Summary
Polarization of effector CD4+ T cells can be influenced by both antigen-specific signals and by pathogen- or adjuvant-induced cytokines, with current models attributing a dominant role to the latter. Here we have examined the relationship between these factors in shaping cell-mediated immunity using intravital imaging of CD4+ T cell interactions with dendritic cells (DCs) exposed to polarizing adjuvants. These studies revealed a close correspondence between strength of T cell receptor (TCR)-dependent signaling and T helper-1 (Th1) vs. Th2 cell fate, with antigen concentration dominating over adjuvant in controlling T cell polarity. Consistent with this finding, at a fixed antigen concentration, adjuvants inducing Th1 cells operated by affecting DC costimulation that amplified TCR signaling. TCR signal strength controlled downstream cytokine receptor expression, linking the two components in a hierarchical fashion. These data reveal how quantitative integration of antigen display and costimulation regulates downstream checkpoints responsible for cytokine-mediated control of effector differentiation.
Introduction
Antigen-activated naïve CD4+ T helper (Th) lymphocytes can differentiate into multiple distinct subsets, defined by expression of surface markers, transcription factors, and effector cytokines. Each subset plays an important and distinct role in mediating or directing the nature of the host response induced upon exposure to a pathogen, interaction with commensals, or vaccination. Past studies have shown a central role for cytokines such as interleukin (IL)-1, 2, 4, 6, 12 21, interferon (IFN)γ or transforming growth factor (TGF)β (Zhu and Paul, 2010) in dictating the differentiation path followed by an antigen-engaged naïve T cell. These findings have led to the widely held view that activation of dendritic cells (DC) by particular pathogen-associated molecular patterns (PAMPs) creates a specific cytokine milieu, which in turn produces qualitatively different intracellular responses that guide CD4+ T cell polarization towards a specific effector phenotype (Medzhitov and Janeway, 1997).
While many of the reports linking cytokine milieu to effector fate choice have been conducted using cells from TCR transgenic animals and in vitro culture systems a substantial body of in vivo evidence also supports the key role played by cytokines in CD4+ T cell polarization (Zhu et al., 2010). Mice deficient in or over-expressing specific cytokines show dramatic changes in the nature of the effector CD4+ T cell that emerge after immunization or infection (Finkelman et al., 2004). Likewise, infection with particular organisms drives polarized effector CD4+ responses and manipulation of the cytokine environment changes the character and efficacy of these pathogen-driven responses (Sacks and Noben-Trauth, 2002), providing in vivo support to a model in which it is the qualitative effects of these soluble mediators that play a dominant role in directing the nature of the cell-mediated immune response.
Despite the widespread acceptance of this qualitative (cytokine-defined) model, there are data showing that quantitative factors, especially the strength of antigen stimulation through the TCR, make important contributions to T cell polarity choice. Both in vitro and in vivo studies (Constant et al., 1995; Hosken et al., 1995; Milner et al., 2010; Yamane et al., 2005) have demonstrated that the extent of signaling through the TCR and associated co-stimulatory receptors can dictate the outcome of differentiation. A high dose of peptide or a strongly agonistic ligand favors development of Th1 (IFNγ-producing) cells whereas stimulation with a low dose of peptide or a weakly agonistic ligand favors Th2 (IL-4, 5, and 13 producing) cells.
As most studies evaluating the role of cytokines in vitro are done at single antigen or anti-TCR antibody concentrations, the quantitative component is generally removed from consideration, giving the appearance that cytokines dominate. In vivo, infections provide a particular degree of antigenic stimulation that is not usually subject to experimental manipulation, making it difficult to parse out the role of signaling strength in experiments that alter the cytokine environment in infected animals. Given that variations in both the cytokine milieu and extent of TCR signaling exist in vivo during infections or upon vaccination, we felt it was important to ask how the cell interprets such complex stimuli and specifically, whether one category of inputs is hierarchically dominant. To this end, we devised a model system in which both the cytokine milieu and the strength of antigen stimulation could be independently varied to explore how quantitative and qualitative aspects of signaling regulate CD4+ T cell differentiation. Dynamic 2-photon microscopy (2P-IVM) was used to directly assess T-DC interaction duration, synapse size, and calcium signaling. By varying both the adjuvant exposure used to activate DC and control their cytokine production and costimulatory capacity, as well as by carefully modulating the peptide-MHC Class II (pMHC) ligand display encountered by the responding T cells, we obtained direct information about how these distinct factors influenced strength of signaling in vivo. Through this crossover experimental design, imaging-based measurements, and assessment of post-priming effector T cell phenotype, we found that strength of signal dominates over adjuvant and cytokines in dictating Th1 vs. Th2 T cell fate. Adjuvants influenced polarization through the effects of costimulation on TCR signaling, with the strength of the combined TCR and costimulatory stimulus in turn controlling cytokine receptor expression. These findings reveal that antigen-dependent events act as upstream regulators of secondary checkpoints leading to the type of cytokine control typically given priority in models of T cell differentiation.
CD4+ T Cells Undergoing Th1 as Compared to Th2 cell Polarization Show Greater T cell-DC Interaction Times
We began our studies by examining the ability of adjuvant treatment of DC to promote Th1 vs. Th2 effector cell development at a fixed moderate concentration of available TCR ligand. DC were pulsed with 0.1μM pigeon cytochrome C peptide (pPCC) in the presence of either lipopolysaccharide (LPS) (LPS-DC) or papain (papain-DC,) and the DC adoptively transferred into CD45.1+ B10.A animals. Eighteen hours later naïve antigen-specific 5CC7 TCR transgenic CD45.2+CD4+ T cells specific for pPCC were transferred into the same animals. Four days later, Th1 vs. Th2 cell differentiation was assessed by measuring IFN-γ or IL-4 production, respectively, by restimulated recovered 5CC7 cells.. LPS-treated DC induced a strongly biased Th1 cell response, whereas interaction with papain-treated DC led to a Th2 cell skewed response (Figure 1A–C). We did not detect cells producing IL-17 under these conditions (data not shown) and note that the fraction of cells showing either a Th1 or Th2 cell effector state is in accord with other in vivo immunization findings (Leon et al., 2012; Tokoyoda et al., 2009; van Panhuys et al., 2008).
Figure 1. Exposure of DC to polarizing adjuvants alters the balance of CD4+ T cell effector fates in concert with changes in cellular interaction times.
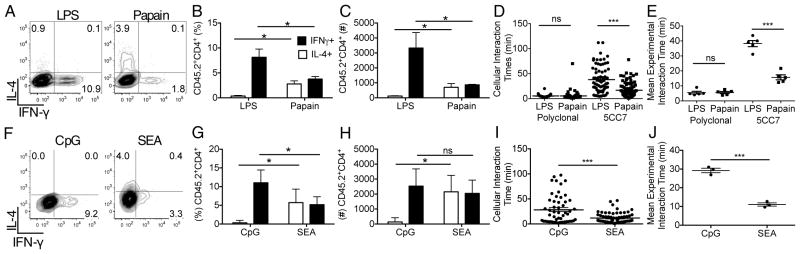
Assessment of 5CC7 cell differentiation following priming with (A–C) LPS- vs. papain-treated DC or (F–H) CpG- vs SEA-treated DC at day four post-adoptive transfer. (D & I) Individual cellular interaction times of polyclonal or 5CC7 T cells interacting with adjuvant treated DC, 0–2hr post-transfer as determined following 2P-IVM imaging. (E & J) Mean cellular interaction times for separate 2P-IVM experiments. (A–C & F–H) Data are representative of three experiments (n=4). (D&I) Data are pooled from five or three animals respectively. (E&J) Data represent the mean interaction times from five or three animals respectively. Error bars represent mean +/− s.e.m. * P<0.05, ** P<0.01, *** P<0.001 as determined by (B–E,G,H) 1 way ANOVA with Tukeys post testing or (I,J) Student’s t test.
To determine if adjuvant treatment altered in vivo trafficking or the the uptake and display of ligand by these cells, thus affecting the intensity of the TCR signaling, DC were pulsed with long chain biotinylated-pPCC (LC-pPCC) in the presence of LPS or papain and LC-pPCC binding to MHCII was assessed. Both LPS-DC and papain-DC displayed similar MHCII and LC-pPCC staining (Figure S1A–G). In addition, similar numbers of adjuvant-treated DC accumulated in the draining LN (dLN), showing that the adjuvant pre-treatment did not alter the migratory capacity of DC (Figure S1H) and no significant difference was detected in MHCII or LC-pPCC staining on DC recovered from the dLN (Figure S1I–L), indicating equivalent antigen presentation in vivo.
While these data suggested that adjuvants did not have a direct effect on quantitative aspects of T cell activation via modification of ligand display, a possible effect on overall strength of TCR signaling was. possible. To explore this issue in vivo, 2P-IVM was employed. The duration of T-DC interactions was measured and the effect of adjuvants evaluated in the context of previously defined sequential stages of T cell-DC interaction (Mempel et al., 2004) in which phase transition has been linked to the strength of TCR-related signaling (Henrickson et al., 2008). DC were fluorescently labeled following incubation with pPCC and LPS or papain and adoptively transferred. Eighteen hrs later 5CC7 CD4+ T cells and wild-type (WT) polyclonal CD4+ T cells labeled with distinct fluorophores were transferred and cellular interactions in the dLN immediately imaged (Movie S1). As previous work has indicated that adjuvants induce differential chemokine production from DCs (Tang et al., 2010), we sought to determine whether adjuvant pretreatment would lead to preferential interaction of 5CC7 cells with LPS- or papain- treated DC. We observed that both WT and 5CC7 cells contacted LPS- and papain-treated DC at similar rates, indicating that a differential chemoattractant potential was not conferred on the DCs by the distinct adjuvant pre-treatments (Figure S2A–C). Polyclonal cells interacting with either LPS-DC or papain-DC exhibited brief browsing behavior as previously reported (Miller et al., 2004), whereas antigen-specific 5CC7 cells exhibited more prolonged interactions with the antigen-bearing DC (Figure 1D,E). A proportion of the 5CC7 contacts involving LPS-treated DC rapidly transitioned to Phase 2-like interactions, with contacts lasting >1hr (Mempel et al., 2004). In contrast, prolonged interactions characteristic of Phase 2-like behavior was virtually absent among 5CC7 cells interacting with papain-DC.
To assess whether such interaction differences were generally associated with adjuvant effects promoting Th1 vs. Th2 cell development at the same TCR ligand density, we compared DCs treated with the Th1 cell-inducing adjuvant CpG oligodeoxynucleotides (CpG) with those treated with the Th2 cell-inducing adjuvant Schistosomal egg antigen (SEA) (Figure 1F–H). Pre-treatment of DC with distinct adjuvants did not alter the chemottractive potential of DCs as the 5CC7 cells showed equal rates of contact with SEA- vs. CpG-treated DC (Figure S2D–F). As seen for the other adjuvant set, DC exposed to the Th1 cell promoting adjuvant showed significantly longer interaction times with 5CC7 cells and a more rapid transition to Phase-2-like behavior as compared to DC exposed to the Th2 cell inducing adjuvant (Figure 1I,J). These data with two distinct adjuvant pairs, taken in the context of previous studies showing more rapid transition to Phase-2 behavior is related to increased TCR signaling (Henrickson et al., 2008), suggest a potential quantitative effect of adjuvant treatment on the TCR-associated signaling capacity of DC displaying equal concentrations of antigen.
To determine whether adjuvant treatment affected cell interaction dynamics at later time points, also suggesting differences in TCR-associated signaling, we imaged the cohort of 5CC7 cells recruited to the LN 0–2hrs post-transfer by blocking further recruitment with anti-CD62L (Mempel et al., 2004). At 12hr post-transfer a significant proportion of 5CC7 cells interacted stably with LPS-DC for the majority of the imaging time, while only a small proportion of 5CC7 cells interacting with papain-DC did so (Figure 2A,C,E,G; S3A&B; Movie S2). At 22hr post-transfer the majority of 5CC7 cells interacting with papain-DC showed Phase-3 behavior, exhibiting increased migration speeds and a mean interaction time similar to that of polyclonal cells (Figure 2B,D,F; Movie S2). In contrast, many long-term interactions were still evident between 5CC7 cells and LPS-DC (Figure 2B,G). Together, these results indicate that incubation of DC with a Th1 cell inducing adjuvant leads to stable long-lived interactions with antigen-specific T cells, whereas DC exposed to a Th2 cell inducing adjuvant show few prolonged interactions with specific T cells and the lymphocytes rapidly progressing to Phase-3 dynamic behavior.
Figure 2. Exposure of DC to polarizing adjuvants alters Phase-2 and Phase-3 interactions.
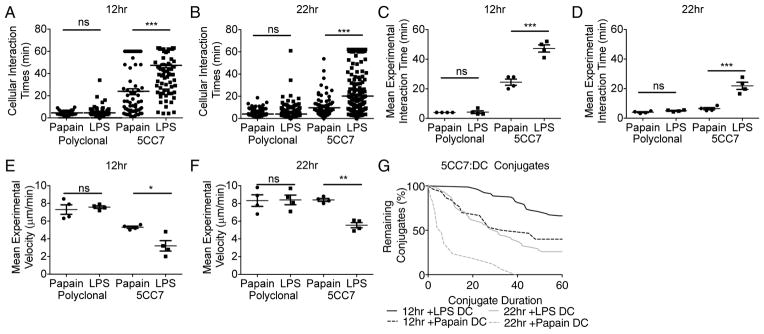
Distribution of interaction times of polyclonal and 5CC7 cells with pPCC loaded papain- or LPS-treated DC at (A) 12hr post-transfer and (B) 22hr post-transfer. Mean experimental interaction times at (C) 12hr post-transfer and (D) 22hr post-transfer. Mean experimental velocities at (E) 12hr and (F) 22hr post-transfer. (G) Percentage of 5CC7 cell:DC conjugates originally observed still remaining over time. Imaging was conducted for 1hr (A,B,G) Represent pooled data from 4 experiments. (C–F) indicate mean +/− s.e.m of data from 4 experiments. *P<0.05, **P<0.01 and ***P<0.001 as determined by 1 way ANOVA with Tukeys post testing.
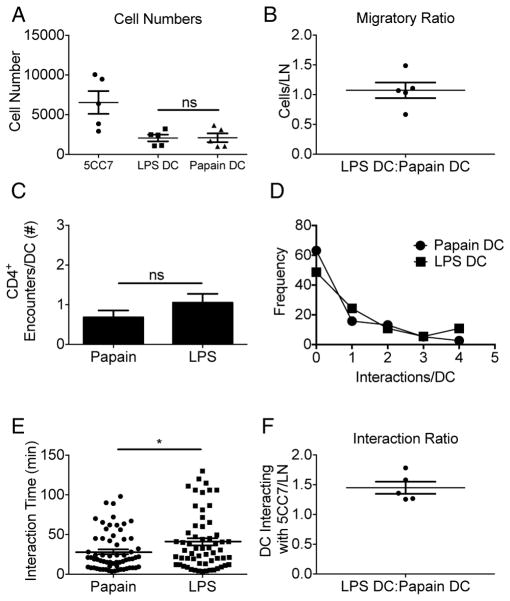
To further determine whether qualitative mediators such as chemokines or cytokines released by DC into the local milieu contribute to the differences in interaction times that we observed, antigen-loaded adjuvant stimulated LPS-DC and papain-DC were labeled with distinct dyes and co-transferred into the same recipient. Eighteen hours post-transfer labeled naïve 5CC7 cells were transferred and immediately after transfer, cellular interactions were imaged (Movie S3). LPS-DC and papain-DC both reached the dLN in equal numbers and showed similar initial rates of interaction with 5CC7 cells (Figure 3A–D). In these mixed transfer conditions, 5CC7 cells exhibited significantly greater interaction times with LPS-DC as compared to papain-DC (Figure 3E) and the transferred T cells were found to be preferentially associated with LPS-DC after two hours of imaging (Figure 3F). These data argue that the distinct interaction durations seen with DC exposed to different adjuvants occur as a result of cis-regulated interactions between T cells and antigen-bearing DC and are not due to the chemokine or cytokine milieu created by the adjuvant-treated DC.
Figure 3. Different adjuvant treatments of DCs alter contact duration but not initial contact frequency with CD4+ T cells.
pPCC loaded papain- and LPS-stimulated DC were alternately labeled and co-transferred into naïve B10.A hosts. Eighteen hrs post transfer 5CC7 cells were adoptively transferred and imaged for 2hr. (A) 5CC7 and DC cell numbers per LN were determined post imaging. (B) Ratio of LPS: papain treated DC per LN. (C) Mean number of CD4+ T cell interactions per DC and (D) frequency distribution of interactions. (E) Interaction times of 5CC7 CD4+ T cells with DCs. (F) Ratio of 5CC7 cells interacting with LPS-DC: papain-DC after 2 hrs. (A–F) Data are indicative of 5 LN. Means +/− s.e.m. *P<0.05, **P<0.01 and ***P<0.001 as determined by Student’s t test.
Th1 Cell Inducing Adjuvants Promote Stronger Ca2+ Signaling and Larger Synapse Size Among Interacting CD4+ T Cells
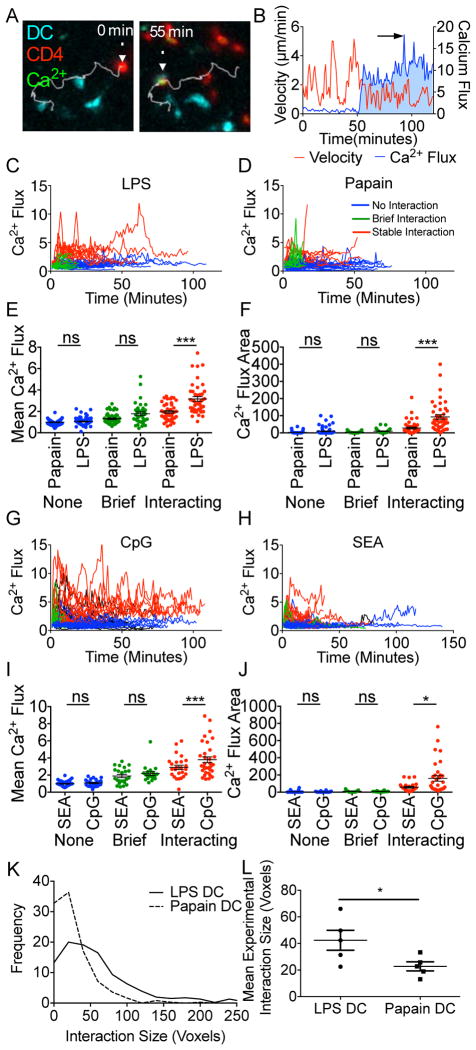
The preceding dynamic imaging studies suggested that adjuvant exposure of DC alters a T cell’s quantitative perception of antigen load. To explore this idea, further we used 2P-IVM to examine Ca2+ flux (Figure 4A,B; Movie S4), which reflects the strength of ligand-induced TCR signaling and is associated with the arrest of migratory behavior (Bhakta et al., 2005). Cells were characterized as ‘non-interacting’ where no DC contact was observed, ‘briefly interacting’ where contact was made without a decrease in cellular velocity (Movie S4), and ‘interacting’ where contact was made and cellular velocity decreased to <2μm/min; Ca2+ flux over time was recorded for each of these types of events (Figure 4C,D,G,H. Movie S4). Mean Ca2+ flux (Figure 4E,I), the area under the Ca2+ trace (Figure 4F,J), and peak Ca2+ flux were then determined (Figure S4A) for the imaged T cells. For all conditions, we saw no significant difference in Ca2+ flux for 5CC7 cells classified as non-interacting or briefly interacting. However, among 5CC7 cells that formed stable interactions, DC cultured with Th1 cell inducing adjuvants induced a significantly higher Ca2+ response. There was a clear positive correlation between extent of the Ca2+ flux evoked by the interaction of T cells with the adjuvant-treated DC (LPS-DC; Figure S4B–D and papain-DC; Figure S4E–G) and the resulting interaction time of the cells, consistent with the previous interpretation of the phase transition data as indicating a difference in proximal TCR signaling by 5CC7 cells contacting DC exposed to distinct adjuvants despite equivalent peptide loading.
Figure 4. Greater Ca2+ signaling and synapse size of CD4+ T cells interacting with DC exposed to Th1vs. Th2cell promoting adjuvants.
DC were loaded with pPCC and treated with either (A–F) LPS or papain or (G–J) CpG or SEA and transferred into naïve B10.A hosts. Eighteen hrs post transfer 5CC7 cells loaded CMTPX and Fluor-4 were adoptively transferred and imaged for 2hr. (A) Representative micrograph of 5CC7 cell tracking during Ca2+ flux analysis by 2P-IVM. (B) 5CC7 cell tracks were analyzed post-2P-IVM and normalized Ca2+ flux (blue) and instantaneous velocity (red) were used to characterize interactions. (C,D,G&H) Individual 5CC7 cell Ca2+ flux tracings for cells interacting with adjuvant treated DC are shown for durations of T cell:DC contacts classified as involving no interaction (blue), brief interactions (green), or stable interactions (red). (E&I) Mean Ca2+ flux and (F&J) integrated Ca2+ flux areas were calculated for individual cell tracks. (K) Distribution of 5CC7 cell:DC interaction interface sizes and (L) experimental mean interaction interface sizes. (E,F&K) five, (I&J) three pooled experiments, (L) means from five separate experiments. Mean +/− s.e.m. * P<0.05, ** P<0.01, *** P<0.001 as determined by 1 way ANOVA with Tukeys post testing.
As a further measure of TCR-dependent interactions, we analyzed the dimensions of the T cell-DC interface (immunological synapse) (Klauschen et al., 2009). At 2hr post transfer, LN were fixed, serially sectioned, imaged, and whole LN were digitally reconstructed (Figure S4H; Movie S5). From these images, DC-T cell interaction interfaces were determined (Figure S4I; Movie S5). LPS-DC showed a significantly larger cellular interface with 5CC7 cells as compared to papain-DC (Figure 4K,L), consistent with the stronger Ca2+ signaling and longer duration interactions observed with LPS-DC. In sum, these data supported a per DC cis-effect of adjuvant on the strength of TCR-associated signaling at a given pMHC density.
Modulation of TCR-dependent Signal Strength Alters T cell Polarization Outcome and Overrides Adjuvant Effects
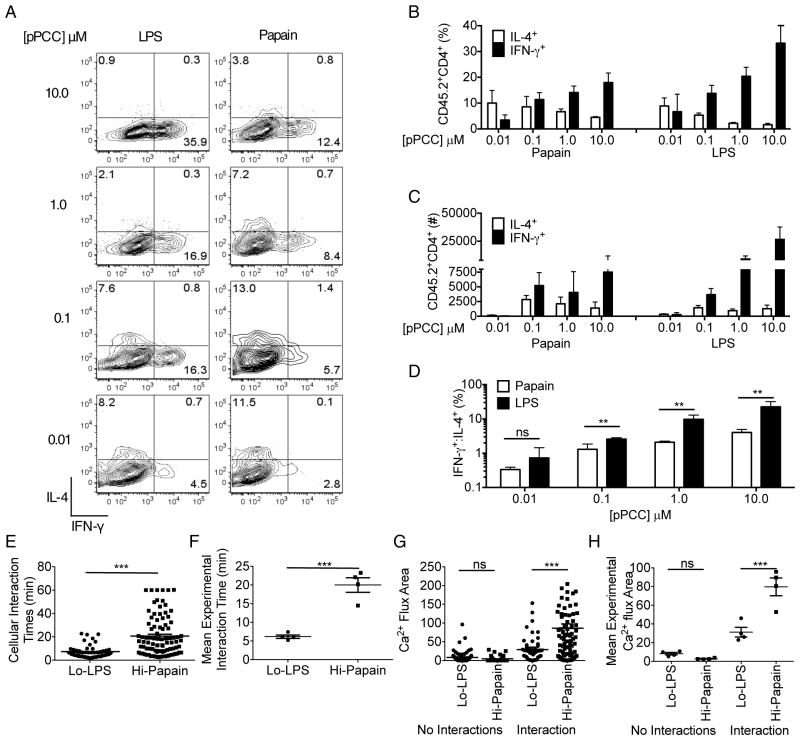
The observations described above revealed that Th1 and Th2 cell polarizing adjuvants influenced the strength and duration of early antigen-induced signals in a manner correlating with past evidence that strong signaling promotes Th1 cell responses whereas weaker stimulation favors Th2 cell development. However, it remained unclear whether these quantitative differences causally determined T cell fate or were simple correlations, with qualitative signals from cytokines induced by adjuvants having a more important role in guiding differentiation. Therefore, we examined whether manipulating the strength of signal by varying the concentration of peptide used to pulse DC would override putative dominant qualitative cytokine signaling mechanisms induced by adjuvant treatment. DC were pulsed with a range of peptide concentrations (10.0μM-0.01μM) in the presence of either LPS or papain, and used to prime 5CC7 cells in vivo. Following priming with the DC exposed to different peptide concentrations, dLN were collected and the relative Th1 vs. Th2 cell differentiation of 5CC7 cells was assessed. A cytokine-dominant qualitative model would predict that changing pPCC amounts on adjuvant–treated DC should result in the same bias of Th cell polarization but with more or fewer cells adopting the expected fate, whereas a quantitative model involving TCR-dominated signaling would predict a change in Th cell fate outcome as peptide concentration was altered.
To assess whether variable loading with synthetic peptides had any effect on the DC other than in terms of pMHC display we examined the surface the activation phenotype of these cells as well as their production of IL-6, IL-10, and IL-12 (Figure S5A–D). These various measures should sensitively report the presence of PAMPs in the peptide preparations that might influence how the DC behave in vivo. We found no significant differences in these various measures with vs. without peptide treatment, providing strong evidence that any changes in T cell fate influenced by varying the peptide loading of the DC reflect the influence of pMHC density.
Following activation with DC pulsed with 10.0μM pPCC, a Th1 cell biased response predominated even after DC treatment with the Th2 cell evoking adjuvant papain (Figure 5A–C). However, as the concentration of pPCC was decreased, the proportion and numbers of IL-4 producing cells increased coincident with a decrease in IFNγ producing cells, such that at 0.01μM pPCC a Th2 cell response was predominantly evoked regardless of the adjuvant used to treat the DC (Figure 5D; S5A). These findings suggest that the strength of proximal signaling induced in the T cell by the DC dominates over any qualitative effects imparted by adjuvants. Consistent with this, 5CC7 cells showed significantly decreased interaction times and Ca2+ fluxes (Figure 5E–H, S5B–D) when recognizing LPS-DC pulsed with a low concentration of pPCC (Lo-LPS: 0.01μM) as compared with papain-DC pulsed with high concentrations of pPCC (Hi-pap: 10.0μM). Thus, the degree of early proximal T cell signaling was more tightly correlated with Th cell differentiation outcome than was the adjuvant used to treat the DC and quantitative features of T-DC interactions appeared to be dominant over qualitative signals arising from adjuvant exposure of the DC.
Figure 5. The magnitude of antigen-dependent signal strength dominates over qualitative effects of adjuvants on DC.
(A) Assessment of 5CC7 cell differentiation following priming with LPS- or papain-treated DC loaded with 10μM-0.01μM pPCC, at day four post-adoptive transfer. (B) Quantification of percent of 5CC7 cells expressing IL-4 or IFNγ, (C) Number of 5CC7 cells expressing IL-4 or IFNγ and (D) ratio of %IFNγ: %IL-4 producers. (E) Cellular interaction times 0–1hr post-transfer of 5CC7 cells with Lo-LPS- or Hi-papain-treated DC. (F) Mean interaction times for individual experiments. (G) Integrated Ca2+ flux areas were calculated for individual cellular tracks. (H) Mean Integrated Ca2+ flux areas for individual experiments. (A–D) are representative of four experiments (n=4). (E&G) Data are pooled from four experiments. (F&H) Data are representative of four experiments. Mean +/− SEM. *P<0.05, **P<0.01 and ***P<0.001. 1 way ANOVA with Tukeys post testing.
Adjuvants Influence Signal Strength Through Effects on Costimulatory Molecule Expression
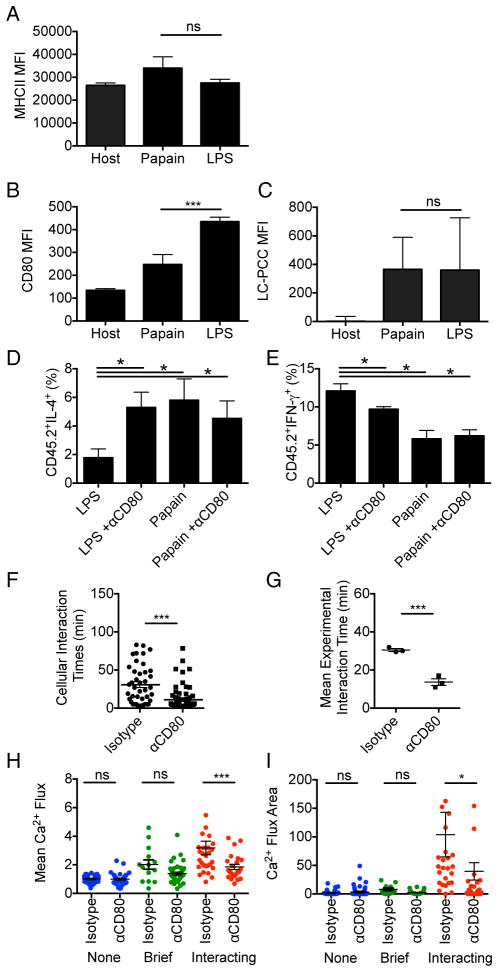
To probe how elevated proximal TCR-related signaling could occur using LPS- or CpG-treated DC presenting similar amounts of pMHC TCR ligand in comparison to papain- or SEA-treated DC, we extended our phenotypic analysis of DC in vitro and ex vivo. There were no significant differences between the DC treatment groups in terms of physical characteristics or in expression of MHCII, intercellular adhesion molecule-1 (ICAM-1), and inducible costimulatory molecule ligand (ICOSL) or presentation of LC-pPCC (Figure 6A,C; S6A–F). One major difference that correlated with outcome did emerge, however; DC treated with Th1 cell inducing adjuvants had higher expression of the CD28 ligands CD80 and CD86 than did those exposed to Th2 cell inducing adjuvants (Figure 6B; S6E,F). CD80 signaling can potentiate Ca2+ flux upon TCR activation (Nurieva et al., 2007) and mediate tighter T cell-DC interactions (Lim et al., 2012), potentially explaining the increased TCR-associated signaling observed following treatment of DC with Th1 cell as compared with Th2 cell inducing adjuvants at a constant pMHC density. To assess this possibility we utilized antibody blockade of CD80 signaling during in vivo activation of 5CC7 cells with LPS-DC or papain-DC. Although only affecting one of several costimulatory ligands on the DC upregulated by LPS treatment, antibody treatment led to a significant decrease in IFNγ production and a corresponding increase in IL-4 production by the recovered 5CC7 cells (Figure 6D,E). 2P-IVM imaging showed that treatment with αCD80 resulted in a decrease both in T cell interaction times with the LPS-DC and in the associated T cell Ca2+ flux (Figure 6F–I), yielding a pattern more similar to that observed under Th2 cell inducing conditions. These findings explain how proximal TCR-related signals can differ with DC exposed to distinct adjuvants even when peptide-MHC display is equalized and indicate that adjuvants can influence T cell polarization through dominant effects on proximal strength of signal involving TCR cooperation with CD28.
Figure 6. T cell interpretation of antigen display is affected by CD80 co-stimulation.
pPCC loaded LPS- or papain-treated DC were isolated from dLN at 24hr post-transfer and analyzed. (A) MHCII, (B) CD80 and (C) PCC peptide presentation reported as MFI post-staining. (D) Percent of 5CC7 cells producing IL-4 and (E) IFNγ at day four post-adoptive transfer, following priming with LPS- or papain-treated DC, +/− blocking with anti-CD80. (F) Cellular interaction times 0–2hr post-transfer of 5CC7 cells with LPS or papain treated. (H) Mean interaction times for individual 2P-IVM experiments. (H) Mean Ca2+ flux values and (I) integrated Ca2+ flux areas were calculated for individual cellular tracks. (A–D) are representative of two experiments (n=4). (E&F) Data are representative of three experiments (n=4). (G,I&J) Data are pooled from three experiments. (H) Data are representative of three experiments. Error bars indicate mean +/− s.e.m. *P<0.05, **P<0.01 and ***P<0.001, as determined by (A–E,H&I) 1 way ANOVA with Tukeys post testing or (F, G)Student’s t test.
TCR-associated Signal Strength Regulates Downstream Cytokine Signaling Checkpoints
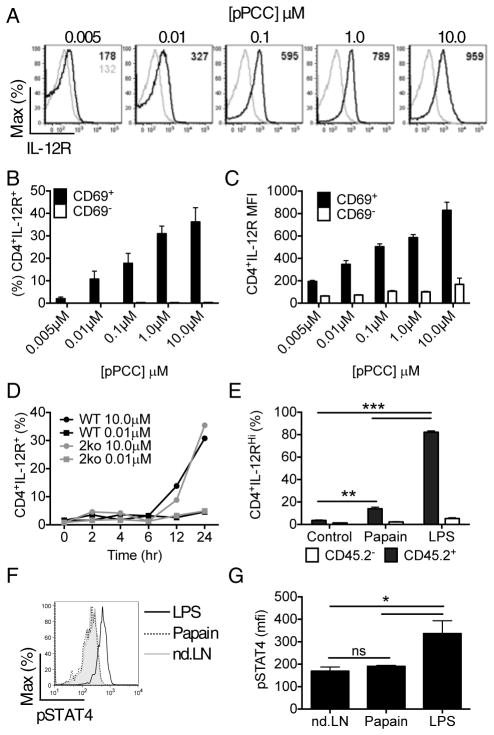
To reconcile our results with previous data showing a crucial role for adjuvant-elicited cytokines in directing effector T cell development, we theorized that TCR signaling may operate upstream of cytokine-mediated checkpoints; that is, signal strength controls T cell responsiveness to polarizing cytokines, which would then drive the molecular events involved in differentiation. IL-12p70 is a critical myeloid-cell-derived cytokine involved in Th1 cell polarization (Zhu et al., 2010), which has been shown to be delivered via the immunological synapse (Pulecio et al., 2010). As the complete IL-12 receptor is not expressed on naïve CD4+ T cells (Desai et al., 1992), we tested if upregulation of the relevant IL-12Rβ2 chain was associated with DC signal strength and the length of interaction with the antigen-bearing DC. DC were pulsed with varying concentrations of pPCC and used to activate 5CC7 cells in vitro. Following activation, increased IL-12Rβ2 expression was found to correlate directly with the concentration of pPCC presented (Figure 7A). Activation was required for the induction of IL-12Rβ2 expression and both the frequency of positive cells and mean fluorescent intensity (MFI) of IL-12Rβ2 expression showed a direct correlation with the dose of antigenic peptide (Figure 7B,C).
Figure 7. Signal strength determines the ability of CD4+ T cells to respond to polarizing cytokines.
5CC7 cells were activated in vitro using P13.9 artificial antigen presenting cells pre-incubated with the concentrations of pPCC indicated. Twenty-four hr post-activation IL-12Rβ2 expression by the T cells was determined by flow cytometry. (A) Representative plots of IL-12Rβ2 expression by stimulated (black lines) or naïve (grey lines) 5CC7cells, (B) Percent of of activated (CD69+) and non-activated (CD69−) 5CC7 cells expressing IL-12Rβ2, (C) comparison of IL-12Rβ2 MFI for activated vs. non-activated 5CC7 cells. WT 5CC7 or 5CC7 Il4−/−Ifng−/− (2ko) cells were stimulated in vitro with P13.9 cells pre-incubated with either 0.01μM or 10μM pPCC and (D) IL-12Rβ2 expression determined. 5CC7 cells were activated in vivo with LPS or papain treated DC and compared to control 5CC7 cells from non-dLN. Ex vivo expression of (E) IL-12Rβ2 and (F & G) pSTAT4 by CD69+ 5CC7 CD4+ T cells was then determined at 24hr post transfer. Data in (A–C) are representative of two experiments (n=4), (D, E, F & G) are representative of three experiments (n=4), means are plotted +/− s.e.m. *P<0.05, **P<0.01 and ***P<0.001 as determined by 1 way ANOVA with Tukeys post testing.
Because our in vivo results indicated that Th1 cell development was associated with long-term T cell-DC interactions, we examined whether there was also a temporal component to the upregulation of IL-12Rβ2. IL-12Rβ2 expression was only induced after >6hrs in culture with high concentrations of pPCC (Figure 7D). To determine if polarizing cytokines materially affected IL-12Rβ2 expression, we compared normal 5CC7 cells with Il4−/−Ifng−/− 5CC7 cells and found that there was no alteration in IL-12Rβ2 expression in the absence of these key mediators (Figure 7D). To investigate the physiological significance of these in vitro findings, we again used antigen-loaded, adjuvant-exposed DC to activate naïve 5CC7 cells in vivo. Following in vivo activation, CD4+ T cell interaction with LPS-DC resulted in higher IL-12Rβ2 expression in comparison to T cell exposure to papain-DC (Figure 7E; S7A–D). Additionally, 5CC7 cells activated by LPS-DC had increased amounts of the phosphorylated transcription factor STAT4 in comparison to those activated by papain-DC (Figure 7F,G). Together, these findings indicate that the strength of antigen-dependent signals, as influenced by pMHC concentration and co-stimulation and the corresponding duration of T-DC interactions, control downstream cytokine response checkpoints that ultimately direct effector polarization.
Discussion
The differentiation of naïve CD4+ T cells into distinct effector subsets plays a major role in governing the outcome of the adaptive immune response. Both qualitative characteristics of the cytokine milieu and quantitative signals imparted through TCR-mediated stimulation can influence the differentiation of specific T helper subsets (Yamane and Paul, 2013). Our data demonstrate that the differentiation of naïve CD4+ T cells into Th1 or Th2 cell effectors involves a series of checkpoints that begins with a quantitative T cell assessment of antigen and co-stimulatory signals, which in turn secondarily control the responsiveness of the T cell to polarizing cytokines. Weak signals are sufficient to activate CD4+ T cells and induce Th2 cell differentiation following brief interactions, potentially through an endogenous Th2 cell differentiation program (Zhu et al., 2012). Conversely, Th1 cell differentiation requires strong signaling and a transition to long-term interactions. Integration of strong signals induces a divergent program of early activation gene expression, including upregulation of IL-12Rβ2, which allows further tuning of the immune response by the cytokine environment. Taken as a whole, these findings reveal how adjuvants not only alter the ability of APC to produce qualitative signals (Medzhitov and Janeway, 1997) affecting CD4+ T cell differentiation, but play a critical and indeed, dominant role in the latter process through effects on co-stimulatory molecule expression that synergizes with antigen to control signals associated with TCR engagement.
This relationship between signal strength and effector fate can be observed in animals with a polyclonal T cell repertoire and absent artificially applied adjuvants, arguing against the present findings being unique to the experimental system we employed. Consistent with our findings showing low signal strength promotes Th2 cell induction, expression of hypomorphic variants of the TCR-proximal signaling adapter LAT leads to spontaneous induction of Th2 cell associated autoimmunity (Mingueneau et al., 2009). Likewise, ZAP70mrd/mrt animals exhibit defective T cell Ca2+ mobilization along with elevated IgG1 and IgE, isotypes characteristic of a Th2 cell response (Siggs et al., 2007). Proteins further downstream in the signaling cascade such as PKCθ and Wiskott Aldrich Syndrome protein (WASp) play opposing roles in the maintenance of the IS(Sims et al., 2007), with PKCθ contributing to termination of stable synaptic interaction with DC and WASp acting as a negative regulator of the PKCθ-mediated symmetry breaking process. In accordance with our data indicating the importance of a temporal element in CD4+ T cell differentiation, activation conditions favoring PKCθ signaling and shorter interactions preferentially induce Th2 cell differentiation (Cannons et al., 2004; Corn et al., 2005; Hilliard et al., 2002; Marsland et al., 2004; Medoff et al., 2006), whereas those that enhance IS stabilization and Ca2+ signaling result in preferential Th1 cell differentiation (Noble et al., 2000). Further, defects in WASp are associated with reduced Th1 cell differentiation (Taylor et al.; Trifari et al., 2006) and development of Th2 cell associated autoimmune disease (Ozcan et al., 2008) and molecules associated with enhanced Ca2+ signaling and IS formation are required for efficient Th1 cell differentiation or the suppression of spontaneous Th2 cell associated disease (Oh-Hora et al., 2008; Tahvanainen et al., 2009; Varga et al., 2010).
Studies of the TCR itself provide further evidence for a primary role of signal strength in controlling T cell fate. Single naïve polyclonal CD4+ T cells can produce an array of effector cells and that the pattern of effector cells generated after in vivo activation with cognate antigen correlated with the TCR-pMHCII dwell time or the amount of pMHCII (Tubo et al., 2013). Depletion of CD4+ T cells with a high affinity for pMHCII from a polyclonal population of cells, left lower affinity T cells that preferentially differentiated into IL-4 producing Th2 cells (Milner et al., 2010). Single cell cloning showed that low affinity cells had fewer preferred complementarity determining region-3 (CDR3) motifs, consistent with findings that the outgrowth of CD4+ T cells under Th2 cell conditions favored cells with elongated TCRα CDR3 motifs that potentially impeded TCR triggering (Boyton et al., 2002). Finally, two strains of transgenic mice created using two different TCR specific for the same self-antigen (gastric ATPase) show biased Th1 vs. Th2 cell-associated disease associated with differences in availability of self-antigen-MHC class II ligands rather than intrinsic affinity for the ligands (Levin et al., 2008). Our present analysis that emphasizes the dominant and upstream role of antigen-dependent strength of signaling in controlling effector polarization provides a coherent, integrated explanation for these many distinct observations.
While our data and these cited studies provide a coherent picture of regulation of Th1–Th2 cell fate choice, we have not yet fully addressed whether the same model applies equally well to other Th cell subsets such as Th17 cell or inducible regulatory T (iTreg) cell. However, there are also clear indications in the literature that strength of TCR signaling influences differentiation along these pathways as well (Fazilleau et al., 2009; Gottschalk et al., 2010). What remains to be determined is the overall scaling of signal input with fate determination for the entire range of Th cell subsets, the relationship of signal strength to induction of other cytokine receptors besides IL-12Rβ2, how polarizing adjuvants or PAMPS contribute to modulation of antigen-specific signaling through other co-stimulatory molecules as we report here for CD80 and 86, and whether there are conditions in which cytokines or metabolites (Arpaia et al., 2013; Furusawa et al., 2013) achieve dominance over the antigen-associated signals. A last point involves understanding how the diversity of TCR affinities and the variation in the amount of antigen presented (self or foreign) shape the overall quality of the emerging response under infectious or steady-state conditions.
A relevant question is how there could be a consistent difference in antigen stimulation strength during helminth infections that typically promote Th2 T cell responses versus bacterial, viral, or unicellular parasitic infections that often promote Th1 T cell responses(Finkelman et al., 2004; Zhu et al., 2010). It is unlikely that the affinity of the TCRs recognizing peptide ligands derived from the two different classes of pathogens (Th1 cell-inducing vs. Th2 cell inducing) is on average different. However, as cited above for the gastritis model(Levin et al., 2008) and shown here using a reductionist system, variation in the amount of pMHC ligand displayed on a DC in concert with the extent of CD80 and CD86 expression can have dramatic effects on T cell effector choice. In the case of worms, they are too large to be phagocytized and they do not reside in intracellular compartments, whereas the Th1-inducing pathogens can be taken up and often require intracellular residence. There is thus a high likelihood of extensive antigen delivery directly to presenting cells in the latter case, whereas for extracellular worms, only smaller amounts of antigen may access the presentation pathway through shed material acquired via endocytic uptake. When this is combined with evolution of the worms to limit detection by sensors controlling the DC activation pathways that promote maturation of the antigen processing machinery(Inaba et al., 2000) and upregulation of CD80 and CD86(Lee and Kim, 2007), it becomes understandable how a broad parsing of the strength of T cell stimulation can occur for these different types of infectious agents and why the immune system would have evolved to link development of the necessary class of CD4+ T cell effector response to such differences in the T cell stimulatory environment.
Many biological systems use differences in the magnitude or duration of signaling to control the qualitative state of the cell (Chen et al., 2001; Purvis and Lahav, 2013). Here we show that the immune system employs a cascading checkpoint mechanism to translate quantitative differences in early antigen and co-stimulatory T cell signaling into qualitative regulation of CD4+ effector T cell differentiation by the cytokines whose role in this process is so well recognized. These findings have important implications for vaccine design in terms of how adjuvants actually mediate their effects and how the balance of antigen amount and choice of adjuvant affect the direct and indirect roles of CD4+ cells in mediating post-vaccination host defense. They also may provide insight into the dominance of different states of adaptive immune polarity during autoimmune processes.
Experimental Procedures
Mice
B10.A CD45.2-, B10.A CD45.2+ 5CC7 TCR–transgenic Rag2−/− and B10.A CD45.2+ 5CC7 TCR–transgenic Rag2−/− IL-4G4/G4 (Hu-Li et al., 2001) x IFNg−/− mice were obtained from Taconic Laboratories through a special NIAID contract. All mice were maintained in SPF conditions at an Association for Assessment and Accreditation of Laboratory Animal Care-accredited animal facility. All procedures were approved by the NIAID Animal Care and Use Committee (NIH, Bethesda, MD).
Peptides
Pigeon cytochrome C (pPCC): KAERADLIAYLKQATAK was from American Peptide Company, long chain biotinylated pigeon cytochrome C (LC-pPCC): biotin-SGGGSGGG-KAERADLIAYLKQATAK was from CPC Scientific.
Adoptive cell transfer
CD11c+ DCs were purified by positive immunomagnetic cell sorting for CD11c (Miltenyi Biotec) as described in product literature from spleens of B10.A CD45.2-, donor mice. Polyclonal CD4+ T cells from LN of B10.A CD45.2- and from TCR transgenic 5CC7 B10.A CD45.2+ mice were purified by negative immunomagnetic cell sorting (Miltenyi Biotec). DC were incubated in vitro in cRPMI (RPMI 1640 supplemented with 10% FCS, 2-ME, glutamine, penicillin, streptomycin, and sodium pyruvate, Lonza) with the adjuvants LPS (1.0μg/ml, Invivogen), papain (100μg/ml, Calbiochem), CpG (25μg/ml, Invivogen) or SEA (40μg/ml) for four hours at 37°C in the presence of various concentrations of peptide as indicated. CD11c+ DC were transferred by s.c. injection into the right rear footpad at 1×106/recipient., CD4+ T cells were transferred by i.v. injection at 2×106/recipient at 18hr post-transfer of CD11c+ DC. Where indicated mice were injected i.v. with 100μg anti-CD62L blocking antibody (MEL-14) at two hours post CD4+ T cell transfer. For CD80 blockade studies mice were injected with either 200μg anti-CD80 blocking Ab (16.10A1) or 200μg isotype control Ab (eBio299Arm) 30 minutes prior to CD4+ T cell transfer.
Ex vivo CD4+ T cell restimulation
At day four post CD4+ T cell transfer intracellular cytokine production was determined following preparation of cell suspensions from dLNs. Cells were restimulated in cRPMI with PMA (100ng/ml, Sigma) and ionomycin (1μg/ml, Sigma) in the presence of monensin (2μM, Sigma) at 37°C for four hours. Cells were surface stained with anti-CD4 (GK1.5, Biolegend) and anti-CD45.2 (104, Biolegend) and live cell staining was performed with LIVE DEAD fixable violet dead cell stain (Invitrogen). Cells were fixed and permeabilized with BD Cytofix Cytoperm kit (BD Pharmingen™) according to the manufacturers instructions. Intracellular cytokine staining was performed with anti-IFN-γ (XMG1.2, Biolegend) and anti-IL-4 (11B11, Biolegend). Flow cytometric data were collected on an LSR II (BD Biosciences) and analyzed with FlowJo software (TreeStar).
2P intravital imaging and calcium analysis
Isoflurane was used to anaesthetize mice prior to exposure of popliteal LN (Baxter; 2.5% for induction, 1~1.5% for maintenance, vaporized in an 80:20 mixture of O2 and air), and subsequent 2P-IVM was performed using a protocol modified from a previous report (Bajenoff et al., 2006). Imaging was conducted on either (i) a Bio-Rad/Zeiss Radiance 2100MP equipped with a Chameleon laser (Coherent) tuned to 800nm, configured with a Nikon 600FN upright microscope equipped with a 20× water immersion lens (NA 0.95, Olympus), and using LaserSharp acquisition control software or (ii) a Zeiss 710 microscope equipped with a Chameleon laser (Coherent) tuned to 800nm in combination with a 20X water-dipping lens (NA 1.0, Zeiss) using Zen 2010 acquisition software. Imaging was conducted in enclosed environmental chambers in which anesthetized mice were warmed by heated air and the surgically exposed LN was kept at 36–37°C with warmed PBS.
To visualize cells, CD11c+ DC were labeled with either 100μM CTB (7-amino-4-chloromethylcoumarin, Invitrogen) or 1.25μM CMFDA (5-chloromethylfluorescein diacetate, Invitrogen) for 20min at 37°C in Hanks buffered salt solution (HBSS). CD4+ T cells were stained with either 1.25μM CMFDA or 1.25μM CMTPX (Invitrogen) for 20min at 37°C in HBSS. For Ca2+ flux assessment cells were co-stained with 1.25μM CMTPX and 2.5μM Fluor-4 (Invitrogen) for 20min at 37°C in HBSS. Following staining with CMTPX cells were further incubated in cRPMI for 30min at 37°C. All dyes were supplied by Molecular Probes. Calcium flux analysis was conducted in a relative fashion by determining the basal ratio of Fluor-4: CMTPX intensity for individual cells following tracking with Imaris Imageworks. At subsequent time points the ratio of Fluor-4 intensity to CMTPX intensity was determined and related to the basal ratio to determine the relative amount of calcium flux present in specific cells. This method was internally validated in each experiment by analysis of CD4+ T cells not interacting with DC, where the ratio of Fluor-4: CMTPX had a mean of ≈1.0.
Static two-photon imaging was conducted following PFA fixation of whole LN, which were subsequently frozen in OCT compound (Tissue Tek) and serially sectioned into 100μm sections. CD4+ T cell- DC interfaces were calculated following the digital reconstruction of whole LN using Imaris Imageworks. Channel specific 3D objects were generated and a pixel co-localization gating strategy was employed to determine the specific voxel size of cell-to-cell interactions.
Analysis of peptide-loaded, adjuvant treated DC
CD11c+ DC were prepared as above for adoptive cell transfer in the presence of adjuvant with either 0.1μm pPCC or 10.0μm LC-pPCC as indicated. DC were then either analyzed immediately by flow cytometry for in vitro analysis or adoptively transferred into the footpad and recovered at 24hr post-transfer for ex vivo analysis. The DC phenotype was determined following staining with MHCII (M5/114.15.2), CD80 (16-10A1), CD86 (GL-1), ICAM-1 (YN1/1.7.4), and ICOSL (HK5.3). LC-pPCC binding was analyzed following staining with Streptavidin-PE (BD Pharmingen™) or Streptavidin-Qdot 605 (Invitrogen), as per Huang et al (Huang et al., 2013). ELISA was conducted for the detection of IL-12 p70 (Biolegend, ELISA Max Delux) and Cytometric Bead Array (BD, Multiplexed Bead Array) was conducted for the detection of IL-6 and IL-12 production as 4hr or 24hr post stimulation in the presence or absence of pPCC or LPS as per the manufacturers instructions. Flow cytometry was conducted on an LSRII (BD) and analyzed using FlowJo software (Tree Star).
CD4+ T cell activation analysis
WT or IL-4G4/G4IFNg−/− 5CC7 CD4+ T cells were activated by in vitro culture in cRPMI with P13.9 fibroblasts stably expressing MHCII, CD80, and ICAM-1 (Ding et al., 1993) that had previously been treated with 25 μg/ml mitomycin C, in the presence of with 0.005μM – 10.0μM pPCC. Alternately, WT 5CC7 CD4+ T cells were activated in vivo with CD11c+ DC treated with either LPS or papain in the presence of 0.1μM pPCC (as per adoptive cell transfer methodology). Following activation for 0–24hr as indicated, CD4+ T cells were harvested and stained for CD4 (GK1.5), CD45.2 (104), CD69 (H1.2F3), IL-12Rβ2 (114) and IL-4R (mIL4R-M1). Flow cytometry was conducted on an LSRII (BD) and analyzed using FlowJo software (Tree Star).
Statistical Methods
1 way ANOVA with Tukeys post testing were used for the statistical analysis of multiple groups. Student’s t test (two-tailed) were used for the statistical analysis of differences between two groups.
Supplementary Material
Highlights.
Antigen-associated signaling by T cells can be measured using intravital imaging.
Strong TCR signals induce Th1 cell differentiation, weak signals induce Th2 cells in vivo.
Adjuvants influence TCR signaling and polarization via effects on costimulation.
TCR signal duration and intensity regulate expression of cytokine receptors.
Acknowledgments
We thank W.E. Paul, J. Zhu, H. Yamane, M. Gerner, R. Gottschalk, Z. Liu, and W. Kastenmueller for their reading and critical discussions of this manuscript. We also thank D. Jankovic for providing reagents. This work was supported by the Intramural Research Program of the NIAID. NVP was supported by the New Zealand FRST and FK was supported by the Grant from the International Human Frontier Science Program (RGY0077/2011).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY. Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature. 2013;504:451–455. doi: 10.1038/nature12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Egen JG, Koo LY, Laugier JP, Brau F, Glaichenhaus N, Germain RN. Stromal cell networks regulate lymphocyte entry, migration, and territoriality in lymph nodes. Immunity. 2006;25:989–1001. doi: 10.1016/j.immuni.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakta NR, Oh DY, Lewis RS. Calcium oscillations regulate thymocyte motility during positive selection in the three-dimensional thymic environment. Nature immunology. 2005;6:143–151. doi: 10.1038/ni1161. [DOI] [PubMed] [Google Scholar]
- Boyton RJ, Zaccai N, Jones EY, Altmann DM. CD4 T cells selected by antigen under Th2 polarizing conditions favor an elongated TCR alpha chain complementarity-determining region 3. Journal of immunology. 2002;168:1018–1027. doi: 10.4049/jimmunol.168.3.1018. [DOI] [PubMed] [Google Scholar]
- Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky GA, Gardner K, Schwartzberg PL. SAP regulates T(H)2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity. 2004;21:693–706. doi: 10.1016/j.immuni.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Chen L, Fischle W, Verdin E, Greene WC. Duration of nuclear NF-kappaB action regulated by reversible acetylation. Science. 2001;293:1653–1657. doi: 10.1126/science.1062374. [DOI] [PubMed] [Google Scholar]
- Constant S, Pfeiffer C, Woodard A, Pasqualini T, Bottomly K. Extent of T cell receptor ligation can determine the functional differentiation of naive CD4+ T cells. The Journal of experimental medicine. 1995;182:1591–1596. doi: 10.1084/jem.182.5.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corn RA, Hunter C, Liou HC, Siebenlist U, Boothby MR. Opposing roles for RelB and Bcl-3 in regulation of T-box expressed in T cells, GATA-3, and Th effector differentiation. Journal of immunology. 2005;175:2102–2110. doi: 10.4049/jimmunol.175.4.2102. [DOI] [PubMed] [Google Scholar]
- Desai BB, Quinn PM, Wolitzky AG, Mongini PK, Chizzonite R, Gately MK. IL-12 receptor. II. Distribution and regulation of receptor expression. Journal of immunology. 1992;148:3125–3132. [PubMed] [Google Scholar]
- Ding L, Linsley PS, Huang LY, Germain RN, Shevach EM. IL-10 inhibits macrophage costimulatory activity by selectively inhibiting the up-regulation of B7 expression. Journal of immunology. 1993;151:1224–1234. [PubMed] [Google Scholar]
- Fazilleau N, McHeyzer-Williams LJ, Rosen H, McHeyzer-Williams MG. The function of follicular helper T cells is regulated by the strength of T cell antigen receptor binding. Nature immunology. 2009;10:375–384. doi: 10.1038/ni.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelman FD, Shea-Donohue T, Morris SC, Gildea L, Strait R, Madden KB, Schopf L, Urban JF., Jr Interleukin-4- and interleukin-13-mediated host protection against intestinal nematode parasites. Immunological reviews. 2004;201:139–155. doi: 10.1111/j.0105-2896.2004.00192.x. [DOI] [PubMed] [Google Scholar]
- Furusawa Y, Obata Y, Fukuda S, Endo TA, Nakato G, Takahashi D, Nakanishi Y, Uetake C, Kato K, Kato T, et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [DOI] [PubMed] [Google Scholar]
- Gottschalk RA, Corse E, Allison JP. TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo. The Journal of experimental medicine. 2010;207:1701–1711. doi: 10.1084/jem.20091999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, Zheng H, Peixoto A, Flynn MP, Senman B, Junt T, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nature immunology. 2008;9:282–291. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliard BA, Mason N, Xu L, Sun J, Lamhamedi-Cherradi SE, Liou HC, Hunter C, Chen YH. Critical roles of c-Rel in autoimmune inflammation and helper T cell differentiation. J Clin Invest. 2002;110:843–850. doi: 10.1172/JCI15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosken NA, Shibuya K, Heath AW, Murphy KM, O’Garra A. The effect of antigen dose on CD4+ T helper cell phenotype development in a T cell receptor-alpha beta-transgenic model. The Journal of experimental medicine. 1995;182:1579–1584. doi: 10.1084/jem.182.5.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu-Li J, Pannetier C, Guo L, Lohning M, Gu H, Watson C, Assenmacher M, Radbruch A, Paul WE. Regulation of expression of IL-4 alleles: analysis using a chimeric GFP/IL-4 gene. Immunity. 2001;14:1–11. doi: 10.1016/s1074-7613(01)00084-x. [DOI] [PubMed] [Google Scholar]
- Huang J, Brameshuber M, Zeng X, Xie J, Li QJ, Chien YH, Valitutti S, Davis MM. A Single Peptide-Major Histocompatibility Complex Ligand Triggers Digital Cytokine Secretion in CD4(+) T Cells. Immunity. 2013;39:846–857. doi: 10.1016/j.immuni.2013.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauschen F, Ishii M, Qi H, Bajenoff M, Egen JG, Germain RN, Meier-Schellersheim M. Quantifying cellular interaction dynamics in 3D fluorescence microscopy data. Nature protocols. 2009;4:1305–1311. doi: 10.1038/nprot.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. Regulation of T(H)2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nature immunology. 2012;13:681–690. doi: 10.1038/ni.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin D, DiPaolo RJ, Brinster C, Revilleza MJ, Boyd LF, Teyton L, Natarajan K, Mage MG, Shevach EM, Margulies DH. Availability of autoantigenic epitopes controls phenotype, severity, and penetrance in TCR Tg autoimmune gastritis. European journal of immunology. 2008;38:3339–3353. doi: 10.1002/eji.200838584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim TS, Goh JK, Mortellaro A, Lim CT, Hammerling GJ, Ricciardi-Castagnoli P. CD80 and CD86 differentially regulate mechanical interactions of T-cells with antigen-presenting dendritic cells and B-cells. PloS one. 2012;7:e45185. doi: 10.1371/journal.pone.0045185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsland BJ, Soos TJ, Spath G, Littman DR, Kopf M. Protein kinase C theta is critical for the development of in vivo T helper (Th)2 cell but not Th1 cell responses. The Journal of experimental medicine. 2004;200:181–189. doi: 10.1084/jem.20032229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medoff BD, Seed B, Jackobek R, Zora J, Yang Y, Luster AD, Xavier R. CARMA1 is critical for the development of allergic airway inflammation in a murine model of asthma. Journal of immunology. 2006;176:7272–7277. doi: 10.4049/jimmunol.176.12.7272. [DOI] [PubMed] [Google Scholar]
- Medzhitov R, Janeway CA., Jr Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- Mempel TR, Henrickson SE, Von Andrian UH. T-cell priming by dendritic cells in lymph nodes occurs in three distinct phases. Nature. 2004;427:154–159. doi: 10.1038/nature02238. [DOI] [PubMed] [Google Scholar]
- Miller MJ, Hejazi AS, Wei SH, Cahalan MD, Parker I. T cell repertoire scanning is promoted by dynamic dendritic cell behavior and random T cell motility in the lymph node. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:998–1003. doi: 10.1073/pnas.0306407101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner JD, Fazilleau N, McHeyzer-Williams M, Paul W. Cutting edge: lack of high affinity competition for peptide in polyclonal CD4+ responses unmasks IL-4 production. Journal of immunology. 2010;184:6569–6573. doi: 10.4049/jimmunol.1000674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingueneau M, Roncagalli R, Gregoire C, Kissenpfennig A, Miazek A, Archambaud C, Wang Y, Perrin P, Bertosio E, Sansoni A, et al. Loss of the LAT adaptor converts antigen-responsive T cells into pathogenic effectors that function independently of the T cell receptor. Immunity. 2009;31:197–208. doi: 10.1016/j.immuni.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Noble A, Truman JP, Vyas B, Vukmanovic-Stejic M, Hirst WJ, Kemeny DM. The balance of protein kinase C and calcium signaling directs T cell subset development. Journal of immunology. 2000;164:1807–1813. doi: 10.4049/jimmunol.164.4.1807. [DOI] [PubMed] [Google Scholar]
- Nurieva RI, Chuvpilo S, Wieder ED, Elkon KB, Locksley R, Serfling E, Dong C. A costimulation-initiated signaling pathway regulates NFATc1 transcription in T lymphocytes. Journal of immunology. 2007;179:1096–1103. doi: 10.4049/jimmunol.179.2.1096. [DOI] [PubMed] [Google Scholar]
- Oh-Hora M, Yamashita M, Hogan PG, Sharma S, Lamperti E, Chung W, Prakriya M, Feske S, Rao A. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nature immunology. 2008;9:432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozcan E, Notarangelo LD, Geha RS. Primary immune deficiencies with aberrant IgE production. The Journal of allergy and clinical immunology. 2008;122:1054–1062. doi: 10.1016/j.jaci.2008.10.023. quiz 1063-1054. [DOI] [PubMed] [Google Scholar]
- Pulecio J, Petrovic J, Prete F, Chiaruttini G, Lennon-Dumenil AM, Desdouets C, Gasman S, Burrone OR, Benvenuti F. Cdc42-mediated MTOC polarization in dendritic cells controls targeted delivery of cytokines at the immune synapse. The Journal of experimental medicine. 2010;207:2719–2732. doi: 10.1084/jem.20100007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis JE, Lahav G. Encoding and decoding cellular information through signaling dynamics. Cell. 2013;152:945–956. doi: 10.1016/j.cell.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacks D, Noben-Trauth N. The immunology of susceptibility and resistance to Leishmania major in mice. Nature reviews Immunology. 2002;2:845–858. doi: 10.1038/nri933. [DOI] [PubMed] [Google Scholar]
- Siggs OM, Miosge LA, Yates AL, Kucharska EM, Sheahan D, Brdicka T, Weiss A, Liston A, Goodnow CC. Opposing functions of the T cell receptor kinase ZAP-70 in immunity and tolerance differentially titrate in response to nucleotide substitutions. Immunity. 2007;27:912–926. doi: 10.1016/j.immuni.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims TN, Soos TJ, Xenias HS, Dubin-Thaler B, Hofman JM, Waite JC, Cameron TO, Thomas VK, Varma R, Wiggins CH, et al. Opposing effects of PKCtheta and WASp on symmetry breaking and relocation of the immunological synapse. Cell. 2007;129:773–785. doi: 10.1016/j.cell.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Tahvanainen J, Kallonen T, Lahteenmaki H, Heiskanen KM, Westermarck J, Rao KV, Lahesmaa R. PRELI is a mitochondrial regulator of human primary T-helper cell apoptosis, STAT6, and Th2-cell differentiation. Blood. 2009;113:1268–1277. doi: 10.1182/blood-2008-07-166553. [DOI] [PubMed] [Google Scholar]
- Tang H, Cao W, Kasturi SP, Ravindran R, Nakaya HI, Kundu K, Murthy N, Kepler TB, Malissen B, Pulendran B. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nature immunology. 2010;11:608–617. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor MD, Sadhukhan S, Kottangada P, Ramgopal A, Sarkar K, D’Silva S, Selvakumar A, Candotti F, Vyas YM. Nuclear role of WASp in the pathogenesis of dysregulated TH1 immunity in human Wiskott-Aldrich syndrome. Sci Transl Med. 2:37ra44. doi: 10.1126/scitranslmed.3000813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokoyoda K, Zehentmeier S, Hegazy AN, Albrecht I, Grun JR, Lohning M, Radbruch A. Professional memory CD4+ T lymphocytes preferentially reside and rest in the bone marrow. Immunity. 2009;30:721–730. doi: 10.1016/j.immuni.2009.03.015. [DOI] [PubMed] [Google Scholar]
- Trifari S, Sitia G, Aiuti A, Scaramuzza S, Marangoni F, Guidotti LG, Martino S, Saracco P, Notarangelo LD, Roncarolo MG, Dupre L. Defective Th1 cytokine gene transcription in CD4+ and CD8+ T cells from Wiskott-Aldrich syndrome patients. Journal of immunology. 2006;177:7451–7461. doi: 10.4049/jimmunol.177.10.7451. [DOI] [PubMed] [Google Scholar]
- Tubo NJ, Pagan AJ, Taylor JJ, Nelson RW, Linehan JL, Ertelt JM, Huseby ES, Way SS, Jenkins MK. Single naive CD4+ T cells from a diverse repertoire produce different effector cell types during infection. Cell. 2013;153:785–796. doi: 10.1016/j.cell.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Panhuys N, Tang SC, Prout M, Camberis M, Scarlett D, Roberts J, Hu-Li J, Paul WE, Le Gros G. In vivo studies fail to reveal a role for IL-4 or STAT6 signaling in Th2 lymphocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12423–12428. doi: 10.1073/pnas.0806372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga G, Nippe N, Balkow S, Peters T, Wild MK, Seeliger S, Beissert S, Krummen M, Roth J, Sunderkotter C, Grabbe S. LFA-1 contributes to signal I of T-cell activation and to the production of T(h)1 cytokines. The Journal of investigative dermatology. 2010;130:1005–1012. doi: 10.1038/jid.2009.398. [DOI] [PubMed] [Google Scholar]
- Yamane H, Paul WE. Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets. Immunological reviews. 2013;252:12–23. doi: 10.1111/imr.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane H, Zhu J, Paul WE. Independent roles for IL-2 and GATA-3 in stimulating naive CD4+ T cells to generate a Th2-inducing cytokine environment. The Journal of experimental medicine. 2005;202:793–804. doi: 10.1084/jem.20051304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G, et al. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity. 2012;37:660–673. doi: 10.1016/j.immuni.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Paul WE. Peripheral CD4+ T-cell differentiation regulated by networks of cytokines and transcription factors. Immunological reviews. 2010;238:247–262. doi: 10.1111/j.1600-065X.2010.00951.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*) Annual review of immunology. 2010;28:445–489. doi: 10.1146/annurev-immunol-030409-101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.