similar disease as seen in the WT recipient controls suggesting γδ T cells do not play a significant role in the mediation or regulation of effector mechanisms in EAE [20].
Unlike the aforementioned data, other reports support the hypothesis that γδ T cells play a pathogenic role during disease [21; 22; 23]. Targeting γδ T cells with monoclonal antibodies during various stages of disease resulted in the inability to detect the cells as well as decreased disease, suggesting γδ T cells play a critical role in the pathogenesis of EAE during both acute and chronic phases [24]. Similarly, in both actively induced and adoptively transferred EAE in B6 mice that genetically lack γδ T cells, EAE disease was significantly reduced [25]. These diverse and conflicting results obtained from animal model studies aimed to dissect the mechanisms of γδ T cell involvement in demyelinating disease could attributed to the use of a variety of mouse strains, inducing antigens and methods of γδ T cell manipulation, namely genetic depletions or monoclonal antibody targeting in vivo.
γδ T cells are a heterogeneous population and perhaps the pleiotropic nature of this cell subset and thus the variety of results from many different studies aimed at determining their role during EAE and MS may be explained by a dichotomy of γδ T cell subset function. We therefore sought to examine a possible dichotomy of these pleiotropic cells within the murine model of MS with the goal of clarifying the previous controversy surrounding the role of γδ T cells in EAE as well as to provide evidence for an alternative method of specifically targeting these cells as a possible treatment for MS. Our study indicates that γδ T cell subsets play opposing roles, such that targeted treatment could optimize the regulation of disease. We show that Vγ4-expressing γδ T cells constitute a significant proportion of IL-17-producing cells in the CNS during EAE pathogenesis and when activated in vivo, exacerbate disease symptoms due to their pathogenic nature. Conversely, the Vγ1 subset plays a protective role and perhaps eliciting function at the priming stage within the spleen rather than in the CNS. Using a γδ T cell reporter mouse we were able to show that in vivo antibody treatment resulted in activation of the γδ T cell subsets and not depletion. Collectively, these data provide some much needed explanation for the contradictory literature surrounding the role of γδ T cells during EAE. We propose that γδ T cell subsets show distinct and opposing functions, such that antibody targeting of these cells may allow a more carefully defined inhibition of the pathogenic response in MS, while maintaining the protective immune mechanisms of these critical immune cells.
2. Materials and Methods
2.1. Mice and peptides
Female SJL/J (Harlan Sprague Dawley), C57BL/6J and Tcrd-/- (The Jackson Laboratory) and Tcrd-eGFP mice [26] were housed under specific pathogen-free conditions in the Northwestern University Animal Facility. All protocols were approved by Northwestern University Animal Care and Use Committee. PLP139–151 (HSLGKWLGHPDKF) and MOG35–55 (MEVGWYRSPFSRVVHLYRNGK), were purchased from Genemed Synthesis (San Francisco, CA).
2.2. Induction of EAE
Chronic EAE (C-EAE) was induced in Tcrd-eGFP, Tcrd-/-, and C57BL/6 mice with 200 μg MOG35-55 with CFA subcutaneously and treated intraperitoneally with 200 ng Pertussis Toxin on days 0 and 2 relative to immunization. Relapsing-remitting EAE (R-EAE) was induced in SJL/J mice with 50 μg PLP139-151 with CFA subcutaneously. Mice were analyzed daily and disease severity scored with the following scale: 0, no symptoms; 1, loss of tail tonicity; 2, unilateral hind limb paralysis; 3, bilateral hind limb paralysis; 4, front limb paralysis and 5, moribund [2].
2.3. In vivo antibody treatment
Mice were treated i.v. with 200 μg anti-γδ TCR (clone UC7), anti-Vγ4 (clone UC3), anti Vγ1 (clone 2.11) or Control Ig (hamster IgG) on days 0 and 2 where day 0 was the day of disease induction for disease course studies. For T cell tracking experiments, Tcrd-eGFP animals were treated with antibody on days 0 and 2, spleen and inguinal lymph nodes were collected at day 7 and FACS analysis was performed.
2.4. Immunofluorescence
After euthanasia, mice were perfused with 4% PFA in PBS and the brain and spinal column were dissected and incubated in fixative overnight at 4°C on a shaker. The tissue was then transferred to 30% sucrose in PBS and allowed to incubate overnight at 4°C followed by placement in cryomold with O.C.T. in and frozen on dry ice for 45minutes. The blocks were stored at -80°C until sectioning. The frozen tissue was sectioned into 10 μm slices and placed onto slides. For immunohistology, slides were first labeled for myelin proteolipid protein (Serotec) 1:200 in PBS + 0.5% normal donkey serum + 0.1% triton-x 100 (PBS+) overnight at 4°C and fixed with cold acetone for 10 minutes prior to labeling. After washing in PBS, slides were incubated in donkey anti-mouse Cy3 (Jackson ImmunoResearch) for 60 minutes at room temperature. Finally, the slides were washed in PBS, incubated in 50 μg/ml DAPI nuclear stain (4′,6-Diamidino-2-phenylindole dihydrochloride (3), Sigma) for 5 minutes, washed and coverslipped with Vector Hard Setting Mounting Medium (Vector Labs). The sections were viewed on the Zeiss LSM 510 META laser scanning confocal microscope located in the Northwestern University Cell Imaging Facility. Confocal stacks were taken at 0.37μm intervals to yield a projection image enabling the visualization of the proximity of γδ GFP+ cells to MBP+ cells. A three dimensional image was rendered using Volocity software.
2.5. Cell Isolation
Mice were anesthetized with Nembutal followed by PBS cardiac perfusion. The cerebellum and spinal cord were chopped with scissors, incubated with collagenase for 30 min at 37°C and physically disrupted over a metal screen. The resulting cellular suspension was resuspended in 30% Percoll, overlayed onto 70% Percoll and centrifuged at 1000 rpm for 25 min at 25°C. The cells at the interfaced were collected, washed and counted. Splenocytes and lymph nodes were isolated by physical disruption, red blood lysis (for spleens only) was performed and cells were washed and counted.
2.6. Flow cytometry and single cell sorting
Single cell suspensions were stained with VID dye (Invitrogen) for live/dead discrimination by manufacturers instructions, blocked with anti-CD16/32 for 15 min at 4°C and incubated with antibodies against cell surface molecules CD11b (M1/70), CD3 (2C11), CD4 (GK1.5), γδ TCR (GL3), Vγ4 (UC3-10A6), CD44 (1M7) and CD69 (H1-2F3) from either BD Pharmingen or eBioscience. The anti-Vγ1 hybridoma (2.11) was a generous gift from Willi Born and was purified and biotinylated by the Northwestern University Monoclonal Antibody Core Facility. Intracellular cytokines were stained using BD Perm/Fix (BD Pharmingen, Franklin Lakes) per the manufacturer's instructions with IFN-γ (XMG-1.2) and IL-17A (eBio17B7). Data were acquired on a Canto II cytometer (BD Biosciences, San Jose, CA) and analyzed with FlowJo software (Tree Star, Ashland, OR). Individual T cell subsets were sorted using MoFlow separation operated by the Cancer Center Flow Core Facility, Northwestern University, Chicago, IL.
2.7. In vitro T cell activation and cytokine detection
2×104 cells (for CNS subsets) or 2×105 cells (for spleen subsets) were incubated in 200 μl in a 96 well plate that had been plate coated with 1 μg/ml 2C11 in PBS overnight. Supernatant was collected and subjected to cytokine analysis by Cytokine Bead Array (BD Bioscience).
2.8. Real Time PCR analysis
Following MoFlow sorting, RNA was isolated from cells using Qiagen RNeasy per manufacturer's instructions. cDNA synthesis was performed using Invitrogen Superscript III per manufacturer's instructions. Gene expression analysis was monitored by real-time PCR with reverse transcription (RT–PCR) using gene-specific primers and probes. Actin Forward: GCT CTG GCT CCT AGC ACC AT, Reverse: GCC ACC GAT CCA CAC AGA GT, Probe: FAM-TCA AGA TCA TTG CTC CTC CTG AGC GC-TAMRA; IL-17A Forward: CTC CAG AAG GCC CTC AGA CTA C Reverse: AGC TTT CCC TCC GCA TTG ACA CAG Probe: FAM-TCT GGG AAG CTC AGT GCC GCC ACC AGC-TAMRA; IL-17F Forward: GAG GAT AAC ACT GTG AGA GTT GAC Reverse: GAG TTC ATG GTG CTG TCT TCC Probe: FAM-AGT TCC CCA TGG GAT TAC AAC ATC ACT C-TAMRA; RORyt Forward: CCG CTG AGA GGG CTT CAC, Reverse: TGC AGG AGT AGG CCA CAT TAC A, Probe: FAM-AAG GGC TTC TTC CGC CGC AGC CAG CAG-TAMRA; IL-21 Forward: ATC CTG AAC TTC TAT CAG CTC CAC, Reverse: GCA TTT AGC TAT GTG CTT CTG TTT C, Probe: FAM-AAG CCA TCA AAC CCT GGA AAC AAT AAG ACA-TAMRA; IL-23R Forward: TCA GTG CTA CAA TCT TCA GAG GAC A, Reverse: GCC AAG AAG ACC ATT CCC GA, Probe: FAM-CCT GCT TCA GGT AAT CAT CAA GAC ATT GGA CTT TT-TAMRA and IL-22 Forward: GAC CAA ACT CAG CAA TCA GCT C Reverse: TCA GAC GCA AGC ATT TCT CAG Probe: FAM-AGA ATG TCA GAA GGC TGA AGG AGA CAG TGA-TAMRA. Primers and probes for GM-CSF, IL-1β, IL-1R and IFN-γ were purchased from Applied Biosciences. For chemokine ligand analysis, real time PCR was analyzed using gene specific primer pairs and sybr green detection (LightCycler FastStart® DNA Master SYBR Green I, Roche). Primer sequences were CCL3 Forward: TTT TGA AAC CAG CAG CCT TT, Reverse: CTC AAG CCC CTG CTC TAC AC; CCL4 Forward: AAC CCC GAG CAA CAC CAT GAA G, Reverse: CCA CAA TAG CAG AGA AAC AGC AAT; CCL5 Forward: GTG CCC ACG TCA AGG AGT AT, Reverse: AGC AAG CAA TGA CAG GGA AG.
2.9. Statistical Analyses
Comparisons of the percentage of animals showing clinical disease were analyzed by X2 using Fisher's exact probability, and two-way ANOVA with a Bonferroni post-test was used to determine statistical differences between mean clinical disease scores. Single comparisons of two means were analyzed by Student's t-test.
3. Results
3.1 γδ T cell subsets accumulate in the CNS of SJL/J mice with PLP139-151-induced EAE correlates with relapsing-remitting disease severity and co-localize with CD4+ T cells
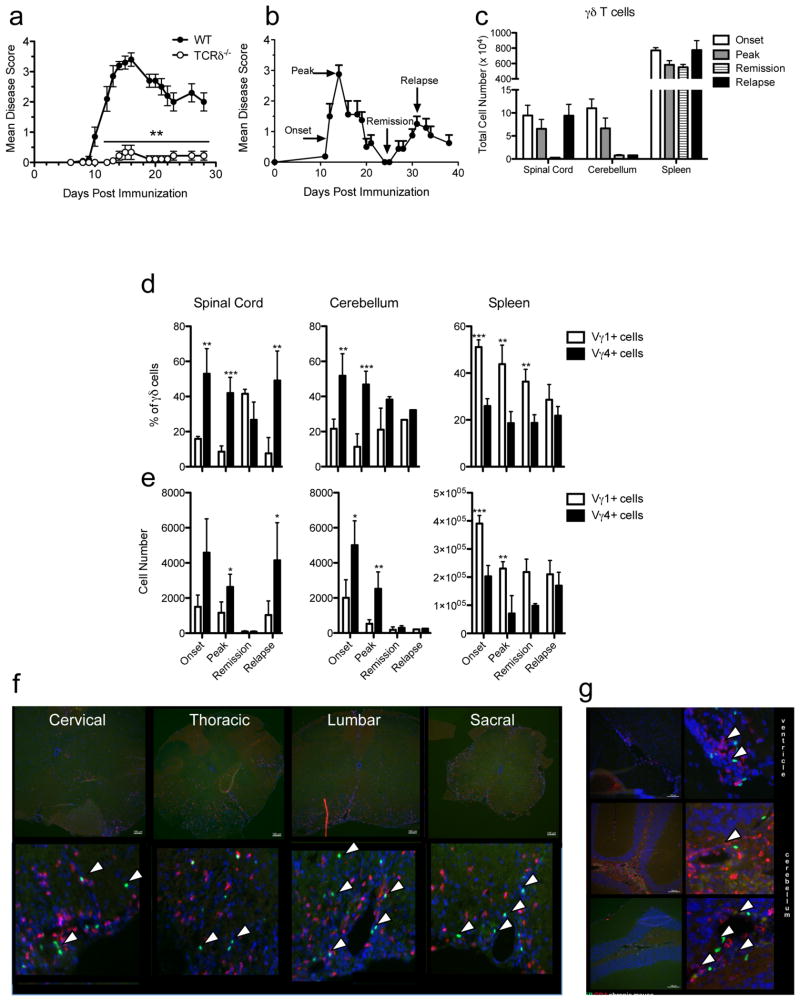
Interestingly, a limited repertoire of γδ T cells, defined by TCR chain usage, have been seen in the CSF and lesions of MS patients, however their function is not understood. The relapsing-remitting EAE model (R-EAE) closely represents the most common form of MS, characterized by repeating bouts of paralytic symptoms interrupted by remissions. Previous studies have examined the role of γδ T cells using multiple EAE models of MS, however the results are conflicting in that these cells have been reported to have both pathologic and protective functions [reviewed in [15]]. Significantly, in our hands, the absence of γδ T cells during MOG35-55-induced EAE in C57BL/6 mice results in essentially total ablation of disease symptoms indicating their importance in disease pathogenesis (Fig. 1a). We next examined which subsets of γδ T cell were activated and present in different lymphoid compartments during PLP139-151-induced R-EAE in SJL/J mice. γδ T cells were identified in the spleen, cerebellum and spinal cord at the following defined phases of disease: onset, peak of acute disease, remission and relapse (Fig. 1b). Interestingly, γδ T cell infiltration in the CNS correlated with disease severity in that there was a dramatic increase in their numbers in the spinal cord during onset, peak and relapse, and during onset and peak in the cerebellum; with cell numbers retracting during remission in both tissues (Fig. 1c). The percentage of T cells that are γδ within the cerebellum and spinal cord during disease is 2-4%, and most elevated in during relapse in the spinal cord (data not shown). However, the total number of γδ T cells in the spleen remains relatively similar throughout the disease course. Interestingly, during the primary relapse, significant γδ T cell infiltration occurs only within the spinal cord, not the cerebellum. The most common circulating γδ T cell subsets defined by their variable region TCR usage, Vγ1 and Vγ4, have recently been identified in models of arthritis and airway inflammation [9; 27]. We therefore investigated the prevalence these subsets in the CNS and peripheral tissues during the various stages of R-EAE. Interestingly, we find during all phases of R-EAE, the γδ T cell subsets show tissue specificity. The Vγ1 subset is dominant in the spleen, comprising 35-50% of the total γδ T cell population at all stages (Fig. 1d), seen most notably earlier during onset and peak disease. This secondary lymphoid organ location is supported by increased expression of L-selectin on the Vγ1 compared to the Vγ4 subset (data not shown). Paradoxically, the Vγ4 subset shows a particular specificity for the CNS with two-fold more Vγ4+ γδ T cells in the cerebellum and spinal cord compared to the Vγ1+ subset, especially during peak disease and relapse in the spinal cord (Fig. 1e). Although the percentage of the Vγ1 subset is higher than the Vγ4 subset in the spinal cord during remission, the total number of cells present in this tissue at this time point is minimal and similar (Vγ1, average: 97 cells and Vγ4, average: 92 cells).
Figure 1.
γδ T cell infiltrates in the CNS correlate with disease symptoms, co-localize with CD4+ cells and γδ T cell subsets show tissue specificity. (a) C-EAE was induced in female WT and Tcrd-/- B6 mice primed subcutaneously with 200 μg MOG33-55/CFA and pertussis toxin. Disease severity was monitored daily as described in Materials and Methods. Results are the mean disease score for at least 9 individual mice per group. (b-g) R-EAE was induced in female SJL/J mice by subcutaneous priming with 50 μg PLP139-151/CFA. Clinical disease was followed for 38 days post-priming. Cells were isolated from the spleen, cerebellum and spinal cord as described in Materials and Methods and cell populations were assessed by flow cytometry. (b) Time points used for cell collection during the clinical course of R-EAE. (c) Total number of γδ T cells in the CNS (spinal cord and cerebellum) and spleen at onset, peak, remission and primary relapse of clinical disease. (d) Percentage of γδ T cell subsets within the total γδ T cell population during the R-EAE disease course in the CNS and spleen. (e) Total number of γδ T cell subsets during the R-EAE disease course in the CNS and spleen. C-EAE was induced in female Tcrd-eGFP mice by subcutaneous priming with 200 μg MOG33-55/CFA and pertussis toxin. The spinal cord (f) and cerebellum (g) was processed for histological analysis on day 20; γδ T cells were visualized by GFP (green), and tissues were also stained with antibodies against CD4 (red) and DAPI (blue). Representative data of one of four experiments (a-e) and representative of 3 individual mice (f-g) are shown. Statistical analysis from unpaired student t test where ***p<0.0005, **p<0.005 and *<0.05.
The reagents available to identify γδ T cells are limited largely to PCR and flow cytometric analyses. Therefore, to examine the anatomical location of γδ T cells in the CNS during R-EAE, we utilized γδ T cell reporter mouse in which γδ T cells can be visualized by GFP fluorescence independently of TCR surface expression. EAE was induced in Tcrd-eGFP mice on the C57BL/6 background using MOG35-55/CFA and the brain and spinal cord tissue was processed and analyzed histologically for infiltrating γδ T cells and conventional αβ CD4+ T cells. CNS-infiltrating γδ T cells (green) are seen in perivascular cuffs both in the spinal cord (Fig. 1f) and the brain (Fig. 1g), in close proximity to autoreactive CD4+ T cells (red). Mycobacterial products within CFA, which is used to induce EAE, have been shown to activate γδ T cells [28]. Importantly, we did not observe CNS infiltration of γδ T cell in animals that had been immunized with MOG35-55/CFA but failed to develop clinical signs of paralysis (data not shown). Together, these data show that accumulation of γδ T cell subsets in the CNS correlates with disease symptoms during R-EAE, but have distinct tissue specificity; the Vγ4 subset is dominant in the CNS, the autoreactive inflammatory site, whereas the Vγ1 subset is the most prevalent in the spleen. The γδ T cells that enter the CNS co-localize with the autoreactive CD4+ cells in the perivascular space and do so independently of microbial activation from CFA and secondarily to CD4+ T cell inflammation.
3.2 γδ T cell subsets play opposing roles during the pathogenesis of disease
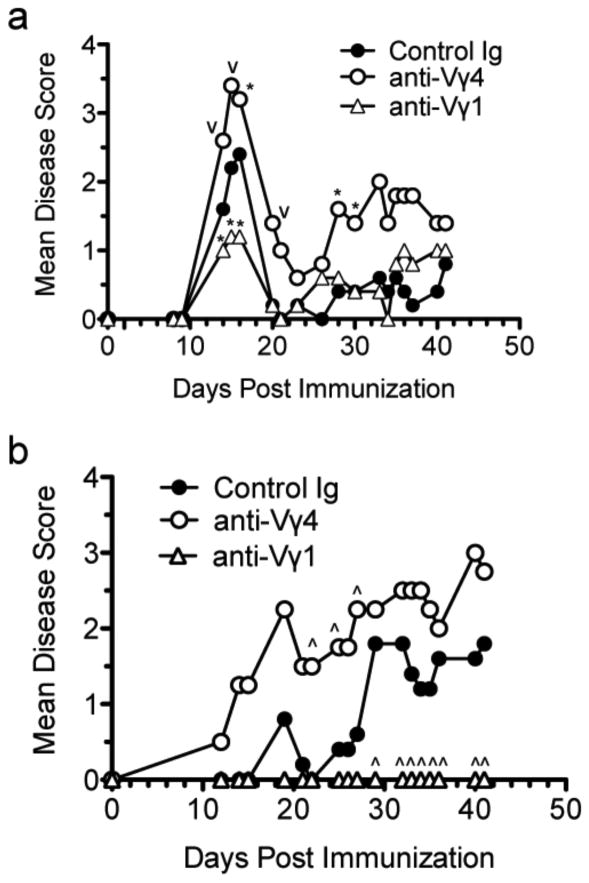
γδ T cells are pleiotropic cells with features common to both innate and adaptive T cells and have diverse functions that may or may not be associated with the subset defined by TCR usage [29; 30]. Indeed, depending on the disease model, the Vγ4 or Vγ1 subset can be pathogenic [9; 27]. To determine whether these subsets have pathogenic or protective roles during R-EAE, we treated animals with antibodies specific for each of the subsets during EAE and monitored the disease course and severity. Disease severity was worsened at all stages during R-EAE in SJL/J mice treated with anti-Vγ4, while, in contrast, treatment with anti-Vγ1 resulted in decreased severity during the peak acute phase (Fig. 2a). Since the previous literature contains conflicting data that could be explained by the use of various strains of animals, we induced C-EAE in B6 mice with MOG35-55/CFA and treated them with the same anti-γδ T cell subset antibodies. Interestingly, the results are similar to what was seen in the R-EAE model, with the anti-Vγ1 treatment totally preventing EAE onset in the B6 mice, while anti-Vγ4 treatment led to significantly enhanced disease. (Fig. 2b). These data indicate that in vivo targeting of the γδ T cell subsets results in opposite effects on the disease course in both relapsing-remitting (SJL/J) and chronic (C57BL/6) models of MS.
Figure 2.
In vivo antibody targeting of the Vγ1 or Vγ4 γδ T cell subsets results in opposing effects on clinical disease outcome in both R-EAE and C-EAE. On day 0, R-EAE was induced in female SJL/J mice primed subcutaneously with 50 μg of PLP139-151/CFA (a) and C-EAE was induced in female C57Bl/6 mice primed subcutaneously with 200 μg MOG33-55/CFA and pertussis toxin (b). 200 μg of purified control Ig, anti-Vγ1 or anti-Vγ4 monoclonal antibody was administered intravenously on days 0 and 2 and disease severity was monitored daily as described in Materials and Methods. Results are representative of at least 2 independent experiments with 5 mice per group. Disease scores significantly different from control Ig-treated mice - ˆp<0.005, *p<0.05 using the unpaired Student's t test.
3.3 In vivo targeting with antibodies against γδ T cells results in activation and downregulation of surface TCR
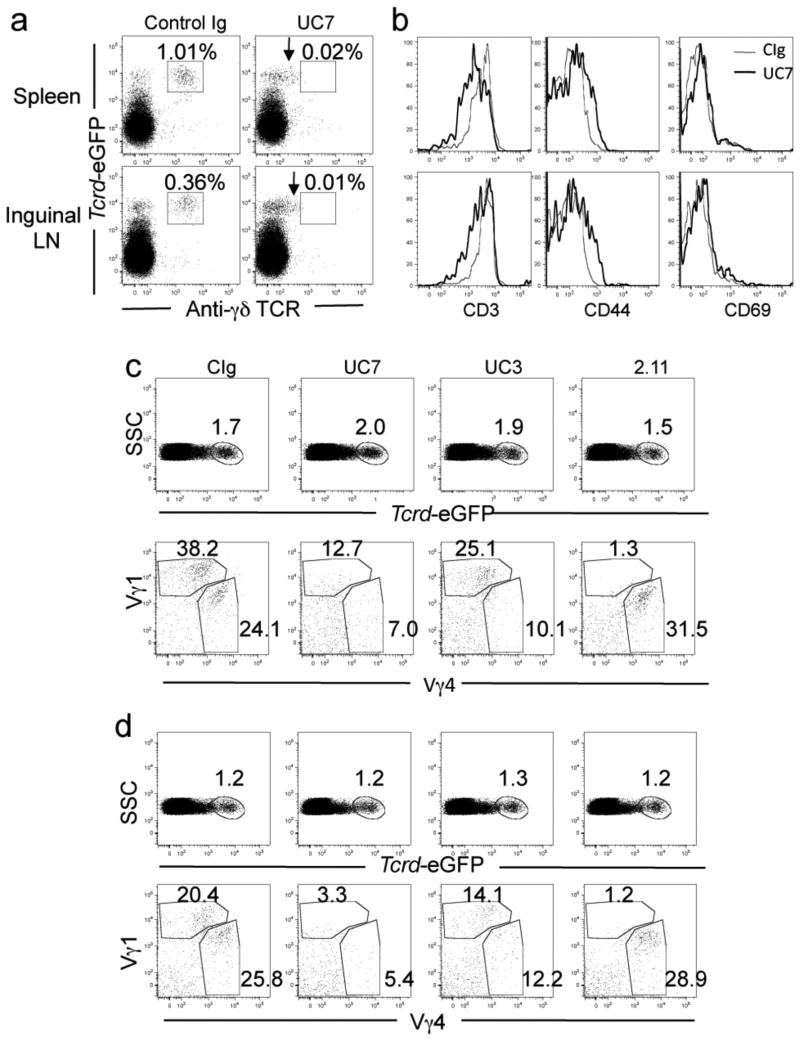
The role of γδ T cells in EAE is controversial due to the variety of models and reagents used to induce disease and modify γδ T cell function. Recently, the use of the γδ T cell reporter mouse has allowed the visualization of γδ T cells without the use of antibodies and has suggested that antibody administration to naïve animals results in downregulation of the TCR, thus rendering the cells “invisible” [31]. To determine whether the clinical outcome we observed using in vivo antibody targeting of the γδ T cell subsets during EAE results in the depletion of γδ T cells and/or downregulation of the surface TCR, we treated Tcrd-eGFP animals with 200 μg anti-pan γδ T cell antibody, UC7, or control Ig intravenously on days 0 and 2 and induced C-EAE via MOG35-55 priming. In the control Ig-treated mice, γδ T cells are double positive for GFP and surface staining for the receptor using the anti-γδ TCR clone GL3 in the spleen and draining (inguinal) lymph nodes, however in the animals treated with the anti-γδ T cell antibody, only GFP+ γδ T cells, and no surface staining, are seen at day 7 post disease induction (Fig. 3a). Interestingly, when treated with anti-γδ TCR in vivo, a population of GFP+ cells that have an intermediate level of TCR surface expression exist, as indicated by the arrows. The GFP+ γδ T cells that have downregulated surface TCR expression are not seen in the control Ig treated animals. Activated T cells commonly downregulate the TCR complex after activation to prevent over-stimulation [32; 33; 34]. Therefore, to determine whether in vivo anti-γδ T cell antibody administration results in γδ T cell activation during EAE induction, we examined CD3 surface expression and the activation markers CD44 and CD69 on the GFP+ γδ T cells following in vivo anti-γδ TCR treatment. CD3 expression is reduced on GFP+ γδ T cells from UC7 treated animals compared to the control treatment following disease induction, which correlates with CD44 and CD69 upregulation (Fig. 3b). In all tissues examined, CD44 upregulation is more significant than the early activation marker, CD69. Collectively, these data show in vivo administration of the UC7 pan anti-γδ TCR antibody during disease induction does not result in depletion of GFP+ γδ T cells, but rather results in the downregulation of the TCR complex, correlating with upregulation of the activation markers CD44 and CD69.
Figure 3.
In vivo antibody targeting activates γδ T cells and downregulates surface TCR expression. C-EAE was induced in Tcrd-eGFP primed subcutaneously with 200 μg MOG33-55/CFA and pertussis toxin as described in Materials and Methods and mice were treated i.v. with 200 μg of the indicated antibodies on days 0 and 2. Cells were isolated from the spleen and inguinal lymph nodes on day 7, γδ TCR-GFP and cell surface molecules were visualized by flow cytometry. (a) GFP and TCR surface staining of T cells from the spleen and draining lymph nodes. Arrow indicates downregulated surface expression. (b) Activation marker expression on GFP+ cells. (c-d top panel) Percent of GFP+ cells in the spleen (c) and inguinal lymph nodes (d). (c-d bottom panel) Surface expression of Vγ1 and Vγ4 TCR from GFP+ cells in the spleen (c) and lymph nodes (d). Data are representative of at least 2 independent experiments and represents the average of 4 mice per group.
To determine whether the antibodies against the specific γδ T cell subsets would reduce the γδ T cell population, Tcrd-eGFP animals were immunized as described above and treated i.v. with 200 μg Control Ig, anti-pan γδ (UC7), anti-Vγ1 TCR (2.11) or anti-Vγ4 TCR (UC3) on days 0 and 2. On day 7, cells from the spleen (Fig. 3c) and draining lymph nodes (Fig. 3d) were examined. The total percentage of GFP positive cells in either the spleen or the inguinal lymph nodes did not change irrespective of the cell type that was targeted (Fig. 3c,d top row). To determine whether the individual subset populations were influenced, the Vγ1 and Vγ4 positive populations within the GFP+ group were analyzed. When the mice were treated with anti-Vγ4, the percentage of this population that is detectable is reduced from 24.1% in the control treated mice to 10.1% in the spleen and from 25.8% to 12.2% in the lymph node. A similar reduction in subset specific surface receptor detection is seen when mice are treated with anti-Vγ1 antibody. The population of Vγ1 positive cells is reduced from 38.2% in the Control treated group to only 1.3% in the spleen and from 20.4% to 1.2% in the lymph node. Interestingly, treatment with the pan-γδ T cell antibody, UC7, results in a reduction in the detection of both subsets in the spleen and lymph node. Taken together, these data indicate that targeting γδ T cells via the Ag-specific receptor on the individual subsets renders the specific cells undetectable by surface TCR expression, reducing expression of the CD3 co-receptor and upregulating expression of activation markers, however the total γδ T cell population as seen by the GFP population remains the same. This is consistent with cell activation, not depletion.
3.4 The Vγ4 subset comprises a significant proportion of IL-17 production during all phases of disease
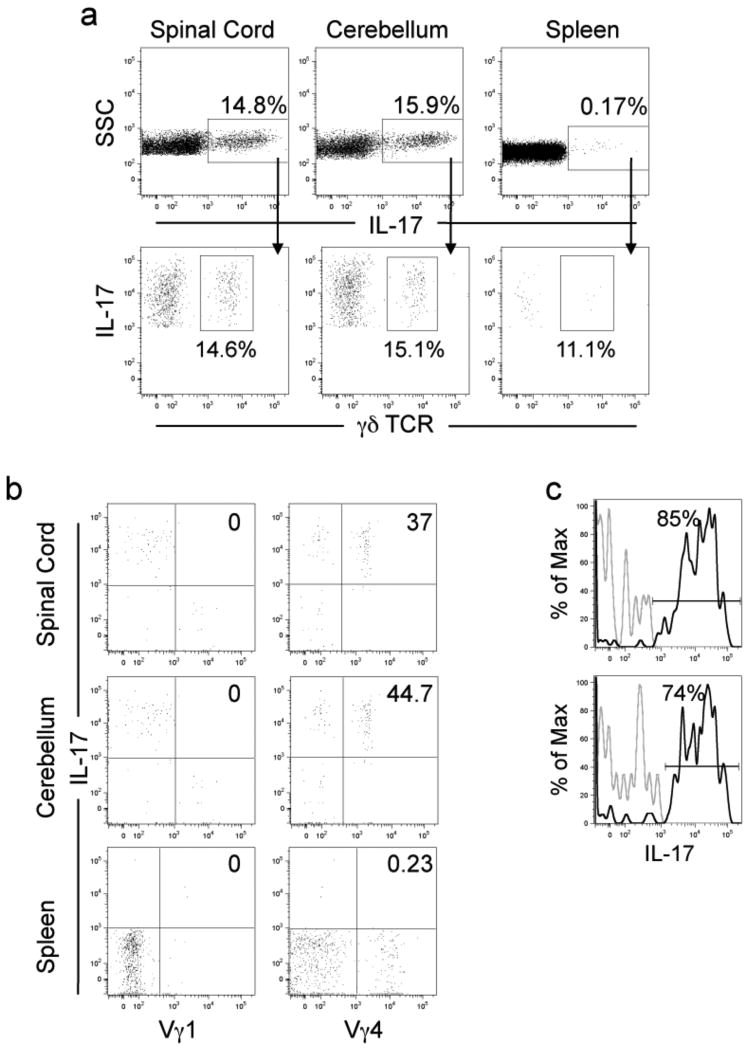
Th17 cells have been recently implicated in many different autoimmune diseases and cytokines that influence Th17 differentiation have been shown to be required for the development of these cells and resulting pathologies. γδ T cells have been identified as dominant IL-17 producers in models of uveitis, airway inflammation, collagen-induced arthritis, glomerulonephritis, stroke, and in response to Mycobacterium tuberculosis [9; 10; 11; 35; 36; 37]. It is not clear whether IL-17 from γδ T cells contributes to EAE pathogenesis. To evaluate whether circulating subsets of γδ T cells produce IL-17 that could contribute to the EAE pathology, we performed intracellular cytokine staining on cells isolated from the CNS and spleen at the peak acute phase of R-EAE. The CNS, spinal cord and cerebellum, but not the spleen have significant percentages of IL-17 producing cells at peak disease and 15-20% of the CNS IL-17 producing cells are γδ T cells (Fig. 4a and Suppl. Fig. 1a). The remaining IL-17 producing cells at peak disease are CD4 and CD8 T cells (Suppl. Fig. 1b). We next sought to determine which of the γδ T cell subsets produced IL-17 using intracellular cytokine staining for both the Vγ1 (left panel) and Vγ4 (right panel) subsets within the γδ T cell gate (Fig. 4b). Although the Vγ1 subset produces no IL-17, the Vγ4 subset produces significant amounts of IL-17 in both the spinal cord and cerebellum. Interestingly, Vγ4 γδ T cells in the CNS produce on average greater than two-fold more per IL-17 per cell than the CD4+ T cells and (Fig. 4a and Suppl. Fig. 1b) more than 75% of the CNS-resident Vγ4 cells produce IL-17 at peak disease (Fig. 4c). These data show that γδ T cells are significant producers of IL-17 in the CNS of mice with EAE and that the Vγ4 subset is the major IL-17 producing γδ T cell subset.
Figure 4. The Vγ4 subset produces a significant proportion of IL-17 in the CNS of SJL/J mice with PLP139-151-induced R-EAE.
R-EAE was induced in female SJL/J mice by subcutaneous priming with 50 μg PLP139-151/CFA as described in Materials in Methods. At peak disease, cells were isolated from the CNS and spleen, and intracellular staining performed and analyzed by flow cytometry. (a) The percentage of total IL-17 producing cells in the spinal cord, cerebellum and spleen (top panel) and the percentage of IL-17 producing cells that are γδ T cells (bottom panel). (b) The percentage of Vγ1 and Vγ4 γδ T cell subset-specific IL-17 producers from the total γδ T cell population. (c) The percentage of Vγ4+ cells that produce IL-17. Data are representative of 3 independent experiments and the mean of 4 animals per tissue.
3.5 γδ T cell subsets display distinct cytokine profiles and the Vγ4 subset expresses multiple transcription factors associated with Th17 differentiation
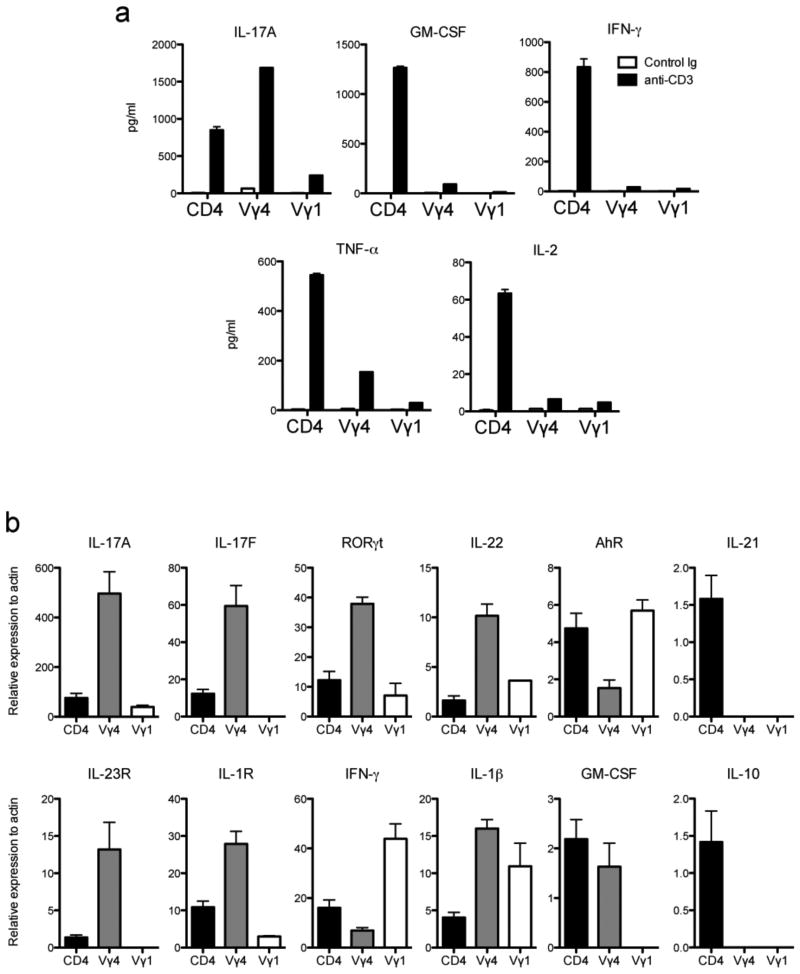
We next wanted to determine what other cytokines are produced by the γδ T cell subsets in the CNS during EAE. First, the Vγ1 and Vγ4 γδ T cell subsets as well as CD4+ T cells were sorted from the CNS at peak disease from PLP139-151/CFA primed SJL/J mice, the cell numbers were normalized, stimulated with anti-CD3 and the supernatants analyzed for secreted cytokines. Consistent with the intracellular cytokine staining data, the sorted Vγ4 subset produced substantial amounts of IL-17, almost two-fold more than the CD4+ T cells (Fig. 5a). This subset also produced other pro-inflammatory cytokines including GM-CSF and TNF-α, albeit not to the levels of the CD4+ population. The Vγ1 subset, conversely, did not produce significant levels of pro-inflammatory cytokines. It is interesting to note that neither the Vγ1 nor the Vγ4 subset produced much IFN-γ, which is in contrast to other published results, however strain differences between these studies could account for this discrepancy.
Figure 5.
The Vγ4 subset expresses multiple Th17 related genes and produces proinflammatory cytokines. R-EAE was induced in female SJL/J mice by subcutaneous priming with 50 μg PLP139-151/CFA. Cells were harvested from the spinal cord and cerebellum at peak disease and CD4+, Vγ1 and Vγ4 T cell subsets were sorted to 99% purity using MoFlow separation. (a) Cytokine analysis via CBA from supernatant of subsets stimulated overnight with plate-coated ant-CD3. (b) Real time PCR gene expression analysis of sorted subsets. Data are representative of at least 2 independent experiments.
We next sought to examine the transcriptional regulation of IL-17 production by γδ T cells from the CNS. γδ T cells have innate properties and therefore may not employ the same mechanisms for regulating cytokine production and differentiation as CD4+ Th17 cells, therefore we examined the expression of transcription factors and cytokines known to be involved in Th17 differentiation within the γδ T cell subsets at the peak of R-EAE. At peak of acute disease, RNA was isolated from CNS-infiltrating CD4+ T cells and γδ T cell subsets sorted to greater than 99% purity and real time PCR was performed on Th17 related genes (Fig. 5b). The Vγ4 subset expresses extremely high levels of IL-17A mRNA, which is consistent with the intracellular cytokine staining (Fig. 4) and secreted protein data (Fig. 5a). Interestingly, this cell subset also expresses high levels of IL-17F, RORγt and IL-22, however the level of aryl hydrocarbon receptor (AhR) and IL-21 expression is reduced compared to the CD4+ population. Collectively, these data show the Vγ4 subset has a distinct pattern of expression of genes compared to autoreactive CNS-infiltrating CD4+ T cells and confirm a recent transcriptome analysis of emergent γδ thymocyte subsets [38]. The Vγ1 subset does not express large amounts of IL-17A, or IL-17F, but does express RORγt, IL-22 and AhR mRNA.
It has been shown that γδ T cells produce IL-17 in response to inflammatory cytokines including IL-1β and IL-23 and perhaps in the absence of TCR stimulation [7]. To determine which γδ T cell subset may respond in such a manner, we examined IL-23R and IL-1R expression on the specific subsets that infiltrate the CNS during peak disease and found that the Vγ4, but not the Vγ1 subset expresses high levels of both receptors (Fig. 5b). Finally, we sought to determine whether the γδ T cell subsets in the CNS express genes for those cytokines that were produced in only minimal amounts when stimulated with anti-CD3. Interestingly, the Vγ1 subset did express IFN-γ and IL-1β mRNA, but did not express GM-CSF or IL-10 mRNA (Fig. 5b).
3.6 Co-localization of γδ T cells and oligodendrocytes in the CNS during pathogenesis
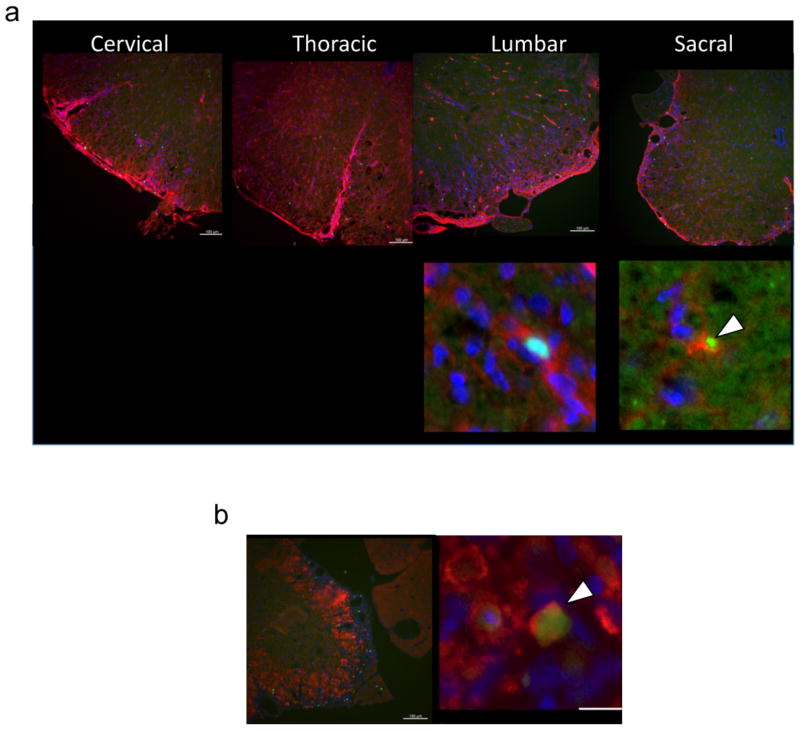
Oligodendrocytes in the CNS are critical for neuronal function and also a target for autoimmune mediated demyelination resulting in the paralytic phenotype seen in MS [1]. γδ T cells from human patients have been shown to directly lyse oligodendrocytes providing a possible pathogenic mechanism for γδ T cells during MS [39]. Mature oligodendrocytes express the cell surface markers CC-1 and MBP [40]. To determine whether γδ T cells interact with oligodendrocytes in the CNS of mice with EAE, we examined the proximity of γδ T cells to oligodendrocytes within the spinal cord during disease using the Tcrd-eGFP reporter animal. Figure 6 shows co-localization of γδ T cells with CC-1 (Fig. 6a) and MBP (Fig. 6b) expressing oligodendrocytes using immunohistological staining. Z-stack analysis of confocal images confirmed these cells are physically interacting (data not shown). These data indicate γδ T cells are in fact interacting with oligodendrocytes in the CNS during R-EAE disease pathogenesis and could suggest that γδ T cells may be partially responsible for oligodendrocyte killing.
Figure 6.
γδ T cells co-localize with oligodendrocytes in CNS lesions. C-EAE was induced in female Tcrd-eGFP mice by subcutaneous priming 200 μg MOG33-55/CFA and pertussis toxin as described in the Materials and Methods. Spinal cord tissue was processed for histological analysis on day 20; γδ T cells were visualized by GFP (green), and tissue were stained with antibodies against CC-1 (a) and MBP (b) in red and DAPI in blue. Data are representative of at least 4 individual mice.
3.7 The Vγ1 subset produces CCR5 ligands upon activation
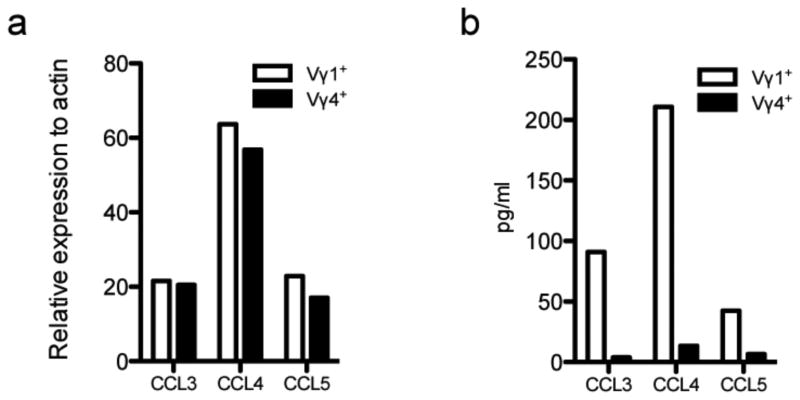
CCR5 is highly expressed on regulatory T cells (Treg), and it has been shown that the CCL4-CCR5 axis is important in regulating the Th17-Treg balance [41]. Therefore, we sought to determine whether the Vγ1 subset could produce ligands for CCR5 upon activation. γδ T cell subsets were sorted from naïve spleens and activated overnight with anti-CD3. The Vγ1 and Vγ4 subset both express CCL3, CCL4 and CCL5 mRNA (Fig. 7a). However, the Vγ1 subset produces significantly more chemokine than the Vγ4 subset and CCL4 is the most prominent CCR5 ligand secreted (Fig. 7b).
Figure 7.
The Vγ1 subset produces high levels of CCR5 ligands. γδ T cell subsets were sorted from naïve SJL/J splenocytes, plated at 1×106 cell/ml and stimulated on plate bound 2C11 (1ug/ml) overnight. RNA was isolated from cells and subjected to real time PCR analysis (a). Supernatant was collected and protein was analyzed by Cytokine Bead Array (b).
4. Discussion
γδ T cells have been identified in both lesions and the CSF of MS patients and display a limited repertoire, however their function is unknown. Past literature using the EAE model of MS is filled with contradictory data as to whether these cells play a pathogenic or protective role [15]. As a population, γδ T cells display a broad functional spectrum that may segregate into the multiple subsets defined by the TCR variable region. In this study we characterize the two main circulating subsets of γδ T cells during EAE and conclude they demonstrate distinct and opposing functions. Using the Tcrd-eGFP animal we are for the first time able to identify γδ T cells in the CNS during EAE independently of surface TCR expression and show that in vivo antibody administration results in activation of the cells and downregulation of TCR surface expression. Subset specific targeting suggests the Vγ1 subset plays a protective role while the Vγ4 subset is pathogenic. Cytokine analysis supports this hypothesis in that multiple proinflammatory mediators are produced by the Vγ4 subset including large amounts of IL-17. These data suggest γδ T cell subsets play distinct and opposing roles and utilize temporally and anatomically distinct regulatory mechanisms during EAE disease progression such that specific targeting of γδ subsets to optimize regulation of autoreactive CNS destruction may prove to be a potential treatment for MS.
The conflicts regarding the potential pathogenic vs. protective role of γδ T cells during EAE are likely due to the variety of models and reagents used. Our results address two possible explanations for the contradictions. First, we definitively show that in vivo antibody treatment activates, rather than depletes γδ T cells during EAE and secondly, that γδ T cell subsets have opposing roles during disease: the Vγ4 subset exacerbates disease whereas the Vγ1 subset has protective properties. Although treatment with these antibodies renders the specific subset “invisible” based on TCR surface expression, the total γδ T cell population remains unchanged suggesting the cells are not depleted but have downregulated the TCR complex, a common event during T cell activation [32; 33; 34]. Anti-γδ T cell antibody treatment downregulates CD3 expression while increasing expression of the activation markers CD44 and CD69. We propose that the confusion in the literature could be partially due to the simultaneous targeting of opposing subsets using pan-γδ T cell antibodies complicated by the use of clones which could have differential affinities for one subset vs. the other. It is also possible different strains of mice have a varying ratio of opposing subsets so that global activation of the population has different affects. Our data corroborates results from another study that used UC7 during EAE resulting in exacerbated disease, but the authors mistakenly concluded that γδ T cells were protective because the cells were undetectable [42]. Other studies have shown that γδ T cell deficient mice display reduced clinical disease [17; 43], and although our studies support these findings, we found that TCRδ-deficient mice were virtually totally resistant to disease (Fig. 1a) perhaps due to different gut microbiota in our colony. Our data utilizing UC7-treated Tcrd-eGFP mice (targeting the pan γδ T cell population) supports the conclusion that activation of the total population of γδ T cells results in an enhanced clinical disease (data not shown). Interestingly, targeting γδ T cells with the GL3 clone during EAE resulted in reduced disease also suggesting a pathogenic role for γδ T cells. However, it is not clear whether these cells are actually depleted or rendered “invisible” [44].
Our results demonstrate an interesting dichotomy among the common circulating Vγ1 and Vγ4 γδ T cell subsets, providing an additional explanation for previous controversial data. The Vγ1 and Vγ4 subsets display a functional dichotomy in other models of inflammation and autoimmunity that is dependent on the disease mechanism [45]. IL-17 produced by the Vγ4 subset likely contributes to the pathogenesis of EAE and collagen induced arthritis, however in response to inhaled OVA, this cell type is protective against airway hyper-reactivity [9; 27,46]. Similarly, the Vγ1 subset promotes IgE production during airway inflammation [27; 46], however our results demonstrate that this subset plays a protective role during EAE. These results suggest that the Vγ1 and Vγ4 subsets' function are ascribed similarly to a Th2 and Th1/17 phenotype, respectively. Interestingly, human data from Vδ1 and Vδ2 γδ T cells also indicates a distinct pattern of gene expression supporting opposing functions for the different subtypes [47].
Specific Ab-induced activation of the Vγ4 subset results in exacerbation of disease at all stages of R-EAE suggesting they either increase in number or their pathogenic function is heightened. CNS-infiltrating Vγ4 cells produce large quantities of IL-17, contributing to the pathogenic environment within this tissue. IL-17 production correlates tightly with autoimmune diseases, is proposed to be critical for pathogenesis [6] and has been implicated in multiple autoimmune diseases including rheumatoid arthritis and MS [48; 49]. IL-17 can act directly on stromal cells to produce inflammation and MS, and has been suggested to potentiate the migration of lymphocytes across the blood brain barrier [50; 51].
The pattern of Th17-related transcription factors and cytokines is distinct from that of the CD4+ T cells found in the CNS at the same time point. Increased expression of RORγt and reduced expression of AhR compared to the CNS CD4+ T cells suggests differential regulation of IL-17 production. IL-17 from Vγ4 cells may not require autocrine IL-21 mediated amplification since IL-21 gene transcripts are undetectable in the subset [52]. The Vγ4 subset in the CNS expresses high levels of IL-22, which is associated with terminal differentiation of Th17 cells. AhR results in the expansion of Th17 cells and although it is not required for Th17 differentiation, its activation is important for further functional differentiation [53]. However, AhR is critical for IL-22 production in Th17 cells [54]. The distinct pattern of expression of these transcription factors suggests differential requirements for IL-22 cytokine production by Vγ4 cells vs. conventional CD4+ Th17.
It has been proposed that IL-17 production from γδ T cells can occur in the absence of additional TCR stimulation in an IL-23- and IL-1β-dependent manner [7]. High levels of IL-23R and IL-1R gene expression in the CNS-infiltrating Vγ4 subset support the hypothesis that γδ T cells produce IL-17 in response to the inflammatory milieu, perhaps partially in absence of antigen. IL-23-dependent activation of γδ T cells resulting in regulation of IL-23R+ γδ T cells has also been shown to regulate CD4+ autoreactive inflammation by rendering the cells refractory to Treg-mediated suppression [19]. Our demonstration of co-localization of γδ T cells and CD4+ T cells in the CNS supports the possibility that activation of the Vγ4 subset through antibody stimulation results in exacerbated disease either via increased pro-inflammatory cytokine production and/or regulation of autoreactive CD4+ cells and Tregs.
The novel use of the Tcrd-eGFP strain has allowed us for the first time to definitively locate γδ T cells in the CNS during EAE pathogenesis. Immune-mediated destruction of myelin-producing oligodendrocytes in MS patients results in the lack of conductivity along neurons resulting in paralysis [55]. γδ T cells isolated from MS patients can directly lyse oligodendrocytes in vitro [39]. Indeed, our study provides evidence that γδ T cells physically interact with oligodendrocytes in the spinal cord during disease. Interestingly, the finding that γδ T cell infiltration is restricted to the spinal cord during relapse correlates tightly with the symptoms of hind limb paralysis. Collectively, the temporal pattern of CNS infiltration, production of pro-inflammatory cytokine and potential ability to lyse oligodendrocytes identify the Vγ4 subset as a suitable target for reducing pathogenesis during MS. Most interestingly, specific activation of the Vγ1 subset results in a reduction in disease severity, suggesting a distinct protective role for this subset. Concomitantly, activated Vγ1 cells produce CCR5 ligands, most prominently CCL4. In addition to directing migration of lymphocytes, chemokines and their receptors have been suggested to regulate T cell differentiation [56]. CCR5 is highly expressed on regulatory T cells (Treg), and it has been shown that the CCL4-CCR5 axis is important in regulating the Th17-Treg balance [41]. Thus, the high levels of CCR5 expression on the Vγ1 subset my relate to its regulatory effects, but this needs to be definitively determined.
Activation of the Vγ1 subset appears to elicit protection early in disease during the acute phase of both R-EAE and C-EAE and delays disease onset during C-EAE, whereas the exacerbation of disease by the Vγ4 subset occurs throughout disease. These results correlate with the observation that the Vγ1 subset is dominant in the periphery and the robust downregulation of the Vγ1 TCR upon 2.11 treatment during early phase of disease (day 7) in the lymph node. The Vγ1 subset is pathogenic in the Th2-dependent model of airway inflammation, thus perhaps during EAE, the Vγ1 subset could ameliorate disease by inducing immune deviation away from Th17 or by the production of CCL4 leading to the promotion of Treg proliferation during the priming phase [27; 41]. Collectively, these data suggest the Vγ1 protective function may dampen the priming of autoreactive T cells and that therapeutic strategies exploiting their protective function may be employed for treatment of MS.
We show that the Vγ4 subset dominates in the CNS. Tissue specificity is common for resident γδ T cells - definitive Vγ chain usage is seen in gut, spleen, thymus, and skin resident murine γδ T cells [57]. Similarly, Vδ1 and Vδ2 γδ T cells are over-expressed in MS plaques [12; 47; 58; 59] during acute disease, but most V and J regions are represented during chronic disease. This pattern suggests an antigen specific expansion during early phases of disease and possible non-specific recruitment thereafter [60]. Junctional sequence analysis also supports the hypothesis that infiltrating γδ T cells are oligoclonal in nature, perhaps indicating specific antigen stimulation [61]. Although the ligands for γδ T cells remain elusive, recognition of self antigens such as heat shock proteins and non-classical MHC I molecules have been speculated as possible targets [14; 62; 63]. Indeed, heat shock protein expression (including hsp60, hsp90 and alpha B crystallin) is upregulated in foamy macrophages and astrocytes within active MS plaques [12; 64]. It is relevant that the inducing antigen(s) in MS has not been identified. It is highly probable that T cell responses (CD4, CD8 and γδ) to epitopes on a number of CNS structural proteins, lipids, as well as proteins upregulated as a result of chronic inflammation (e.g. heat shock proteins) are critically involved in chronic disease progression. It is this de novo activation in response to endogenously released CNS epitopes via a process termed epitope spreading which we have studied over the past 20 years [65; 66]. Broadening the immune response during disease progression due to release of a wide variety of endogenous CNS antigens suggests that regulation of ongoing disease pathogenesis may have to target not just conventional αβ TCR expressing effector cells, but also γδ T cells [67]. Specific expansion of the Vγ4 subset in the CNS suggests this subset may respond to a self-antigen in the CNS and respond by production of pro-inflammatory cytokines and perhaps the direct lysis of oligodendrocytes. Given that data from MS patients suggests a limited repertoire of γδ T cells in the CSF and lesions along with our data indicating γδ T cell subsets play opposing roles and show tissue specificity during EAE, it is intriguing to speculate that specific targeting of γδ T cell subsets could be employed to regulate autoimmune destruction of CNS tissue during MS.
Supplementary Material
Supplementary Figure 1. Analysis of CNS infiltrating cells that produce IL-17. R-EAE was induced in female SJL/J mice primed subcutaneously with 50 μg PLP139-151/CFA as described in Materials and Methods. At peak disease, cells were isolated from the CNS and spleen, and intracellular cytokine staining performed and analyzed by flow cytometry. (a) The percentage of total γδ T cells that produce IL-17 in the spinal cord and cerebellum at onset, peak and relapse. (b) The percentage of lymphocytes which produce IL-17 and are CD4+, CD8+ or γδ+ T cells. Data are representative of 3 independent experiments and the mean of 4 animals per tissue.
Highlights.
γδ IL-17-producing T cells are pathogenic in SJL/J mice with EAE
a γδ T cells are found in CNS inflammatory lesions and contact oligodendrocytes
The Vγ4 subset exclusively produces IL-17 in the CNS
The Vγ1 subset plays a protective role in regulating EAE priming in the periphery.
Transcriptional regulation of IL-17 in Vγ4 cells differs from CD4+ Th17 cells
Acknowledgments
This work was supported by grants from the Myelin Repair Foundation and NIH Grant NS-026543. We thank Dr. Liang Zhou for his assistance with real time PCR primers and for helpful discussions. We also thank Dr. William Karpus for the generous gift of PCR primers for chemokines.
Abbreviations
- CNS
central nervous system
- mAb
monoclonal antibody
- MS
multiple sclerosis
- OVA
ovalbumin
- PLP
myelin proteolipid protein
- R-EAE
relapsing experimental autoimmune encephalomyelitis
- TCR
T cell receptor
Footnotes
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steinman L, Martin R, Bernard C, Conlon P, Oksenberg JR. Multiple sclerosis: deeper understanding of its pathogenesis reveals new targets for therapy. Annu Rev Neurosci. 2002;25:491–505. doi: 10.1146/annurev.neuro.25.112701.142913. [DOI] [PubMed] [Google Scholar]
- 2.Smith CE, Miller SD. Multi-peptide coupled-cell tolerance ameliorates ongoing relapsing EAE associated with multiple pathogenic autoreactivities. J Autoimmun. 2006;27:218–31. doi: 10.1016/j.jaut.2006.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- 4.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 6.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–30. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–41. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Stark MA, Huo Y, Burcin TL, Morris MA, Olson TS, Ley K. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 2005;22:285–94. doi: 10.1016/j.immuni.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 9.Roark CL, French JD, Taylor MA, Bendele AM, Born WK, O'Brien RL. Exacerbation of collagen-induced arthritis by oligoclonal, IL-17-producing gamma delta T cells. J Immunol. 2007;179:5576–83. doi: 10.4049/jimmunol.179.8.5576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murdoch JR, Lloyd CM. Resolution of Allergic Airway Inflammation and Airway Hyperreactivity is Mediated by IL-17 Producing gamma-delta T Cells. Am. J. Respir. Crit Care Med. 2010 doi: 10.1164/rccm.200911-1775OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shichita T, Sugiyama Y, Ooboshi H, Sugimori H, Nakagawa R, Takada I, Iwaki T, Okada Y, Iida M, Cua DJ, Iwakura Y, Yoshimura A. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury. Nat Med. 2009;15:946–50. doi: 10.1038/nm.1999. [DOI] [PubMed] [Google Scholar]
- 12.Wucherpfennig KW, Newcombe J, Li H, Keddy C, Cuzner ML, Hafler DA. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci USA. 1992;89:4588–92. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sobel RA, Kuchroo VK. The immunopathology of acute experimental allergic encephalomyelitis induced with myelin proteolipid protein. T cell receptors in inflammatory lesions. J Immunol. 1992;149:1444–51. [PubMed] [Google Scholar]
- 14.Haregewoin A, Soman G, Hom RC, Finberg RW. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989;340:309–12. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- 15.Blink SE, Miller SD. The contribution of gammadelta T cells to the pathogenesis of EAE and MS. Curr Mol Med. 2009;9:15–22. doi: 10.2174/156652409787314516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ponomarev ED, Novikova M, Yassai M, Szczepanik M, Gorski J, Dittel BN. Gamma delta T cell regulation of IFN-gamma production by central nervous system-infiltrating encephalitogenic T cells: correlation with recovery from experimental autoimmune encephalomyelitis. J Immunol. 2004;173:1587–95. doi: 10.4049/jimmunol.173.3.1587. [DOI] [PubMed] [Google Scholar]
- 17.Ponomarev ED, Dittel BN. Gamma delta T cells regulate the extent and duration of inflammation in the central nervous system by a Fas ligand-dependent mechanism. J Immunol. 2005;174:4678–87. doi: 10.4049/jimmunol.174.8.4678. [DOI] [PubMed] [Google Scholar]
- 18.Olivares-Villagomez D, Wang Y, Lafaille JJ. Regulatory CD4(+) T cells expressing endogenous T cell receptor chains protect myelin basic protein-specific transgenic mice from spontaneous autoimmune encephalomyelitis. J Exp Med. 1998;188:1883–94. doi: 10.1084/jem.188.10.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Petermann F, Rothhammer V, Claussen MC, Haas JD, Blanco LR, Heink S, Prinz I, Hemmer B, Kuchroo VK, Oukka M, Korn T. Gamma delta T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33:351–63. doi: 10.1016/j.immuni.2010.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clark RB, Lingenheld EG. Adoptively transferred EAE in gamma delta T cell-knockout mice. J Autoimmun. 1998;11:105–10. doi: 10.1006/jaut.1997.0180. [DOI] [PubMed] [Google Scholar]
- 21.Rajan AJ, Klein JD, Brosnan CF. The effect of gammadelta T cell depletion on cytokine gene expression in experimental allergic encephalomyelitis. J Immunol. 1998;160:5955–62. [PubMed] [Google Scholar]
- 22.Odyniec A, Szczepanik M, Mycko MP, Stasiolek M, Raine CS, Selmaj KW. Gammadelta T cells enhance the expression of experimental autoimmune encephalomyelitis by promoting antigen presentation and IL-12 production. J Immunol. 2004;173:682–94. doi: 10.4049/jimmunol.173.1.682. [DOI] [PubMed] [Google Scholar]
- 23.Cardona AE, Teale JM. Gamma/delta T cell-deficient mice exhibit reduced disease severity and decreased inflammatory response in the brain in murine neurocysticercosis. J Immunol. 2002;169:3163–71. doi: 10.4049/jimmunol.169.6.3163. [DOI] [PubMed] [Google Scholar]
- 24.Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J Immunol. 1996;157:941–9. [PubMed] [Google Scholar]
- 25.Spahn TW, Issazadah S, Salvin AJ, Weiner HL. Decreased severity of myelin oligodendrocyte glycoprotein peptide 33 - 35-induced experimental autoimmune encephalomyelitis in mice with a disrupted TCR delta chain gene. Eur J Immunol. 1999;29:4060–71. doi: 10.1002/(SICI)1521-4141(199912)29:12<4060::AID-IMMU4060>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 26.Prinz I, Sansoni A, Kissenpfennig A, Ardouin L, Malissen M, Malissen B. Visualization of the earliest steps of gammadelta T cell development in the adult thymus. Nat Immunol. 2006;7:995–1003. doi: 10.1038/ni1371. [DOI] [PubMed] [Google Scholar]
- 27.Hahn YS, Taube C, Jin N, Sharp L, Wands JM, Aydintug MK, Lahn M, Huber SA, O'Brien RL, Gelfand EW, Born WK. Different potentials of gamma delta T cell subsets in regulating airway responsiveness: V gamma 1+ cells, but not V gamma 4+ cells, promote airway hyperreactivity, Th2 cytokines, and airway inflammation. J Immunol. 2004;172:2894–902. doi: 10.4049/jimmunol.172.5.2894. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien RL, Happ MP, Dallas A, Palmer E, Kubo R, Born WK. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989;57:667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- 29.Allison JP, Havran WL. The immunobiology of T cells with invariant gamma delta antigen receptors. Ann Rev Immunol. 1991;9:679–705. doi: 10.1146/annurev.iy.09.040191.003335. [DOI] [PubMed] [Google Scholar]
- 30.O'Brien RL, Roark CL, Jin N, Aydintug MK, French JD, Chain JL, Wands JM, Johnston M, Born WK. gammadelta T-cell receptors: functional correlations. Immunol Rev. 2007;215:77–88. doi: 10.1111/j.1600-065X.2006.00477.x. [DOI] [PubMed] [Google Scholar]
- 31.Koenecke C, Chennupati V, Schmitz S, Malissen B, Forster R, Prinz I. In vivo application of mAb directed against the gammadelta TCR does not deplete but generates “invisible” gammadelta T cells. Eur J Immunol. 2009;39:372–9. doi: 10.1002/eji.200838741. [DOI] [PubMed] [Google Scholar]
- 32.Valitutti S, Muller S, Cella M, Padovan E, Lanzavecchia A. Serial triggering of many T-cell receptors by a few peptide-MHC complexes. Nature. 1995;375:148–151. doi: 10.1038/375148a0. [DOI] [PubMed] [Google Scholar]
- 33.Bachmann MF, Oxenius A, Speiser DE, Mariathasan S, Hengartner H, Zinkernagel RM, Ohashi PS. Peptide-induced T cell receptor down-regulation on naive T cells predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur J Immunol. 1997;27:2195–203. doi: 10.1002/eji.1830270912. [DOI] [PubMed] [Google Scholar]
- 34.Itoh Y, Hemmer B, Martin R, Germain RN. Serial TCR engagement and down-modulation by peptide:MHC molecule ligands: relationship to the quality of individual TCR signaling events. J Immunol. 1999;162:2073–80. [PubMed] [Google Scholar]
- 35.Cui Y, Shao H, Lan C, Nian H, O'Brien RL, Born WK, Kaplan HJ, Sun D. Major role of gamma delta T cells in the generation of IL-17+ uveitogenic T cells. J Immunol. 2009;183:560–7. doi: 10.4049/jimmunol.0900241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–9. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 37.Turner JE, Krebs C, Tittel AP, Paust HJ, Meyer-Schwesinger C, Bennstein SB, Steinmetz OM, Prinz I, Magnus T, Korn T, Stahl RA, Kurts C, Panzer U. IL-17A Production by Renal gammadelta T Cells Promotes Kidney Injury in Crescentic GN. J Am Soc Nephrol. 2012 doi: 10.1681/ASN.2012010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Narayan K, Sylvia KE, Malhotra N, Yin CC, Martens G, Vallerskog T, Kornfeld H, Xiong N, Cohen NR, Brenner MB, Berg LJ, Kang J. Intrathymic programming of effector fates in three molecularly distinct gammadelta T cell subtypes. Nat Immunol. 2012;13:511–8. doi: 10.1038/ni.2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Freedman MS, Ruijs TC, Selin LK, Antel JP. Peripheral blood gamma-delta T cells lyse fresh human brain-derived oligodendrocytes. Ann Neurol. 1991;30:794–800. doi: 10.1002/ana.410300608. [DOI] [PubMed] [Google Scholar]
- 40.Lin W, Bailey SL, Ho H, Harding HP, Ron D, Miller SD, Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kroetz DN, Deepe GS., Jr CCR5 dictates the equilibrium of proinflammatory IL-17+ and regulatory Foxp3+ T cells in fungal infection. J Immunol. 2010;184:5224–31. doi: 10.4049/jimmunol.1000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi Y, Kawai K, Ito K, Honda H, Sobue G, Yoshikai Y. Aggravation of murine experimental allergic encephalomyelitis by administration of T-cell receptor gammadelta-specific antibody. J Neuroimmunol. 1997;73:169–174. doi: 10.1016/s0165-5728(96)00187-7. [DOI] [PubMed] [Google Scholar]
- 43.Wohler JE, Smith SS, Zinn KR, Bullard DC, Barnum SR. Gammadelta T cells in EAE: early trafficking events and cytokine requirements. Eur J Immunol. 2009;39:1516–26. doi: 10.1002/eji.200839176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rajan AJ, Gao YL, Raine CS, Brosnan CF. A pathogenic role for gamma delta T cells in relapsing-remitting experimental allergic encephalomyelitis in the SJL mouse. J Immunol. 1996;157:941–949. [PubMed] [Google Scholar]
- 45.Huber SA, Graveline D, Born WK, O'Brien RL. Cytokine production by Vgamma(+)-T-cell subsets is an important factor determining CD4(+)-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J Virol. 2001;75:5860–9. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang Y, Jin N, Roark CL, Aydintug MK, Wands JM, Huang H, O'Brien RL, Born WK. The influence of IgE-enhancing and IgE-suppressive gammadelta T cells changes with exposure to inhaled ovalbumin. J Immunol. 2009;183:849–55. doi: 10.4049/jimmunol.0804104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kress E, Hedges JF, Jutila MA. Distinct gene expression in human Vdelta1 and Vdelta2 gammadelta T cells following non-TCR agonist stimulation. Mol Immunol. 2006;43:2002–11. doi: 10.1016/j.molimm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 48.Kim HR, Cho ML, Kim KW, Juhn JY, Hwang SY, Yoon CH, Park SH, Lee SH, Kim HY. Up-regulation of IL-23p19 expression in rheumatoid arthritis synovial fibroblasts by IL-17 through PI3-kinase-, NF-kappaB- and p38 MAPK-dependent signalling pathways. Rheumatology (Oxford, England) 2007;46:57–64. doi: 10.1093/rheumatology/kel159. [DOI] [PubMed] [Google Scholar]
- 49.Tzartos JS, Friese MA, Craner MJ, Palace J, Newcombe J, Esiri MM, Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–55. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kebir H, Kreymborg K, Ifergan I, Dodelet-Devillers A, Cayrol R, Bernard M, Giuliani F, Arbour N, Becher B, Prat A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat Med. 2007;13:1173–5. doi: 10.1038/nm1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–55. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Esser C, Rannug A, Stockinger B. The aryl hydrocarbon receptor in immunity. Trends Immunol. 2009;30:447–54. doi: 10.1016/j.it.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Dumoutier L, Van Roost E, Ameye G, Michaux L, Renauld JC. IL-TIF/IL-22: genomic organization and mapping of the human and mouse genes. Genes Immun. 2000;1:488–94. doi: 10.1038/sj.gene.6363716. [DOI] [PubMed] [Google Scholar]
- 55.Simons M, Trotter J. Wrapping it up: the cell biology of myelination. Curr Opin Neurobiol. 2007;17:533–40. doi: 10.1016/j.conb.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 56.Luther SA, Cyster JG. Chemokines as regulators of T cell differentiation. Nat Immunol. 2001;2:102–7. doi: 10.1038/84205. [DOI] [PubMed] [Google Scholar]
- 57.Haas W, Pereira P, Tonegawa S. Gamma/delta cells. Ann Rev Immunol. 1993;11:637–85. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- 58.Sobel RA, Kuchroo VK. The immunopathology of acute experimental allergic encephalomyelitis induced with myelin proteolipid protein. J Immunol. 1992;149:1444–1451. [PubMed] [Google Scholar]
- 59.Hvas J, Oksenberg JR, Fernando R, Steinman L, Bernard CC. Gamma delta T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J Neuroimmunol. 1993;46:225–34. doi: 10.1016/0165-5728(93)90253-u. [DOI] [PubMed] [Google Scholar]
- 60.Olive C. Gamma delta T cell receptor variable region usage during the development of experimental allergic encephalomyelitis. J Neuroimmunol. 1995;62:1–7. doi: 10.1016/0165-5728(95)00081-c. [DOI] [PubMed] [Google Scholar]
- 61.Shimonkevitz R, Colburn C, Burnham JA, Murray RS, Kotzin BL. Clonal expansions of activated gamma/delta T cells in recent- onset multiple sclerosis. Proc Natl Acad Sci USA. 1993;90:923–927. doi: 10.1073/pnas.90.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chien YH, Konigshofer Y. Antigen recognition by gammadelta T cells. Immunol Rev. 2007;215:46–58. doi: 10.1111/j.1600-065X.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 63.Spada FM, Grant EP, Peters PJ, Sugita M, Melian A, Leslie DS, Lee HK, van Donselaar E, Hanson DA, Krensky AM, Majdic O, Porcelli SA, Morita CT, Brenner MB. Self-recognition of CD1 by gamma/delta T cells: implications for innate immunity. J Exp Med. 2000;191:937–48. doi: 10.1084/jem.191.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Noort JM, van Sechel AC, Bajramovic JJ, el Ouagmiri M, Polman CH, Lassmann H, Ravid R. The small heat-shock protein alpha B-crystallin as candidate autoantigen in multiple sclerosis. Nature. 1995;375:798–801. doi: 10.1038/375798a0. [DOI] [PubMed] [Google Scholar]
- 65.Vanderlugt CL, Miller SD. Epitope spreading in immune-mediated diseases: implications for immunotherapy. Nat Rev Immunol. 2002;2:85–95. doi: 10.1038/nri724. [DOI] [PubMed] [Google Scholar]
- 66.McMahon EJ, Bailey SL, Castenada CV, Waldner H, Miller SD. Epitope spreading initiates in the CNS in two mouse models of multiple sclerosis. Nat Med. 2005;11:335–339. doi: 10.1038/nm1202. [DOI] [PubMed] [Google Scholar]
- 67.Steinman L. New targets for treatment of multiple sclerosis. J Neurol Sci. 2008;274:1–4. doi: 10.1016/j.jns.2008.06.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Analysis of CNS infiltrating cells that produce IL-17. R-EAE was induced in female SJL/J mice primed subcutaneously with 50 μg PLP139-151/CFA as described in Materials and Methods. At peak disease, cells were isolated from the CNS and spleen, and intracellular cytokine staining performed and analyzed by flow cytometry. (a) The percentage of total γδ T cells that produce IL-17 in the spinal cord and cerebellum at onset, peak and relapse. (b) The percentage of lymphocytes which produce IL-17 and are CD4+, CD8+ or γδ+ T cells. Data are representative of 3 independent experiments and the mean of 4 animals per tissue.