Abstract
Chemokines, a large family of small chemoattractive cytokines, and their receptors play an integral role in the regulation of the immune response and homeostasis. The ability of chemokines to attract specific populations of immune cells sets them apart from other chemoattractants. Chemokines produced within the gastrointestinal mucosa, are critical players in directing the balance between physiological and pathophysiological inflammation in health, inflammatory bowel disease and the progression to colon cancer. In addition to the well-characterized role of chemokines in directed trafficking of immune cells to the gut mucosa, the expression of chemokine receptors on the cells of the epithelium makes them active participants in the chemokine signaling network. Recent findings demonstrate an important role for chemokines and chemokine receptors in epithelial barrier repair and maintenance as well as an intricate involvement in limiting metastasis of colonic carcinoma. Increased recognition of the association between barrier defects and inflammation and the subsequent progression to cancer in inflammatory bowel disease thus implicates chemokines as key regulators of mucosal homeostasis and disease pathogenesis.
Mucosal immunity
The healthy gastrointestinal mucosa is the largest repository of immune cells within the human body 1. The constitutive presence and trafficking of immunocytes into the mucosal compartment has been termed physiologic inflammation and reflects production of chemokines by cells within the gastrointestinal mucosa 1-6. The inflammatory bowel diseases (IBD), including ulcerative colitis (UC) and Crohn's disease (CD), are chronic inflammatory diseases of the gastrointestinal tract that in genetically susceptible individuals are believed to arise out of fundamental dysregulation of the immune system in response to environmental triggers 7-9. A growing body of work suggests that the chronic inflammation seen during IBD results from defects in the ability to properly regulate the immune system in response to the enteric microbiota 7;10-12. These defects may include alterations in pattern recognition receptors expressed by epithelial cells lining the gastrointestinal mucosal surface critical for identifying microorganisms 8;13-17. In addition to recognition of the gut microbiota, disease pathogenesis may also reflect defects in immune regulation, increased influx of inflammatory cells, or diminished barrier integrity 18-23. These factors are likely inter-related in that the additional influx of immune cells may elicit damage, resulting in epithelial and mucosal lesions, through increased production of an array of bioactive molecules. Alternatively, increased immunocyte trafficking may reflect a primary defect in barrier integrity, exacerbating pathogenesis of IBD by facilitating the entry of noxious luminal stimuli into the sub-epithelial mucosal and submucosal layers and thus leading to increased trafficking of inflammatory cells.
Further compounding IBD disease pathogenesis, patients with UC and CD are increasingly at risk for developing cancers of the colon and rectum 24-27. Consistent with the model proposed by Itzkowitz and Yio 28 mucosal inflammation promotes the development of colon carcinoma through combinations of genetic and epigenetic mutations in an array of regulatory epithelial genes, further altering or exacerbating mucosal inflammation or mucosal wound healing responses 29;30. The current model states that innate immune components, especially signaling through the pro-inflammatory NF-κB transcription factor, plays critical roles in connecting IBD to carcinoma development 31-33. While these data are compelling, the causative cancer promoting genes dysregulated by these pathways in metaplastic epithelia remain to be fully established. Thus, regulation of host defense genes within the cells of the intestinal epithelial lining appears to play a key role in physiologic and pathophysiologic inflammation and may foster the progression to colon cancer in patients with unchecked mucosal immune responses.
The intestinal epithelium
Epithelial cells lining the mucosal surface of the gastrointestinal tract function in digestion and absorption of essential nutrients as well as the regulated secretion of electrolytes and macromolecules 34;35. These cells also comprise a dynamic physical interface between the external luminal environment and the body's interior and thus represent a central mechanism of the innate immune system preventing or limiting the entry of food-and water-borne antigens and microbes 4. The cells that comprise the mucosal barrier are a self-renewing system undergoing continuous replacement from pluripotent stem cells located near the base of the crypts of Lieberkühn. Daughters of these stem cells undergo terminal differentiation into absorptive enterocytes, Goblet cells or enteroendocrine cells as they migrate toward the crypt surface, or differentiate to Paneth cells as they migrate to the crypt base in the small intestine 36-41. Intestinal epithelial cells migrate with increasing velocity along the basement membrane toward the small intestinal villus tip or colonic surface, whereupon those cells lose the ability to adhere to the basement membrane and undergo programmed cell death as they are subsequently shed into the intestinal lumen 37;42-44.
As highlighted in studies of IBD pathogenesis and colitis-associated cancer, epithelial cells actively participate as a dynamic front-line defense response to external stimuli, playing an integral role in innate and adaptive mucosal immunity 45-48. Intestinal epithelial cells thus, participate in several distinct host defense mechanisms limiting pathogen or antigen entry and the progression to colon cancer. Epithelial host defense functions include regulated secretion of electrolytes that are key to flushing noxious stimuli from the bowel lumen 35. Alternatively, modulation of epithelial growth, apoptosis and differentiation function as repair mechanisms to maintain barrier integrity and limit entry of environmental luminal stimuli 6. Further, the epithelium is a dynamic partner in mucosal immune responses through the regulated production of chemokines, cytokines, growth factors and antimicrobial molecules essential to mucosal inflammation and host defense 1;49-52.
Chemokines
Chemokines are a large family of small, 8-14kDa, secreted chemotactic cytokines with well-recognized roles in adhesion and directional homing of immune and inflammatory cells 53-55. These molecules have been divided into four subfamilies based on the arrangement of highly conserved cysteine residues in the amino-terminus of the protein (Table 1). The largest chemokine subfamilies are the CXC chemokines in which the amino-terminal cysteines are separated by an intervening amino acid and the CC subfamily where the amino-terminal two cysteines are adjacent. Chemokines of the CXC subfamily can further be subdivided based on the amino-terminal presence or absence of a glutamate-leucine-arginine (ELR) amino acid motif which are potently chemoattractive for neutrophils and possess angiogenic properties 56. Two additional subfamilies include XCL1/lymphotactin, which possess a single amino-terminal cysteine residue 57, and CX3CL1/fractalkine in which three intervening amino acids separate the cysteines 58.
Table 1.
Nomenclature and characteristics of chemokines and chemokine receptors.1
| Systematic Name | Common synonym 2 | Class/Expression | Receptor | Cells Attracted |
|---|---|---|---|---|
| CXCL1 | GROα | ELR+, Inducible | CXCR2, CXCR1 | neutrophils |
| CXCL2 | GROβ | ELR+, Inducible | CXCR2 | neutrophils |
| CXCL3 | GROγ | ELR+, Inducible | CXCR2 | neutrophils |
| CXCL4 | PF4 | ELR-, Inducible | ?3 | fibroblasts, endothelial cells |
| CXCL5 | ENA-78 | ELR+, Inducible | CXCR2 | neutrophils |
| CXCL6 | GCP-2 | ELR+, Inducible | CXCR2 | neutrophils, macrophages |
| CXCL7 | NAP-2 | ELR+, Inducible | CXCR2 | neutrophils |
| CXCL8 | IL-8 | ELR+, Inducible | CXCR1, CXCR2 | neutrophils, macrophages |
| CXCL9 | Mig | ELR-, Inducible | CXCR3 | Th1 T cells, NK cells |
| CXCL10 | IP-10 | ELR-, Inducible | CXCR3 | Th1 T cells, NK cells |
| CXCL11 | I-TAC | ELR-, Inducible | CXCR3 | Th1 T cells, NK cells |
| CXCL12 | SDF-1 | ELR-, Constitutive | CXCR4, CXCR7 | CD34+ progenitor cells and most hematopoietic cell types |
| CXCL13 | BCA-1 | ELR-, Constitutive | CXCR5 | naive B cells, CD4+ T cells |
| CXCL14 | BRAK | ELR-, Constitutive | ? | monocytes, immature dendritic cells, NK cells |
| CXCL16 | SR-PSOX | ELR-, Constitutive | CXCR6 | naïve CD8+ T cells, subsets of activated memory CD4+ T cells, NK-T cells |
|
| ||||
| CCL1 | I-309 | Inducible | CCR8 | monocytes, T cells, B cells |
| CCL2 | MCP-1 | Inducible | CCR2 | monocytes, T cells, NK cells, immature dendritic cells |
| CCL3 | MIP-1α | Inducible | CCR1, CCR5 | monocytes, macrophages, NK cells, T cells, Th1 T cells, Th2 T cells, immature dendritic cells |
| CCL4 | MIP-1β | Inducible | CCR5, CCR8 | monocytes, macrophages, T cells, Th1 T cells, immature dendritic cells |
| CCL5 | RANTES | Inducible | CCR1, CCR3, CCR5 | monocytes, macrophages, eosinophils, NK cells, T cells, Th1 T cells, immature dendritic cells |
| CCL7 | MCP-3 | Inducible | CCR1, CCR2, CCR3 | monocytes, macrophages, NK cells, T cells, immature dendritic cells |
| CCL8 | MCP-2 | Inducible | CCR3 | eosinophils, basophils, mast cells, Th2 T cells |
| CCL11 | Eotaxin | Inducible | CCR3 | eosinophils, basophils, mast cells, Th2 T cells |
| CCL13 | MCP-4 | Inducible | CCR2, CCR3 | monocytes, T cells, NK cells, immature dendritic cells, eosinophils, basophils, mast cells, Th2 T cells |
| CCL14 | HCC-1 | ? | CCR1 | monocytes, macrophages, NK cells, T cells |
| CCL15 | HCC-2 | ? | CCR1, CCR3 | monocytes, macrophages, NK cells, T cells, eosinophils, basophils, mast cells, Th2 T cells |
| CCL16 | HCC-4 | ? | CCR1 | monocytes, macrophages NK cells, T cells |
| CCL17 | TARC | Inducible/constitutive | CCR4 | Th2 T cells, macrophages |
| CCL18 | PARC | Constitutive | ? | T cells |
| CCL19 | MIP-3β/ELC | Constitutive | CCR7 | T cells, B cells, mature dendritic cells |
| CCL20 | MIP-3α/LARC | Inducible/Constitutive | CCR6 | T cells, B cells, immature dendritic cells |
| CCL21 | SLC | Constitutive | CCR7 | T cells, B cells, mature dendritic cells |
| CCL22 | MDC | Inducible/Constitutive | CCR4 | Th2 T cells, macrophages |
| CCL23 | MPIF1 | CCR1 | ||
| CCL24 | Eotaxin-2 | Inducible | CCR3 | eosinophils, basophils, mast cells, Th2 T cells |
| CCL25 | TECK | Constitutive | CCR9 | thymocytes, T cells |
| CCL26 | Eotaxin-3 | Inducible | CCR3 | eosinophils, basophils, mast cells, Th2 T cells |
| CCL27 | CTACK | Constitutive | CCR10 | T cells, monocytes, B cells, immature dendritic cells |
| CCL28 | MEC | Constitutive | CCR10, CCR3 | T cells |
|
| ||||
| XCL1 | lymphotactin | ? | XCR1 | T cells, natural killer cells |
|
| ||||
| CX3CL1 | fractalkine | Inducible/Constitutive | CX3CR1 | T cells, monocytes, neutrophils |
CXCL15, CCL6, CCL9/CCL10, and CCL12 represent the mouse chemokines lungkine, C10, MRP-2, and MCP-5 for which the human orthologs have yet to be identified.
abbreviations used: BCA, B cell-activating chemokine; BRAK, breast and kidney chemokine; CTACK, cutaneous T cell-attracting chemokine; ENA, epithelial neutrophil attractant; ELC, Epstein-Barr virus-induced receptor ligand chemokine; GCP, granulocyte chemoattractant protein; GRO, growth-related oncogene; HCC, hemofiltrate CC-chemokine; IP, γ-interferon-induced protein; I-TAC, interferon-inducible T cell α chemoattractant; LARC, liver and activation-related chemokine; MCP, monocyte chemoattractant protein; MDC, macrophage-derived chemokine; MEC, mucosa-associated epithelial chemokine; MIG, monokine induced by γ-interferon; MIP, macrophage inflammatory protein; NAP, neutrophil activating protein; PARC, pulmonary and activation-regulated chemokine; PF, platelet-factor; RANTES, regulated on activation normal T cell expressed and secreted; SDF, stromal cell-derived factor; SLC, secondary lymphoid tissue chemokine; SR-PSOX, scavenger receptor for phosphatidylserine and oxidized lipoprotein; TARC, thymus and activation related chemokine; TECK, thymus expressed chemokine.
Question mark indicates unknown receptor or regulation pattern.
Chemokines have also been classified based on function and expression pattern into an inflammatory, or inducible, subfamily regulated by proinflammatory stimuli important in innate and adaptive immune responses. A homeostatic, or constitutive, chemokine subfamily plays a correspondingly key immune surveillance role in lymphocyte and dendritic cell trafficking between primary and secondary lymphatic tissues 59. These molecules are highly basic proteins capable of adhering to glycosaminoglycans on cell surfaces further establishing localized foci of elevated chemoattractant concentrations 60. In the vascular lumen, under elevated sheer stress from blood flow, endothelial-produced chemokines signal circulating leukocytes upon binding to the cognate chemokine receptor expressed by the target cells. Functionally, activation of the chemokine receptor results in increased affinity of leukocyte integrins for endothelial adhesion molecules as a first step in leukocyte diapedesis into the tissue space 61;62. In addition, subsets of chemokines such as CX3CL1 and CXCL16 possess transmembrane-domains that tether the chemokine ligand to the cell membrane thus further aiding the ability of those chemokines to establish focal regions of high ligand concentration and foster intimate contact with receptor expressing target cells 58;63.
Chemokine Receptors
Chemokines exert their actions through the binding and activation of specific 7 transmembrane G-protein coupled receptors located within the cell membrane of target cells 54. Much like their ligands, chemokine receptors possess a conserved structure and possess 20-80% amino acid identity 64. These receptors are largely linked to Gαi heterotrimeric G proteins and regulate cell migration 54. Binding of the chemokine ligand to its cognate receptor activates the heterotrimeric G protein complex, resulting in the dissociation of the Gα subunit from the Gβγ subunits and establishing two distinct signaling modules capable of activating intracellular second messenger proteins. Increased flux in intracellular calcium and in turn chemotaxis of those cells is prominent amongst those signaling pathways activated by ligand engagement to chemokine receptors 53.
This shared biologic feature of chemokine signaling has classically been characterized by directed leukocyte and lymphocyte migration, and hematopoietic progenitor cell trafficking 53;54. Within this generalized function, specificity is dictated by expression patterns of chemokine receptors on defined subsets of target cells and through temporal and spatial regulation of ligand expression. The prototypic role for chemokines in leukocyte activation and trafficking was expanded following the determination that subsets of chemokine receptors function as co-receptors for human immunodeficiency virus (HIV) infection 65;66.
Subsequent analyses of the cell types expressing chemokine receptors lead to the description of even further functions, ascribing roles for specific subsets of chemokines in angiogenesis or angiostasis 67-69, immune and non-immune tissue development 70-75, recruitment of endothelial progenitor cells 76, and epithelial wound healing 77-81. Redundancy of ligand for chemokine receptors is a key characteristic feature of this family of immune mediators. Thus, for several different chemokines, distinct ligands may bind to a single specific receptor, or alternatively, a single ligand may bind to separate chemokine receptors, leading to a high level of redundancy in chemokine receptor function, particularly in inflammatory responses 53;54. Ligand-receptor selectivity may also determine the level of chemokine receptor desensitization by distinct chemokine ligand subsets, suggesting a hierarchical relationship in receptor signaling. These interactions may be of importance in elucidating the intricate multifactorial relationships that exist during immune and inflammatory responses as well as lymphoid organ development.
Chemokines in mucosal inflammation
Chemokines are ubiquitous mediators of inflammation and host defense with a central role in lymphocyte recirculation and immune surveillance as well as leukocyte trafficking. In addition, chemokines play a role in several diseases, from viral diseases, to cancer metastasis, to inflammation and autoimmune diseases 65;66;82-85. Within the gastrointestinal mucosa, physiologic and pathophysiologic inflammation in the intestine reflects a network of inter-dependent relationships that is susceptible to inappropriate activation, despite multiple checks and balances and chemokine ligand-receptor redundancy. The diseases that comprise IBD are not simple inflammatory diseases. Instead, disease pathogenesis reflects an integrated activation of signaling events within and between components of the intestine including the epithelial cells, nervous system, immune cells and extracellular matrix. The relationship between intestinal epithelial cells and immune cells is an important factor in the intestinal immune response 6. An array of cytokines and chemokines are upregulated during and likely playing key participatory roles in immunocyte infiltration into the pathologically inflamed gut mucosa 48;61.
As previously reviewed, several reports have established the current paradigm, schematically summarized in Figure 1A, for chemokines, especially those of the inflammatory/inducible group, in mucosal inflammation and their regulation in IBD 46;48;61;86. Thus, an array of inflammatory chemokines including CXCL8, CCL2, CCL20, CCL5 amongst others, are elevated in human specimens from CD and UC patients and likely reflect mechanisms that result in increased trafficking and localization of monocytes, dendritic cells, natural killer cells, and T lymphocytes to the gut mucosa 48;61;87-94. As a notable example, several reports link expression of the CCR6 ligand CCL20 to the cells of the intestinal epithelium in vitro and in vivo 94-96 with directing the trafficking of dendritic cells to the subjacent lamina propria and to the subepithelial dome of Peyer's patches 97-100.
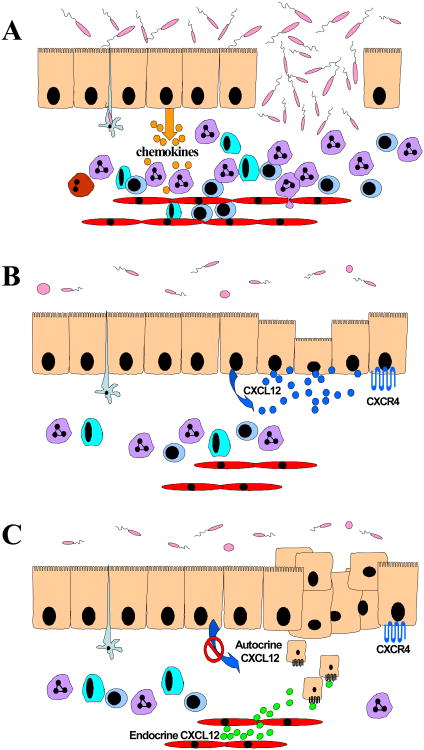
Figure 1. Expanded role for epithelial-derived chemokines in mucosal inflammation, wound healing, and carcinogenesis.
A. Chemokines likely regulate immune cell trafficking in the inflammatory bowel diseases. Increased expression and production of chemokines by epithelial cells in the damaged or intact mucosal barrier direct the elevated trafficking of leukocytes and lymphocytes of the innate and adaptive immune response to the gastrointestinal mucosa. B. Potential roles for chemokines in repair of the epithelial barrier. The homeostatic chemokine CXCL12 is constitutively expressed in the cells of the human intestinal epithelium. In culture model systems CXCL12 activates the canonical restitutive epithelial migration signaling pathway and aids in increased closure of wounded epithelial monolayers. C. Chemokines differentially participate in the progression from dysplastic epithelium to frank tumor and metastasis. Epigenetic silencing of CXCL12 in the colonic carcinoma cells confers metastasis-proficient phenotype to those cells, allowing them to respond to endocrine chemokine gradients (green circles).
Chemokines largely of the constitutive or homeostatic group, play key roles in lymphocyte and leukocyte recirculation and, more broadly have been shown to participate in development and organization of mesenteric lymph nodes, Peyer's patches, cryptopatches and establishment of the intraepithelial lymphoid compartment 99;101-104. Following their development in primary lymph tissues, naïve antigen-inexperienced lymphocytes as well as leukocytes circulate through secondary lymphatic tissues sampling antigens and limiting entry of microbial pathogens 62. The role for homeostatic chemokines and their receptors in mucosal lymphoid development and trafficking of immunocytes to the lamina propria in the gut has been extensively reviewed elsewhere 48;105. Notably, expression of the chemokine receptor CCR7 plays a critical role in the organization of secondary lymphoid tissue, including Peyer's patches in the gastrointestinal tract 70;106. Similarly, genetic deficiency of CXCL13 or its receptor CXCR5 results in impaired organization of intestinal Peyer's patches 75;107. Those data are consistent with a role for this homeostatic chemokine in lymphoid organogenesis and organization within the gastrointestinal mucosa. Additionally, intestinal epithelial cells of the small intestine produce the chemokine CCL25, while those of the large intestine selectively secrete CCL28, with each regulating the trafficking of specific T cell subsets to the mucosa of those organs respectively 102;108;109. Alterations in the receptors for those ligands, CCR9 and CCR10, are similarly associated with marked changes in lymphocyte recirculation into the gastrointestinal mucosa, impaired organization of Peyer's patches, and other mucosal-associated lymphoid tissue 103;110. Development and organization of gastrointestinal mucosal lymphatic tissue is not solely regulated through signaling of members of the constitutive group of chemokines as genetic ablation of CCR6 in knockout mice similarly leads to profound alterations in dendritic cell trafficking to the epithelial barrier as well as organization of isolated lymphoid follicles 98;100;111;112.
Although those data suggest a role for homeostatic/constitutive chemokines and their receptors in development of secondary lymphatic tissues within the gastrointestinal mucosa, their role in the pathogenesis of IBD is less clearly understood. Thus, changes in expression levels of homeostatic chemokines, including CCL19, CCL21, CCL25, CCL28 as well as CXCL12 and CXCL13 have been shown to be increased in pathologically-inflamed human small intestine or colon 113-117. Given the role for these molecules in neo-lymphorganogenesis and lymphocyte recirculation expression of constitutive/homeostatic chemokines may therefore participate in re-organization of peripheral lymphoid structures observed in the chronically inflamed mucosa of IBD patients 114;118.
CXCL12 and CXCR4
Due to its high degree of amino acid conservation and broad eukaryotic expression, CXCL12 has been termed a primordial homeostatic/constitutive chemokine 119. Within the gastrointestinal tract, expression of CXCL12 has been noted by the cells of the human colonic and small intestinal epithelium 120. These data, together with previous reports detailing the expression of its cognate receptor CXCR4 by those cells 92;121, suggests an autocrine/paracrine signaling arc between the cells of the mucosal epithelial barrier. In agreement with their concomitant expression in endothelial and epithelial tissues of the small and large intestine both CXCR4 and CXCL12 are similarly expressed in other mucosal epithelia including the liver and mammary gland 69;122;123. In mice, CXCL12, initially termed stromal cell-derived factor-1, and CXCR4, initially termed fusin, appear to be the only chemokine or chemokine receptor critical for life, as genetic deficiency in either of those genes is embryonic lethal 71;72;124. Phenotypic changes in CXCR4 and CXCL12 knockout embryos include marked defects in cardiac and gut vascular development and hematopoiesis 71;72. The comparable phenotypic defects observed in those animals suggested this receptor and ligand comprised a monogamous signaling unit. Recent work, however, suggests CXCL12 is also capable of binding to the newly characterized chemokine receptor CXCR7 which is similarly necessary for life 125;126.
In addition, CXCL12 was among the first chemokines shown to inhibit HIV-1 entry through occupancy of the CXCR4 viral co-receptor expressed on T cells, and perhaps by cells of the intestinal epithelium 120;127. Consistent with other members of the homeostatic/constitutive chemokine group, CXCL12 activation of CXCR4 regulates trafficking of hematopoietic stem cells, naïve lymphocytes and leukocytes 128;129 and has roles in cell-type specific mitogenesis 130-132, carcinoma metastasis 82;85;122, and rheumatoid arthritis 133. Together, these findings implicate CXCL12 and CXCR4 in the broad regulation not only of lymphocyte recirculation, but also tissue morphogenesis, tumor metastasis and inflammatory disease.
Chemokine receptors at the epithelial barrier
Studies in chemokine receptor knockout mice assessed the roles for those receptors in modulating mucosal inflammation. Mice lacking the CCR5 chemokine receptor appeared refractory to induction of colitis associated with addition of dextran sodium sulfate (DSS) to the drinking water 134. Alternatively, blockade of CCR2, or a combinatorial approach blocking both CCR5 and CXCR3 using neutralizing antibodies similarly lead to decreased colitis in the DSS model 134. However, mucosal protection in the DSS model of colitis reflected abrogation of leukocyte trafficking to the gut mucosa following the concomitant pharmacological blockade of the receptors CCR2, CCR5 and CXCR3 135. Broad expression of those receptors by monocytes and T lymphocytes, cells with a well known role in the pathogenesis of human IBD, were protective through modulation of immunocyte trafficking into the mucosa 135. The conclusion that decreased trafficking of those cells is beneficial to limiting disease pathogenesis was further strengthened in recent reports in which gut T lymphocytes were shown to exacerbate epithelial barrier defects, in turn worsening gut inflammation in a murine model of colitis 136;137.
While studies implicate regulated epithelial cell expression of chemokines with increased trafficking of immunocytes to the gastrointestinal mucosa in IBD and other inflammatory disorders, expression and functional analysis of chemokine receptors by those cells is more limited. Expression of chemokine receptors by intestinal epithelial cells was noted in conjunction with the prominence of chemokine ligand production by those cells 92;121. Much like the analysis of the varying chemokine ligands, initial reports ascribed a limited functional role for chemokine receptors to the modulation of the intestinal epithelial inflammatory response through regulation of additional chemokines or expression of cellular adhesion molecules important in leukocyte transepithelial migration 92;121.
Thus, prior reports largely ascribe a limited role for chemokines in the directed infiltration of damage-provoking or, alternatively damage-exacerbating, immune cells into the gut mucosa in IBD. However, recent evidence from our laboratory, and others, endorse expanding that model and assign a role for chemokine receptor signaling in the maintenance of the epithelial barrier by stimulating the migratory repair process, termed restitution, of wounded epithelium 79;81;138. Specifically, in addition to CXCL8, studies in cell culture systems, as modeled in Figure 1B, demonstrate that CXCL12 binding to CXCR4 regulates epithelial cell restitution, which is critical for repair of the barrier subsequent to inflammatory injury 79;81.
Epithelial wound healing
Limitations in epithelial barrier integrity have long been associated with IBD. Consistent with the morphoregulation hypothesis of Edelman 139 epithelial wound repair mechanisms parallel those important in barrier morphogenesis and require the spatial and temporal integration and coordination of epithelial migration, proliferation and maturation 140-144. Mucosal architecture and integrity rely upon regulated epithelial migration out of the crypt toward the lumen surface 37. The process of epithelial migration is chemotactic locomotion and does not reflect ‘pressure’ generated from continually dividing stem cells in the crypt 139;145;146. It has been shown that deletion of extracellular mediators profoundly alters mucosal structure and epithelial maturation in transgenic mice, leading to inflammation and adenoma formation 40;147-151. Restitution, defined as the rapid migration of epithelial cells over the site of injury independent of proliferation, is a key step in mucosal repair 140;142;144;152. Epithelial cells surrounding the injury subsequently initiate proliferation and differentiation gene programs to complete the repair and repolarize into a mature enterocyte. Epithelial restitution and repair processes are of vital importance to homeostatic turnover characteristic of the healthy mucosa and is an essential function within normal epithelial migration 153.
Within the gastrointestinal tract several growth factors, cytokines, hormones, neuropeptides, and polyamines 154;141;143;146;149;155;156 as well as luminal peptides and probiotics have been shown to participate in epithelial restitution in vitro and in vivo 142;157;158. The inflammatory chemokine CXCL8 was shown to stimulate epithelial carcinoma cell migration 138;159 and the cytokine TGFβ1 has been shown to participate both in restitution and also constitutive barrier formation 149. TGFβ1 and its receptor, TGFβRII, share many features in common with the homeostatic chemokine CXCL12 and its cognate receptor CXCR4. Namely, both ligand and receptor are widely expressed by a multiplicity of cellular targets in vivo and both of these receptor-ligand pairs have been shown to mediate cell type specific signaling of differentiation, migration, extracellular matrix formation, and immune responsiveness 65;72;160.
Restitution is dependent largely upon Rho-mediated modifications to the actin cytoskeleton 147;161-166. Deficiencies in specific F-actin regulatory signaling pathways leads to aberrant epithelial migration and differentiation in vitro and in vivo 41;161;163;167-171. In contrast to TGFβ1 directed epithelial migration, the chemokine receptor CXCR4 is coupled to heterotrimeric G-proteins and, in specific target cell subsets, directly activates the monomeric RhoA GTPase to initiate actin polymerization, and in turn leukocyte migration 82;129.
The signaling pathways regulated by chemokine receptors upon ligand engagement parallel, in part, those intracellular effectors of the epithelial restitution pathway. Thus, in vitro assays of restitution using wounded monolayers of intestinal epithelial cells indicate epithelial sheet migration across the denuded barrier is dependent upon activation of the Rho GTPase and in turn formation of F-actin filaments at the leading edge 152;161;164. The importance of these signaling pathways in healing of micro-ulcers in vivo has recently been shown in mouse models of restitution 172 and support not only the relevancy of data from the cell culture systems but also the key role for the canonical Rho-ROCK/MLCK-actin signaling module in epithelial restitution and barrier integrity. As the role for chemokines as cardinal mediators of directed cell movement in epithelial restitution had not been established, our laboratory has begun investigating the function of chemokine receptors expressed at the mucosal epithelial cell surface. Studies using cell culture systems indicate that, much like TGFβ1, engagement of CXCL12 to CXCR4 elicits a rapid increase in epithelial restitution via activation of the RhoGTPase and the downstream polymerization of F-actin in accordance with the known epithelial migration paradigm 79;81. Moreover, we noted that this homeostatic chemokine not only was capable of directing sheet migration across wounded epithelial monolayers but was a potent chemotactic signal for single cell suspensions of human intestinal epithelial cells. In support of those data, a report examining the functions of CCR6 indicate that ligand stimulation modulates p130Cas of the focal adhesion complex, suggesting chemokines may have broader impact on epithelial cell adhesion and migration 173. Together, these data parallel the function of chemokines in leukocytes and suggested that CXCL12 and CXCR4 might play a role in movement of metastatic carcinoma cells out of the gastrointestinal mucosa.
Tumor metastasis
Among the most serious complications of IBD is the increased risk of colon cancer and its associated malignancy. Given their well defined roles in leukocyte trafficking, it was intuitive that chemokine receptor expression on carcinoma cells could aid in their metastasis to sites of constitutive as well acute inflammatory sites of high chemokine ligand production. Indeed, in accordance with our studies 79 there is increasing evidence implicating several chemokine signaling networks in directed metastasis of colorectal carcinoma. Specifically, CXCR3 and CCR7 expression on the surface of colorectal carcinoma cells appears to aid in the specific homing of those cells to regional lymph nodes while CXCR4 and CCR6 seem to be more specific to liver metastasis where expression of the specific CXCL12 and CCL20 ligands, respectively, are readily abundant 174;175. Thus, differential chemokine receptor expression by metaplastic carcinoma cells may play key roles in the sequential steps of primary tumor growth, invasion, vascular entry, cell homing and exit at ectopic tissues 85. Consistent with that notion, we have shown that expression of an array of chemokine receptors of the inflammatory group is extensive and variable amongst a battery of colonic carcinoma cell lines 92.
Cancer is a hyperproliferative disorder that involves morphological cellular transformation, dysregulation of apoptosis, uncontrolled cellular proliferation, invasion, angiogenesis, and metastasis 176. Clinical and epidemiologic studies have suggested a strong association between chronic infection, inflammation, and cancer 32;33;177;178. Several chemokines are known to be prominently regulated by inflammatory mediators such as the transcription factor NF-κB, and have dysregulated expression patterns in IBD as well colorectal carcinoma. A large majority of research has focused on the role of chemokines in the pathologic recruitment of immune and vascular cells into chronically inflamed mucosa and tumors. Given the identification of chemokine receptor expression on normal intestinal epithelium as well as in colorectal carcinoma, these cells are not only producers of chemokine ligands, but are also targets of chemokine signaling. For example, CXCL8, a chemokine ligand known to upregulated in colitis and carcinoma, signaling through CXCR1 has been linked to the epithelial-mesenchymal transition in colonic carcinoma 179.
In addition to chemokines of the inflammatory group, the homeostatic primordial signaling axis CXCR4-CXCL12 has been shown to be the major chemokine network pathologically hijacked by metastasizing carcinoma cells 82;122;123;180-183. These data are strengthened by studies showing increased CXCR4 expression in colorectal carcinomas correlated with increased metastasis and decreased clinical prognosis 184;185. Similarly, CXCL12 stimulation of mammary cancer cells via CXCR4 and CXCR7, the newly identified receptor for CXCL12 and CXCL11, has been shown to promote tumor cell proliferation and survival 186;187. However, recent work from our laboratory indicates CXCL12 is epigenetically silenced in colorectal cancer and that constitutive over-expression of CXCL12 in carcinoma cells increased caspase activity and decreased tumor growth and metastasis 188. Similar findings were obtained in mammary carcinoma, implying that pathological silencing of CXCL12 is not confined to colorectal cancer 123. Aberrant loss of homeostatic chemokine expression may thus facilitate disease progression and worsening disease prognosis by abolishing the autocrine/paracrine signaling arc of the healthy gastrointestinal epithelium (Figure 1C). Moreover, pathologic silencing of CXCL12 has been detected in ulcerative colitis samples (unpublished observations) suggesting that that small subset of cells may be at a selective advantage for metastasis in colitis-related carcinoma even though an overall modulation of CXCL12 has not been noted in UC 189. Further studies are needed to elucidate the full importance of non-chemotactic roles for chemokines in IBD and colitis-associated carcinoma, especially how paracrine and autocrine chemokine signaling networks are modulated in the epithelial compartment.
Future Directions
Inflammatory bowel disease, radiation injury, colorectal cancer as well as infectious enterocolitis and therapeutic drugs have long been associated with defects in epithelial integrity. Therefore, factors that contribute to wound healing are of clinical importance as possible therapeutic modality to restore barrier homeostasis. While additional work is needed, especially in in vivo models of colitis, the information from cell culture systems implicate chemokines as equally-potent in stimulating restitutive migration as the prototypical trefoil factors and growth factors. Chronic dysregulation of chemokines and/or chemokine receptors appears to aid in the latter steps of disease progression including de novo establishment of mucosa-associated lymphoid tissue, as well as carcinoma cell metastasis. Studying the genetic, epigenetic and immunological mechanisms modulating expression of chemokines and chemokine receptors will shed light on the critical roles for those molecules in the progression from physiologic to pathophysiologic inflammation and neoplasia in the human colon.
Acknowledgments
The authors thank Luke Drury, Soonyean Hwang, Amanda Cooper, Dr. Priscilla Johanesen, and Dr. David Binion, Director of the IBD Center, Medical College of Wisconsin, for ongoing discussions, collaborations and critical reading of this manuscript. Financial support was provided by the National Institutes of Health (NIDDK 062022) and FIRST Award from the Crohn's and Colitis Foundation of America.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kagnoff MF. Immunology of the Digestive Tract. In: Johnson LR, editor. Physiology of the Grastrointestinal Tract. New York: Raven Press; 1987. pp. 1699–728. [Google Scholar]
- 2.Moser B, Loetscher P. Lymphocyte traffic control by chemokines. Nat Immunol. 2001;2:123–28. doi: 10.1038/84219. [DOI] [PubMed] [Google Scholar]
- 3.Williams IR. Chemokine receptors and leukocyte trafficking in the mucosal immune system. Immunol Res. 2004;29:283–92. doi: 10.1385/IR:29:1-3:283. [DOI] [PubMed] [Google Scholar]
- 4.Macdonald TT, Monteleone G. Immunity, inflammation, and allergy in the gut. Science. 2005;307:1920–1925. doi: 10.1126/science.1106442. [DOI] [PubMed] [Google Scholar]
- 5.Salmi M, Jalkanen S. Lymphocyte homing to the gut: attraction, adhesion, and commitment. Immunol Rev. 2005;206:100–13. 100–13. doi: 10.1111/j.0105-2896.2005.00285.x. [DOI] [PubMed] [Google Scholar]
- 6.Dahan S, Roth-Walter F, Arnaboldi P, Agarwal S, Mayer L. Epithelia: lymphocyte interactions in the gut. Immunol Rev. 2007;215:243–53. 243–53. doi: 10.1111/j.1600-065X.2006.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kobayashi KS, Chamaillard M, Ogura Y, et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science. 2005;307:731–34. doi: 10.1126/science.1104911. [DOI] [PubMed] [Google Scholar]
- 9.Maeda S, Hsu LC, Liu H, et al. Nod2 mutation in Crohn's disease potentiates NF-kappaB activity and IL-1beta processing. Science. 2005;307:734–38. doi: 10.1126/science.1103685. [DOI] [PubMed] [Google Scholar]
- 10.Darfeuille-Michaud A, Neut C, Barnich N, et al. Presence of adherent Escherichia coli strains in ileal mucosa of patients with Crohn's disease. Gastroenterology. 1998;115:1405–13. doi: 10.1016/s0016-5085(98)70019-8. [DOI] [PubMed] [Google Scholar]
- 11.O'Neill LA. Immunology. After the toll rush. Science. 2004;303:1481–82. doi: 10.1126/science.1096113. [DOI] [PubMed] [Google Scholar]
- 12.Eckmann L, Karin M. NOD2 and Crohn's disease: loss or gain of function? Immunity. 2005;22:661–67. doi: 10.1016/j.immuni.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Rakoff-Nahoum S, Medzhitov R. Role of the innate immune system and host-commensal mutualism. Curr Top Microbiol Immunol. 2006;308:1–18. 1–18. doi: 10.1007/3-540-30657-9_1. [DOI] [PubMed] [Google Scholar]
- 14.Bonen DK, Cho JH. The genetics of inflammatory bowel disease. Gastroenterology. 2003;124:521–36. doi: 10.1053/gast.2003.50045. [DOI] [PubMed] [Google Scholar]
- 15.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 16.Oostenbrug LE, Drenth JP, de Jong DJ, et al. Association between Toll-like receptor 4 and inflammatory bowel disease. Inflamm Bowel Dis. 2005;11:567–75. doi: 10.1097/01.mib.0000161305.81198.0f. [DOI] [PubMed] [Google Scholar]
- 17.De Jager PL, Franchimont D, Waliszewska A, et al. The role of the Toll receptor pathway in susceptibility to inflammatory bowel diseases. Genes Immun. 2007;8:387–97. doi: 10.1038/sj.gene.6364398. [DOI] [PubMed] [Google Scholar]
- 18.Teahon K, Smethurst P, Levi AJ, Menzies IS, Bjarnason I. Intestinal permeability in patients with Crohn's disease and their first degree relatives. Gut. 1992;33:320–323. doi: 10.1136/gut.33.3.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollander D, Vadheim CM, Brettholz E, Petersen GM, Delahunty T, Rotter JI. Increased intestinal permeability in patients with Crohn's disease and their relatives. A possible etiologic factor Ann Intern Med. 1986;105:883–85. doi: 10.7326/0003-4819-105-6-883. [DOI] [PubMed] [Google Scholar]
- 20.Yacyshyn BR, Meddings JB. CD45RO expression on circulating CD19+ B cells in Crohn's disease correlates with intestinal permeability. Gastroenterology. 1995;108:132–37. doi: 10.1016/0016-5085(95)90017-9. [DOI] [PubMed] [Google Scholar]
- 21.Buhner S, Buning C, Genschel J, et al. Genetic basis for increased intestinal permeability in families with Crohn's disease: role of CARD15 3020insC mutation? Gut. 2006;55:342–47. doi: 10.1136/gut.2005.065557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gitter AH, Wullstein F, Fromm M, Schulzke JD. Epithelial barrier defects in ulcerative colitis: characterization and quantification by electrophysiological imaging. Gastroenterology. 2001;121:1320–1328. doi: 10.1053/gast.2001.29694. [DOI] [PubMed] [Google Scholar]
- 23.Sanders DS. Mucosal integrity and barrier function in the pathogenesis of early lesions in Crohn's disease. J Clin Pathol. 2005;58:568–72. doi: 10.1136/jcp.2004.021840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 25.Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res. 2001;61:3573–77. [PubMed] [Google Scholar]
- 26.Herszenyi L, Miheller P, Tulassay Z. Carcinogenesis in inflammatory bowel disease. Dig Dis. 2007;25:267–69. doi: 10.1159/000103898. [DOI] [PubMed] [Google Scholar]
- 27.Jess T, Loftus EV, Jr, Velayos FS, et al. Risk factors for colorectal neoplasia in inflammatory bowel disease: a nested case-control study from Copenhagen county, Denmark and Olmsted county, Minnesota. Am J Gastroenterol. 2007;102:829–36. doi: 10.1111/j.1572-0241.2007.01070.x. [DOI] [PubMed] [Google Scholar]
- 28.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am J Physiol Gastrointest Liver Physiol. 2004;287:G7–17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 29.Rashid A, Issa JP. CpG island methylation in gastroenterologic neoplasia: a maturing field. Gastroenterology. 2004;127:1578–88. doi: 10.1053/j.gastro.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Karin M, Lawrence T, Nizet V. Innate immunity gone awry: linking microbial infections to chronic inflammation and cancer. Cell. 2006;124:823–35. doi: 10.1016/j.cell.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 31.Greten FR, Eckmann L, Greten TF, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–96. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 32.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–36. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 33.Clevers H. At the crossroads of inflammation and cancer. Cell. 2004;118:671–74. doi: 10.1016/j.cell.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 34.Sanderson IR. Nutritional factors and immune functions of gut epithelium. Proc Nutr Soc. 2001;60:443–47. doi: 10.1079/pns2001122. [DOI] [PubMed] [Google Scholar]
- 35.Barrett KE, Keely SJ. Chloride secretion by the intestinal epithelium: molecular basis and regulatory aspects. Annu Rev Physiol. 2000;62:535–72. doi: 10.1146/annurev.physiol.62.1.535. [DOI] [PubMed] [Google Scholar]
- 36.Creamer B, Shorter RG, Bamforth J. The turnover and shedding of epithelial cells. I. The turnover in the gastro-intestinal tract. Gut. 1961;2:110–8. 110–118. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. V. Unitarian Theory of the origin of the four epithelial cell types. Am J Anat. 1974;141:537–61. doi: 10.1002/aja.1001410407. [DOI] [PubMed] [Google Scholar]
- 38.Booth C, Potten CS. Gut instincts: thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–99. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt GH, Winton DJ, Ponder BA. Development of the pattern of cell renewal in the crypt-villus unit of chimaeric mouse small intestine. Development. 1988;103:785–90. doi: 10.1242/dev.103.4.785. [DOI] [PubMed] [Google Scholar]
- 40.Hermiston ML, Wong MH, Gordon JI. Forced expression of E-cadherin in the mouse intestinal epithelium slows cell migration and provides evidence for nonautonomous regulation of cell fate in a self-renewing system. Genes Dev. 1996;10:985–96. doi: 10.1101/gad.10.8.985. [DOI] [PubMed] [Google Scholar]
- 41.Stappenbeck TS, Gordon JI. Rac1 mutations produce aberrant epithelial differentiation in the developing and adult mouse small intestine. Development. 2000;127:2629–42. doi: 10.1242/dev.127.12.2629. [DOI] [PubMed] [Google Scholar]
- 42.Potten CS, Allen TD. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977;60:272–77. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- 43.Bullen TF, Forrest S, Campbell F, et al. Characterization of epithelial cell shedding from human small intestine. Lab Invest. 2006;86:1052–63. doi: 10.1038/labinvest.3700464. [DOI] [PubMed] [Google Scholar]
- 44.Hofmann C, Obermeier F, Artinger M, et al. Cell-cell contacts prevent anoikis in primary human colonic epithelial cells. Gastroenterology. 2007;132:587–600. doi: 10.1053/j.gastro.2006.11.017. [DOI] [PubMed] [Google Scholar]
- 45.Kraehenbuhl JP, Pringault E, Neutra MR. Review article: Intestinal epithelia and barrier functions. Aliment Pharmacol Ther. 1997;11(Suppl 3):3–8. doi: 10.1111/j.1365-2036.1997.tb00803.x. discussion 8-9.:3-8. [DOI] [PubMed] [Google Scholar]
- 46.Kagnoff MF, Eckmann L. Epithelial cells as sensors for microbial infection. J Clin Invest. 1997;100:6–10. doi: 10.1172/JCI119522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–G499. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- 48.Papadakis KA, Targan SR. The role of chemokines and chemokine receptors in mucosal inflammation. Inflamm Bowel Dis. 2000;6:303–13. doi: 10.1002/ibd.3780060408. [DOI] [PubMed] [Google Scholar]
- 49.Perdue MH, Forstner JF, Roomi NW, Gall DG. Epithelial response to intestinal anaphylaxis in rats: goblet cell secretion and enterocyte damage. Am J Physiol. 1984;247:G632–G637. doi: 10.1152/ajpgi.1984.247.6.G632. [DOI] [PubMed] [Google Scholar]
- 50.Croitoru K, Ernst PB. Leukocytes in the intestinal epithelium: an unusual immunological compartment revisited. Reg Immunol. 1992;4:63–69. [PubMed] [Google Scholar]
- 51.Perdue MH, McKay DM. Integrative immunophysiology in the intestinal mucosa. Am J Physiol. 1994;267:G151–G165. doi: 10.1152/ajpgi.1994.267.2.G151. [DOI] [PubMed] [Google Scholar]
- 52.Ouellette AJ. IV. Paneth cell antimicrobial peptides and the biology of the mucosal barrier. Am J Physiol. 1999;277:G257–G261. doi: 10.1152/ajpgi.1999.277.2.G257. [DOI] [PubMed] [Google Scholar]
- 53.Rollins BJ. Chemokines. Blood. 1997;90:909–28. [PubMed] [Google Scholar]
- 54.Murphy PM, Baggiolini M, Charo IF, et al. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol Rev. 2000;52:145–76. [PubMed] [Google Scholar]
- 55.Rossi D, Zlotnik A. The biology of chemokines and their receptors. Annu Rev Immunol. 2000;18:217–42. doi: 10.1146/annurev.immunol.18.1.217. [DOI] [PubMed] [Google Scholar]
- 56.Hebert CA, Vitangcol RV, Baker JB. Scanning mutagenesis of interleukin-8 identifies a cluster of residues required for receptor binding. J Biol Chem. 1991;266:18989–94. [PubMed] [Google Scholar]
- 57.Kelner GS, Kennedy J, Bacon KB, et al. Lymphotactin: a cytokine that represents a new class of chemokine. Science. 1994;266:1395–99. doi: 10.1126/science.7973732. [DOI] [PubMed] [Google Scholar]
- 58.Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385:640–644. doi: 10.1038/385640a0. [DOI] [PubMed] [Google Scholar]
- 59.Cyster JG. Chemokines and cell migration in secondary lymphoid organs. Science. 1999;286:2098–102. doi: 10.1126/science.286.5447.2098. [DOI] [PubMed] [Google Scholar]
- 60.Johnson Z, Proudfoot AE, Handel TM. Interaction of chemokines and glycosaminoglycans: a new twist in the regulation of chemokine function with opportunities for therapeutic intervention. Cytokine Growth Factor Rev. 2005;16:625–36. doi: 10.1016/j.cytogfr.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 61.MacDermott RP, Sanderson IR, Reinecker HC. The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 1998;4:54–67. doi: 10.1097/00054725-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 62.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 63.Matloubian M, David A, Engel S, Ryan JE, Cyster JG. A transmembrane CXC chemokine is a ligand for HIV-coreceptor Bonzo. Nat Immunol. 2000;1:298–304. doi: 10.1038/79738. [DOI] [PubMed] [Google Scholar]
- 64.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 65.Deng H, Liu R, Ellmeier W, et al. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–66. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 66.Dragic T, Litwin V, Allaway GP, et al. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–73. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 67.Belperio JA, Keane MP, Arenberg DA, et al. CXC chemokines in angiogenesis. J Leukoc Biol. 2000;68:1–8. [PubMed] [Google Scholar]
- 68.Heidemann J, Ogawa H, Dwinell MB, et al. Angiogenic effects of interleukin 8 (CXCL8) in human intestinal microvascular endothelial cells are mediated by CXCR2. J Biol Chem. 2003;278:8508–15. doi: 10.1074/jbc.M208231200. [DOI] [PubMed] [Google Scholar]
- 69.Heidemann J, Ogawa H, Rafiee P, et al. Mucosal angiogenesis regulation by CXCR4 and its ligand CXCL12 expressed by human intestinal microvascular endothelial cells. Am J Physiol Gastrointest Liver Physiol. 2004;286:G1059–G1068. doi: 10.1152/ajpgi.00417.2003. [DOI] [PubMed] [Google Scholar]
- 70.Forster R, Schubel A, Breitfeld D, et al. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell. 1999;99:23–33. doi: 10.1016/s0092-8674(00)80059-8. [DOI] [PubMed] [Google Scholar]
- 71.Nagasawa T, Hirota S, Tachibana K, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–38. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 72.Tachibana K, Hirota S, Iizasa H, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–94. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 73.Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–99. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- 74.Peled A, Petit I, Kollet O, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283:845–48. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 75.Voigt I, Camacho SA, de Boer BA, Lipp M, Forster R, Berek C. CXCR5-deficient mice develop functional germinal centers in the splenic T cell zone. Eur J Immunol. 2000;30:560–567. doi: 10.1002/1521-4141(200002)30:2<560::AID-IMMU560>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 76.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 77.Nanney LB, Mueller SG, Bueno R, Peiper SC, Richmond A. Distributions of melanoma growth stimulatory activity of growth-regulated gene and the interleukin-8 receptor B in human wound repair. Am J Pathol. 1995;147:1248–60. [PMC free article] [PubMed] [Google Scholar]
- 78.Fedyk ER, Jones D, Critchley HO, Phipps RP, Blieden TM, Springer TA. Expression of stromal-derived factor-1 is decreased by IL-1 and TNF and in dermal wound healing. J Immunol. 2001;166:5749–54. doi: 10.4049/jimmunol.166.9.5749. [DOI] [PubMed] [Google Scholar]
- 79.Smith JM, Johanesen PA, Wendt MK, Binion DG, Dwinell MB. CXCL12 activation of CXCR4 regulates mucosal host defense through stimulation of epithelial cell migration and promotion of intestinal barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;288:G316–G326. doi: 10.1152/ajpgi.00208.2004. [DOI] [PubMed] [Google Scholar]
- 80.Avniel S, Arik Z, Maly A, et al. Involvement of the CXCL12/CXCR4 pathway in the recovery of skin following burns. J Invest Dermatol. 2006;126:468–76. doi: 10.1038/sj.jid.5700069. [DOI] [PubMed] [Google Scholar]
- 81.Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87:807–17. doi: 10.1038/labinvest.3700595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muller A, Homey B, Soto H, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 83.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–78. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 84.Godessart N, Kunkel SL. Chemokines in autoimmune disease. Curr Opin Immunol. 2001;13:670–675. doi: 10.1016/s0952-7915(01)00277-1. [DOI] [PubMed] [Google Scholar]
- 85.Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–65. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dwinell MB, Johanesen PA, Smith JM. Immunobiology of epithelial chemokines in the intestinal mucosa. Surgery. 2003;133:601–7. doi: 10.1067/msy.2003.143. [DOI] [PubMed] [Google Scholar]
- 87.Reinecker HC, Loh EY, Ringler DJ, Mehta A, Rombeau JL, MacDermott RP. Monocyte-chemoattractant protein 1 gene expression in intestinal epithelial cells and inflammatory bowel disease mucosa. Gastroenterology. 1995;108:40–50. doi: 10.1016/0016-5085(95)90006-3. [DOI] [PubMed] [Google Scholar]
- 88.Maghazachi AA. Role of the heterotrimeric G proteins in stromal-derived factor-1alpha-induced natural killer cell chemotaxis and calcium mobilization. Biochem Biophys Res Commun. 1997;236:270–274. doi: 10.1006/bbrc.1997.6937. [DOI] [PubMed] [Google Scholar]
- 89.Ina K, Kusugami K, Yamaguchi T, et al. Mucosal interleukin-8 is involved in neutrophil migration and binding to extracellular matrix in inflammatory bowel disease. Am J Gastroenterol. 1997;92:1342–46. [PubMed] [Google Scholar]
- 90.Banks C, Bateman A, Payne R, Johnson P, Sheron N. Chemokine expression in IBD. Mucosal chemokine expression is unselectively increased in both ulcerative colitis and Crohn's disease. J Pathol. 2003;199:28–35. doi: 10.1002/path.1245. [DOI] [PubMed] [Google Scholar]
- 91.Huang GT, Eckmann L, Savidge TC, Kagnoff MF. Infection of human intestinal epithelial cells with invasive bacteria upregulates apical intercellular adhesion molecule-1 (ICAM)-1) expression and neutrophil adhesion. J Clin Invest. 1996;98:572–83. doi: 10.1172/JCI118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Dwinell MB, Eckmann L, Leopard JD, Varki NM, Kagnoff MF. Chemokine receptor expression by human intestinal epithelial cells. Gastroenterology. 1999;117:359–67. doi: 10.1053/gast.1999.0029900359. [DOI] [PubMed] [Google Scholar]
- 93.Berin MC, Dwinell MB, Eckmann L, Kagnoff MF. Production of MDC/CCL22 by human intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1217–G1226. doi: 10.1152/ajpgi.2001.280.6.G1217. [DOI] [PubMed] [Google Scholar]
- 94.Izadpanah A, Dwinell MB, Eckmann L, Varki NM, Kagnoff MF. Regulated MIP-3alpha/CCL20 production by human intestinal epithelium: mechanism for modulating mucosal immunity. Am J Physiol Gastrointest Liver Physiol. 2001;280:G710–G719. doi: 10.1152/ajpgi.2001.280.4.G710. [DOI] [PubMed] [Google Scholar]
- 95.Kwon JH, Keates S, Bassani L, Mayer LF, Keates AC. Colonic epithelial cells are a major site of macrophage inflammatory protein 3alpha (MIP-3alpha) production in normal colon and inflammatory bowel disease. Gut. 2002;51:818–26. doi: 10.1136/gut.51.6.818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Katchar K, Kelly CP, Keates S, O'brien MJ, Keates AC. MIP-3alpha neutralizing monoclonal antibody protects against TNBS-induced colonic injury and inflammation in mice. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1263–G1271. doi: 10.1152/ajpgi.00409.2006. [DOI] [PubMed] [Google Scholar]
- 97.Iwasaki A, Kelsall BL. Localization of distinct Peyer's patch dendritic cell subsets and their recruitment by chemokines macrophage inflammatory protein (MIP)-3alpha, MIP-3beta, and secondary lymphoid organ chemokine. J Exp Med. 2000;191:1381–94. doi: 10.1084/jem.191.8.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cook DN, Prosser DM, Forster R, et al. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity. 2000;12:495–503. doi: 10.1016/s1074-7613(00)80201-0. [DOI] [PubMed] [Google Scholar]
- 99.Lugering A, Kucharzik T, Soler D, Picarella D, Hudson JT, III, Williams IR. Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6. J Immunol. 2003;171:2208–15. doi: 10.4049/jimmunol.171.5.2208. [DOI] [PubMed] [Google Scholar]
- 100.Lugering A, Floer M, Westphal S, et al. Absence of CCR6 inhibits CD4+ regulatory T-cell development and M-cell formation inside Peyer's patches. Am J Pathol. 2005;166:1647–54. doi: 10.1016/S0002-9440(10)62475-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muller G, Hopken UE, Lipp M. The impact of CCR7 and CXCR5 on lymphoid organ development and systemic immunity. Immunol Rev. 2003;195:117–35. doi: 10.1034/j.1600-065x.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 102.Wurbel MA, Philippe JM, Nguyen C, et al. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. Eur J Immunol. 2000;30:262–71. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 103.Wurbel MA, Malissen M, Guy-Grand D, et al. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–32. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 104.Pan J, Kunkel EJ, Gosslar U, et al. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–49. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 105.Campbell JJ, Butcher EC. Chemokines in tissue-specific and microenvironment-specific lymphocyte homing. Curr Opin Immunol. 2000;12:336–41. doi: 10.1016/s0952-7915(00)00096-0. [DOI] [PubMed] [Google Scholar]
- 106.Hopken UE, Wengner AM, Loddenkemper C, et al. CCR7 deficiency causes ectopic lymphoid neogenesis and disturbed mucosal tissue integrity. Blood. 2007;109:886–95. doi: 10.1182/blood-2006-03-013532. [DOI] [PubMed] [Google Scholar]
- 107.Ansel KM, Ngo VN, Hyman PL, et al. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000;406:309–14. doi: 10.1038/35018581. [DOI] [PubMed] [Google Scholar]
- 108.Lazarus NH, Kunkel EJ, Johnston B, Wilson E, Youngman KR, Butcher EC. A common mucosal chemokine (mucosae-associated epithelial chemokine/CCL28) selectively attracts IgA plasmablasts. J Immunol. 2003;170:3799–805. doi: 10.4049/jimmunol.170.7.3799. [DOI] [PubMed] [Google Scholar]
- 109.Wurbel MA, Malissen M, Guy-Grand D, Malissen B, Campbell JJ. Impaired accumulation of antigen-specific CD8 lymphocytes in chemokine CCL25-deficient intestinal epithelium and lamina propria. J Immunol. 2007;178:7598–606. doi: 10.4049/jimmunol.178.12.7598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pabst O, Ohl L, Wendland M, et al. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. J Exp Med. 2004;199:411–16. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Varona R, Villares R, Carramolino L, et al. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest. 2001;107:R37–R45. doi: 10.1172/JCI11297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McDonald KG, McDonough JS, Wang C, Kucharzik T, Williams IR, Newberry RD. CC chemokine receptor 6 expression by B lymphocytes is essential for the development of isolated lymphoid follicles. Am J Pathol. 2007;170:1229–40. doi: 10.2353/ajpath.2007.060817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Papadakis KA, Prehn J, Moreno ST, et al. CCR9-positive lymphocytes and thymus-expressed chemokine distinguish small bowel from colonic Crohn's disease. Gastroenterology. 2001;121:246–54. doi: 10.1053/gast.2001.27154. [DOI] [PubMed] [Google Scholar]
- 114.Carlsen HS, Baekkevold ES, Morton HC, Haraldsen G, Brandtzaeg P. Monocyte-like and mature macrophages produce CXCL13 (B cell-attracting chemokine 1) in inflammatory lesions with lymphoid neogenesis. Blood. 2004;104:3021–27. doi: 10.1182/blood-2004-02-0701. [DOI] [PubMed] [Google Scholar]
- 115.Ogawa H, Iimura M, Eckmann L, Kagnoff MF. Regulated production of the chemokine CCL28 in human colon epithelium. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1062–G1069. doi: 10.1152/ajpgi.00162.2004. [DOI] [PubMed] [Google Scholar]
- 116.Middel P, Raddatz D, Gunawan B, Haller F, Radzun HJ. Increased number of mature dendritic cells in Crohn's disease: evidence for a chemokine mediated retention mechanism. Gut. 2006;55:220–227. doi: 10.1136/gut.2004.063008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Saruta M, Yu QT, Avanesyan A, Fleshner PR, Targan SR, Papadakis KA. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn's disease. J Immunol. 2007;178:3293–300. doi: 10.4049/jimmunol.178.5.3293. [DOI] [PubMed] [Google Scholar]
- 118.Kawashima D, Oshitani N, Jinno Y, et al. Augmented expression of secondary lymphoid tissue chemokine and EBI1 ligand chemokine in Crohn's disease. J Clin Pathol. 2005;58:1057–63. doi: 10.1136/jcp.2004.024828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shirozu M, Nakano T, Inazawa J, et al. Structure and chromosomal localization of the human stromal cell-derived factor 1 (SDF1) gene. Genomics. 1995;28:495–500. doi: 10.1006/geno.1995.1180. [DOI] [PubMed] [Google Scholar]
- 120.Agace WW, Amara A, Roberts AI, et al. Constitutive expression of stromal derived factor-1 by mucosal epithelia and its role in HIV transmission and propagation. Curr Biol. 2000;10:325–28. doi: 10.1016/s0960-9822(00)00380-8. [DOI] [PubMed] [Google Scholar]
- 121.Jordan NJ, Kolios G, Abbot SE, et al. Expression of functional CXCR4 chemokine receptors on human colonic epithelial cells. J Clin Invest. 1999;104:1061–69. doi: 10.1172/JCI6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wendt MK, Johanesen PA, Kang-Decker N, Binion DG, Shah V, Dwinell MB. Silencing of epithelial CXCL12 expression by DNA hypermethylation promotes colonic carcinoma metastasis. Oncogene. 2006;25:4986–97. doi: 10.1038/sj.onc.1209505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wendt MK, Cooper AN, Dwinell MB. Epigenetic silencing of CXCL12 increases the metastatic potential of mammary carcinoma cells. Oncogene. 2007 Sep 3; doi: 10.1038/sj.onc.1210751. [DOI] [PubMed] [Google Scholar]
- 124.Ma Q, Jones D, Borghesani PR, et al. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–53. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burns JM, Summers BC, Wang Y, et al. A novel chemokine receptor for SDF-1 and I-TAC involved in cell survival, cell adhesion, and tumor development. J Exp Med. 2006;203:2201–13. doi: 10.1084/jem.20052144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sierro F, Biben C, Martinez-Munoz L, et al. Disrupted cardiac development but normal hematopoiesis in mice deficient in the second CXCL12/SDF-1 receptor, CXCR7. Proc Natl Acad Sci U S A. 2007;104:14759–64. doi: 10.1073/pnas.0702229104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Oberlin E, Amara A, Bachelerie F, et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–35. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 128.Loetscher M, Geiser T, O'Reilly T, Zwahlen R, Baggiolini M, Moser B. Cloning of a human seven-transmembrane domain receptor, LESTR, that is highly expressed in leukocytes. J Biol Chem. 1994;269:232–37. [PubMed] [Google Scholar]
- 129.Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T and B cells for development and effector function. J Leukoc Biol. 1999;65:6–15. doi: 10.1002/jlb.65.1.6. [DOI] [PubMed] [Google Scholar]
- 130.Hesselgesser J, Taub D, Baskar P, et al. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595–98. doi: 10.1016/s0960-9822(98)70230-1. [DOI] [PubMed] [Google Scholar]
- 131.Scotton CJ, Wilson JL, Milliken D, Stamp G, Balkwill FR. Epithelial cancer cell migration: a role for chemokine receptors? Cancer Res. 2001;61:4961–65. [PubMed] [Google Scholar]
- 132.Han Y, He T, Huang DR, Pardo CA, Ransohoff RM. TNF-alpha mediates SDF-1 alpha-induced NF-kappa B activation and cytotoxic effects in primary astrocytes. J Clin Invest. 2001;108:425–35. doi: 10.1172/JCI12629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Robledo MM, Bartolome RA, Longo N, et al. Expression of functional chemokine receptors CXCR3 and CXCR4 on human melanoma cells. J Biol Chem. 2001;276:45098–105. doi: 10.1074/jbc.M106912200. [DOI] [PubMed] [Google Scholar]
- 134.Andres PG, Beck PL, Mizoguchi E, et al. Mice with a selective deletion of the CC chemokine receptors 5 or 2 are protected from dextran sodium sulfate-mediated colitis: lack of CC chemokine receptor 5 expression results in a NK1.1+ lymphocyte-associated Th2-type immune response in the intestine. J Immunol. 2000;164:6303–12. doi: 10.4049/jimmunol.164.12.6303. [DOI] [PubMed] [Google Scholar]
- 135.Tokuyama H, Ueha S, Kurachi M, et al. The simultaneous blockade of chemokine receptors CCR2, CCR5 and CXCR3 by a non-peptide chemokine receptor antagonist protects mice from dextran sodium sulfate-mediated colitis. Int Immunol. 2005;17:1023–34. doi: 10.1093/intimm/dxh284. [DOI] [PubMed] [Google Scholar]
- 136.Hyun JG, Lee G, Brown JB, et al. Anti-interferon-inducible chemokine, CXCL10, reduces colitis by impairing T helper-1 induction and recruitment in mice. Inflamm Bowel Dis. 2005;11:799–805. doi: 10.1097/01.mib.0000178263.34099.89. [DOI] [PubMed] [Google Scholar]
- 137.Clayburgh DR, Barrett TA, Tang Y, et al. Epithelial myosin light chain kinase-dependent barrier dysfunction mediates T cell activation-induced diarrhea in vivo. J Clin Invest. 2005;115:2702–15. doi: 10.1172/JCI24970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sturm A, Baumgart DC, d'Heureuse JH, Hotz A, Wiedenmann B, Dignass AU. CXCL8 modulates human intestinal epithelial cells through a CXCR1 dependent pathway. Cytokine. 2005;29:42–48. doi: 10.1016/j.cyto.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 139.Edelman GM. Morphoregulation. Dev Dyn. 1992;193:2–10. doi: 10.1002/aja.1001930103. [DOI] [PubMed] [Google Scholar]
- 140.Johnson LR, McCormack SA. Healing of Gastrointestinal Mucosa: Involvement of Polyamines. News Physiol Sci. 1999;14:12–17. doi: 10.1152/physiologyonline.1999.14.1.12. [DOI] [PubMed] [Google Scholar]
- 141.Dignass AU, Sturm A. Peptide growth factors in the intestine. Eur J Gastroenterol Hepatol. 2001;13:763–70. doi: 10.1097/00042737-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 142.Podolsky DK. Review article: healing after inflammatory injury--coordination of a regulatory peptide network. Aliment Pharmacol Ther. 2000;14(Suppl 1):87–93. doi: 10.1046/j.1365-2036.2000.014s1087.x. [DOI] [PubMed] [Google Scholar]
- 143.Basson MD. Gut mucosal healing: is the science relevant? Am J Pathol. 2002;161:1101–5. doi: 10.1016/S0002-9440(10)64385-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mammen JM, Matthews JB. Mucosal repair in the gastrointestinal tract. Crit Care Med. 2003;31:S532–S537. doi: 10.1097/01.CCM.0000081429.89277.AF. [DOI] [PubMed] [Google Scholar]
- 145.Kaur P, Potten CS. Cell migration velocities in the crypts of the small intestine after cytotoxic insult are not dependent on mitotic activity. Cell Tissue Kinet. 1986;19:601–10. doi: 10.1111/j.1365-2184.1986.tb00761.x. [DOI] [PubMed] [Google Scholar]
- 146.Polk DB, Tong W. Epidermal and hepatocyte growth factors stimulate chemotaxis in an intestinal epithelial cell line. Am J Physiol. 1999;277:C1149–C1159. doi: 10.1152/ajpcell.1999.277.6.C1149. [DOI] [PubMed] [Google Scholar]
- 147.Blay J, Brown KD. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J Cell Physiol. 1985;124:107–12. doi: 10.1002/jcp.1041240117. [DOI] [PubMed] [Google Scholar]
- 148.Miettinen PJ, Berger JE, Meneses J, et al. Epithelial immaturity and multiorgan failure in mice lacking epidermal growth factor receptor. Nature. 1995;376:337–41. doi: 10.1038/376337a0. [DOI] [PubMed] [Google Scholar]
- 149.Beck PL, Rosenberg IM, Xavier RJ, Koh T, Wong JF, Podolsky DK. Transforming growth factor-beta mediates intestinal healing and susceptibility to injury in vitro and in vivo through epithelial cells. Am J Pathol. 2003;162:597–608. doi: 10.1016/s0002-9440(10)63853-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wong MH, Hermiston ML, Syder AJ, Gordon JI. Forced expression of the tumor suppressor adenomatosis polyposis coli protein induces disordered cell migration in the intestinal epithelium. Proc Natl Acad Sci U S A. 1996;93:9588–93. doi: 10.1073/pnas.93.18.9588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–7. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- 152.McCormack SA, Viar MJ, Johnson LR. Migration of IEC-6 cells: a model for mucosal healing. Am J Physiol. 1992;263:G426–G435. doi: 10.1152/ajpgi.1992.263.3.G426. [DOI] [PubMed] [Google Scholar]
- 153.Schmidt GH, Wilkinson MM, Ponder BA. Cell migration pathway in the intestinal epithelium: an in situ marker system using mouse aggregation chimeras. Cell. 1985;40:425–29. doi: 10.1016/0092-8674(85)90156-4. [DOI] [PubMed] [Google Scholar]
- 154.Dignass A, Lynch-Devaney K, Kindon H, Thim L, Podolsky DK. Trefoil peptides promote epithelial migration through a transforming growth factor beta-independent pathway. J Clin Invest. 1994;94:376–83. doi: 10.1172/JCI117332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Youakim A, Ahdieh M. Interferon-gamma decreases barrier function in T84 cells by reducing ZO-1 levels and disrupting apical actin. Am J Physiol. 1999;276:G1279–G1288. doi: 10.1152/ajpgi.1999.276.5.G1279. [DOI] [PubMed] [Google Scholar]
- 156.Nishiyama R, Sakaguchi T, Kinugasa T, et al. Interleukin-2 receptor beta subunit-dependent and -independent regulation of intestinal epithelial tight junctions. J Biol Chem. 2001;276:35571–80. doi: 10.1074/jbc.M106013200. [DOI] [PubMed] [Google Scholar]
- 157.Madsen K, Cornish A, Soper P, et al. Probiotic bacteria enhance murine and human intestinal epithelial barrier function. Gastroenterology. 2001;121:580–591. doi: 10.1053/gast.2001.27224. [DOI] [PubMed] [Google Scholar]
- 158.Mashimo H, Wu DC, Podolsky DK, Fishman MC. Impaired defense of intestinal mucosa in mice lacking intestinal trefoil factor. Science. 1996;274:262–65. doi: 10.1126/science.274.5285.262. [DOI] [PubMed] [Google Scholar]
- 159.Wilson AJ, Byron K, Gibson PR. Interleukin-8 stimulates the migration of human colonic epithelial cells in vitro. Clin Sci (Lond) 1999;97:385–90. [PubMed] [Google Scholar]
- 160.Zhao Y, Young SL. Requirement of transforming growth factor-beta (TGF-beta) type II receptor for TGF-beta-induced proliferation and growth inhibition. J Biol Chem. 1996;271:2369–72. doi: 10.1074/jbc.271.5.2369. [DOI] [PubMed] [Google Scholar]
- 161.Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–11. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Bement WM, Forscher P, Mooseker MS. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J Cell Biol. 1993;121:565–78. doi: 10.1083/jcb.121.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nusrat A, Giry M, Turner JR, et al. Rho protein regulates tight junctions and perijunctional actin organization in polarized epithelia. Proc Natl Acad Sci U S A. 1995;92:10629–33. doi: 10.1073/pnas.92.23.10629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–96. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Turner JR. ‘Putting the squeeze’ on the tight junction: understanding cytoskeletal regulation. Semin Cell Dev Biol. 2000;11:301–8. doi: 10.1006/scdb.2000.0180. [DOI] [PubMed] [Google Scholar]
- 166.Ray RM, Patel A, Viar MJ, et al. RhoA inactivation inhibits cell migration but does not mediate the effects of polyamine depletion. Gastroenterology. 2002;123:196–205. doi: 10.1053/gast.2002.34216. [DOI] [PubMed] [Google Scholar]
- 167.Martin P, Lewis J. Actin cables and epidermal movement in embryonic wound healing. Nature. 1992;360:179–83. doi: 10.1038/360179a0. [DOI] [PubMed] [Google Scholar]
- 168.Wong MH, Rubinfeld B, Gordon JI. Effects of forced expression of an NH2-terminal truncated beta-Catenin on mouse intestinal epithelial homeostasis. J Cell Biol. 1998;141:765–77. doi: 10.1083/jcb.141.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jou TS, Schneeberger EE, Nelson WJ. Structural and functional regulation of tight junctions by RhoA and Rac1 small GTPases. J Cell Biol. 1998;142:101–15. doi: 10.1083/jcb.142.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Guo X, Rao JN, Liu L, et al. Regulation of adherens junctions and epithelial paracellular permeability: a novel function for polyamines. Am J Physiol Cell Physiol. 2003;285:C1174–C1187. doi: 10.1152/ajpcell.00015.2003. [DOI] [PubMed] [Google Scholar]
- 171.Ivanov AI, McCall IC, Parkos CA, Nusrat A. Role for actin filament turnover and a myosin II motor in cytoskeleton-driven disassembly of the epithelial apical junctional complex. Mol Biol Cell. 2004;15:2639–51. doi: 10.1091/mbc.E04-02-0163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Russo JM, Florian P, Shen L, et al. Distinct temporal-spatial roles for rho kinase and myosin light chain kinase in epithelial purse-string wound closure. Gastroenterology. 2005;128:987–1001. doi: 10.1053/j.gastro.2005.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang CC, Ogawa H, Dwinell MB, McCole DF, Eckmann L, Kagnoff MF. Chemokine receptor CCR6 transduces signals that activate p130Cas and alter cAMP-stimulated ion transport in human intestinal epithelial cells. Am J Physiol Cell Physiol. 2005;288:C321–C328. doi: 10.1152/ajpcell.00171.2004. [DOI] [PubMed] [Google Scholar]
- 174.Cerda SR, Bissonnette M, Scaglione-Sewell B, et al. PKC-delta inhibits anchorage-dependent and -independent growth, enhances differentiation, and increases apoptosis in CaCo-2 cells. Gastroenterology. 2001;120:1700–1712. doi: 10.1053/gast.2001.24843. [DOI] [PubMed] [Google Scholar]
- 175.Kuhns DB, Young HA, Gallin EK, Gallin JI. Ca2+-dependent production and release of IL-8 in human neutrophils. J Immunol. 1998;161:4332–39. [PubMed] [Google Scholar]
- 176.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 177.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–45. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 178.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Bates RC, DeLeo MJ, III, Mercurio AM. The epithelial-mesenchymal transition of colon carcinoma involves expression of IL-8 and CXCR-1-mediated chemotaxis. Exp Cell Res. 2004;299:315–24. doi: 10.1016/j.yexcr.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 180.Volin MV, Joseph L, Shockley MS, Davies PF. Chemokine receptor CXCR4 expression in endothelium. Biochem Biophys Res Commun. 1998;242:46–53. doi: 10.1006/bbrc.1997.7890. [DOI] [PubMed] [Google Scholar]
- 181.Phillips RJ, Burdick MD, Lutz M, Belperio JA, Keane MP, Strieter RM. The stromal derived factor-1/CXCL12-CXC chemokine receptor 4 biological axis in non-small cell lung cancer metastases. Am J Respir Crit Care Med. 2003;167:1676–86. doi: 10.1164/rccm.200301-071OC. [DOI] [PubMed] [Google Scholar]
- 182.Schrader AJ, Lechner O, Templin M, et al. CXCR4/CXCL12 expression and signalling in kidney cancer. Br J Cancer. 2002;86:1250–1256. doi: 10.1038/sj.bjc.6600221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Ben Baruch A. Organ selectivity in metastasis: regulation by chemokines and their receptors. Clin Exp Metastasis. 2007 doi: 10.1007/s10585-007-9097-3. [DOI] [PubMed] [Google Scholar]
- 184.Ottaiano A, Franco R, Aiello TA, et al. Overexpression of both CXC chemokine receptor 4 and vascular endothelial growth factor proteins predicts early distant relapse in stage II-III colorectal cancer patients. Clin Cancer Res. 2006;12:2795–803. doi: 10.1158/1078-0432.CCR-05-2142. [DOI] [PubMed] [Google Scholar]
- 185.Kim J, Takeuchi H, Lam ST, et al. Chemokine receptor CXCR4 expression in colorectal cancer patients increases the risk for recurrence and for poor survival. J Clin Oncol. 2005;23:2744–53. doi: 10.1200/JCO.2005.07.078. [DOI] [PubMed] [Google Scholar]
- 186.Sozzani S, Allavena P, D'Amico G, et al. Differential regulation of chemokine receptors during dendritic cell maturation: a model for their trafficking properties. J Immunol. 1998;161:1083–86. [PubMed] [Google Scholar]
- 187.Bacon KB, Flores-Romo L, Life PF, et al. IL-8-induced signal transduction in T lymphocytes involves receptor-mediated activation of phospholipases C and D. J Immunol. 1995;154:3654–66. [PubMed] [Google Scholar]
- 188.Angiolillo AL, Sgadari C, Taub DD, et al. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J Exp Med. 1995;182:155–62. doi: 10.1084/jem.182.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Katsuta T, Lim C, Shimoda K, et al. Interleukin-8 and SDF1-alpha mRNA expression in colonic biopsies from patients with inflammatory bowel disease. Am J Gastroenterol. 2000;95:3157–64. doi: 10.1111/j.1572-0241.2000.03289.x. [DOI] [PubMed] [Google Scholar]