Abstract
Modern approaches to the investigation of the molecular mechanisms underlying human cognitive disease often include multidisciplinary examination of animal models engineered with specific mutations that spatially and temporally restrict expression of a gene of interest. This approach not only makes possible the development of animal models that demonstrate phenotypic similarities to their respective human disorders, but has also allowed for significant progress towards understanding the processes that mediate synaptic function and memory formation in the nondiseased state. Examples of successful mouse models where genetic manipulation of the mouse resulted in recapitulation of the symptomatology of the human disorder and was used to significantly expand our understanding of the molecular mechanisms underlying normal synaptic plasticity and memory formation are discussed in this article. These studies have broadened our knowledge of several signal transduction cascades that function throughout life to mediate synaptic physiology. Defining these events is key for developing therapies to address disorders of cognitive ability.
Keywords: Alzheimer’s disease, Angelman syndrome, autism, hippocampus, knockout mouse, neurofibromatosis type 1, Reelin, Rubinstein-Taybi syndrome, secretin, synaptic plasticity
Scientific investigation of the molecular mechanisms underlying human cognition underwent a renaissance with the first report in 1989 of homologous recombination in embryonic stem cells to generate gene-targeted mice. The advent of murine knockout technology was so revolutionary to the field that Mario R Capecchi, Martin J Evans and Oliver Smithies were awarded the Nobel Prize in Physiology or Medicine in 1997. Knockout technology promoted the search for disease-causing genes by identifying specific genes that were altered in individuals who were grouped with specific disorders. While gene discovery was daunting for incredibly complex disorders such as autism or schizophrenia, which involve multiple genes and environmental factors, the identification of single gene disruptions that result in human disorders were highly suitable for using the newly perfected knockout strategy. The past two decades have seen the number of reported knockout mouse strains increase exponentially. Now it is possible to introduce specific mutations that can be both spatially and temporally controlled in the CNS, thereby enhancing our ability to understand not only the basic function of genes, but the temporal windows within which diseases that result from gene alterations can be treated.
This article describes a snapshot of a few human cognitive disorders that have benefited from the use of knockout technologies. Attention was paid to present a selection of mouse models that represent examples of:
-
▪
Human disorders resulting from a single gene mutation/disruption;
-
▪
Where genetic manipulation of the mouse resulted in a model that recapitulated the symptomatology of the human disorder;
-
▪
Where the mouse model has significantly expand our understanding of the disorder and the molecular mechanisms underlying normal synaptic plasticity and memory formation.
However, these examples are not intended to reflect the whole spectrum of human cognitive disorders. Specifically, these mouse models were used to determine phenotype similarities, developmental characteristics, gross and micro-structural morphological changes and measurements of synaptic function (Table 1). The characterization of these models often facilitated the elucidation of potential molecular mechanisms underlying the disorder. It should also be noted that the examples given later are hippocampus-centric in their respective analyses for the fundamental reason that this region of the brain exhibits well-characterized patterns of synaptic transmission and plasticity, is intimately involved in many forms of memory and represents the majority of published studies on overall synaptic function.
Table 1.
Overview of six genes that are important for normal learning and memory.
| Gene | Protein | Disorder | Hippocampal function CA1 LTP | Cognitive function |
|
|---|---|---|---|---|---|
| Loss of function | Gain of function | Loss of function | |||
| NF1 | NF1 | NF1 | ↓ | ND | ↓ HPWM, ↓ attention and ↓ CFC |
| UBE3A | Ube3a | Angelman syndrome | ↓ | ND | ↓ HPWM and ↓ CFC |
| SCT | Secretin | Autism | ↓ | ND | ↓ HPWN and ↓ CFC |
| CREBBP | CBP | Rubinstein–Taybi syndrome | Normal | ↑ | ↓ social recognition and ↓ reversal learning |
| RELN | Reelin | Schizophrenia and lissencephaly | ↓ | ↑ | ↓ CFC and ↑ PPI |
| APOE | ApoE | Alzheimer’s disease | E4 > E2 > E3 | HPWM results unclear and ↑ anxiety (E4 > E2/E3) | |
NF1, Ube3a (an E3 ubiquiton ligase, also known as E6-AP), secretin, CBP, Reelin, and apoE are listed alongside their associated disorders. Moreover, changes in hippocampal function are described for mouse models where the genes have either been deleted or decreased, focusing on changes in CA1 LTP; for targeted replacement apoE isoform-expressing mice, relative changes are conveyed. Specific changes in cognitive function are also included, predominantly focused on spatial learning (determined using the hidden platform water maze) and hippocampus-dependent CFC.
CBP: CREB binding protein; CFC: Contextual fear conditioning; HPWM: Hidden platform water maze; LTP: Long-term potentiation; ND: No data; NF1: Neurofibromatosis type 1; PPI: Prepulse inhibition.
Hippocampus & the measurement of synaptic plasticity
The hippocampus represents an amazing feature of the mammalian CNS. It is one of the evolutionarily oldest regions of the brain and bridges other ancient brain regions, such as the amygdala, to the higher cognitive areas of the cortex [1]. The hippocampus contributes to the other CNS structures forming the limbic system and is part of the hippocampal formation, which includes the dentate gyrus, subiculum and entorhinal cortex (Figure 1). The hippocampus can signal to the entorhinal cortex and the amygdala, which in turn project into numerous brain regions, affecting aspects of behavior, learning ability and memory formation [2]. While the entorhinal cortex is a major source of projections into the hippocampus, the hippocampus also receives information from the amygdala, cingulate cortex and temporal lobe, and has outputs to the cortex and other higher brain regions [3]. Thus, disruption in hippocampal function can affect interpretation of inputs and alter the modulation of output signals to numerous important modalities.
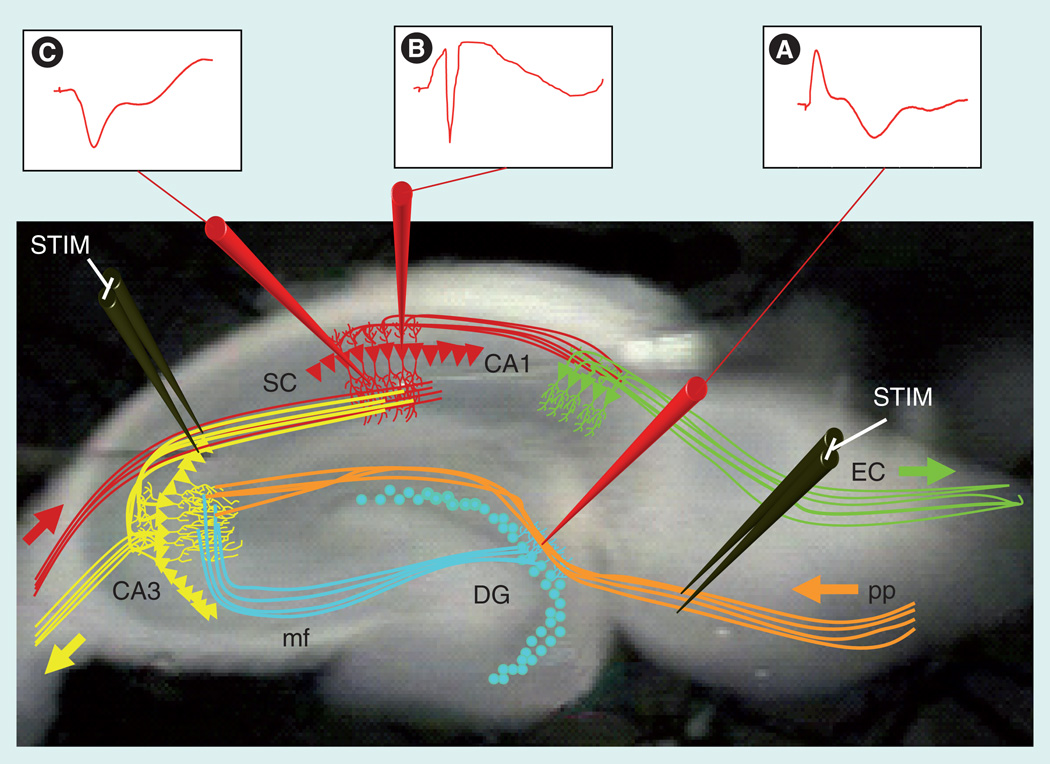
Figure 1. The hippocampal trisynaptic pathway and electrophysiology recording.
The hippocampus consists of a trisynaptic pathway that can be maintained through acute lateral slicing of the mouse brain. Using this technique, the ex vivo slices can be artificially stimulated and recordings can be made across well-known sites of synaptic connections. The hippocampal DG receives major inputs from the EC through activation of the pp. (A) STIM of the pp can be recorded as an excitatory postsynaptic potential (EPSP) in the dendritic field of the DG. The granule cells of the DG synapse onto the dendrites of the pyramidal cells composing area CA3 via the mf. The pyramidal neurons of CA3 project to area CA1 via the SC synapses to the pyramidal neurons of area CA1. Presynaptic STIM of CA3 axons results in EPSPs in area CA1 recorded from either (B) the cell body layer or (C) dendritic fields of CA1. The primary output of the hippocampus is to the subiculum in area CA1, and subsequent signaling exits the hippocampus to the EC.
DG: Dentate gyrus; EC: Entorhinal cortex; mf: Mossy fiber; pp: Perforant path; SC; Schaffer collateral; STIM: Stimulation.
The CA1 area of the hippocampus displays two major forms of long-term potentiation (LTP) [4,5] and at least one form of long-term depression [5,6]. Each of these can be initiated by exogenous tetanic stimulation, resulting in varying degrees of measurable increases (i.e., LTP) or decreases (i.e., long-term depression) in the size of the dendritic excitatory postsynaptic potentials or measured in the cell bodies as population spikes (Figure 1). Hippocampal LTP can last from minutes in acute ex vivo hippocampal slices to weeks in the living animal [7–11]. Induction of LTP in area CA1 that most closely resembles the proposed in vivo mechanisms requires presynaptic neurotransmitter release, N-methyl-d-aspartate receptor (NMDAR) activation and subsequent upregulation of AMPA receptor expression and function [12–15]. The opening of NMDARs requires both glutamate binding and membrane depolarization. Downstream of receptor activation are numerous signal transduction pathways, all of which integrate into a larger coincidence detection system that is believed to be important for reaching a threshold for the processes of synaptic plasticity [16]. Thus, genetic mutations that disrupt cognitive ability can represent dysfunction in presynaptic neurotransmitter release, extracellular signaling, membrane-bound receptor function, intracellular signaling pathways or genetic changes following stimulation.
In the study of mammalian models of human cognitive disorders or those disease states that involve disruption in learning and memory, synaptic plasticity is a major part of the characterization of the model. Furthermore, synaptic plasticity is frequently associated with behavioral testing of learning and memory and is often used as a quantifiable measurement of the efficacy of a therapeutic agent on the specific model. A large body of evidence demonstrates that LTP and memory are supported by similar mechanisms. For example, blockade of NMDARs effectively blocks LTP and impairs learning in rodents in hippocampal-dependent memory tasks [17–20]. Conversely, stimulation of the hippocampus to the extent that makes most of the synaptic connections in area CA1 plastic can inhibit normal spatial learning and memory [21], suggesting an intimate relationship between the processes of hippocampal synaptic function and those underlying learning and memory. The following sections provide examples of the use of mouse models with specific genetic mutations resulting in neuronal molecular disruption associated with alterations in synaptic function and/or learning and memory.
ApoE & lipoprotein receptors: Alzheimer’s disease
For more than a decade, the allelic variation of apoE (APOE) has been known to be associated with the risk of developing Alzheimer’s disease (AD) [22,23]. The human population expresses three APOE alleles that differ by the presence or absence of a cysteine or arginine amino acid at positions 112 and 158 in their protein product: APOE*2 (Cysll2 and Cysl58), APOE*3 (Cysll2 amd Argl58), and APOE*4 (Argll2 and Argl58). The APOE*3 allele is found in approximately 78% of the US population and is often considered to be the neutral allele regarding disease risk relative to APOE*2 and APOE*4. APOE*4 is found in 14% of the US population and is linked to an increased risk of developing sporadic AD, as well as a decreased age of onset compared with APOE*3. By contrast, APOE*2 is found in the remaining 8% of the US population and is found to decrease AD risk compared with those expressing the APOE*3 allele [22,24].
In the CNS, the APOE protein product, apoE, binds to the seven identified mammalian members of the highly conserved low-density lipoprotein receptor (LDLR) family [25]. Mice deficient for at least two of the apoE receptors, apoE receptor 2 (apoER2) and the very LDLR (VLDLR), show defects in both learning and memory, as well as hippocampal LTP [26–30].
The apoER2 and VLDLRs are linked to several signal transduction pathways including the Src, PI3K and CDK5 signal transduction pathways (Figure 2) [31–33]. While apoE also associates with these important signaling receptors, relatively little is known about apoE signaling in the CNS in general, or how apoE isoforms may specifically affect synaptic function.
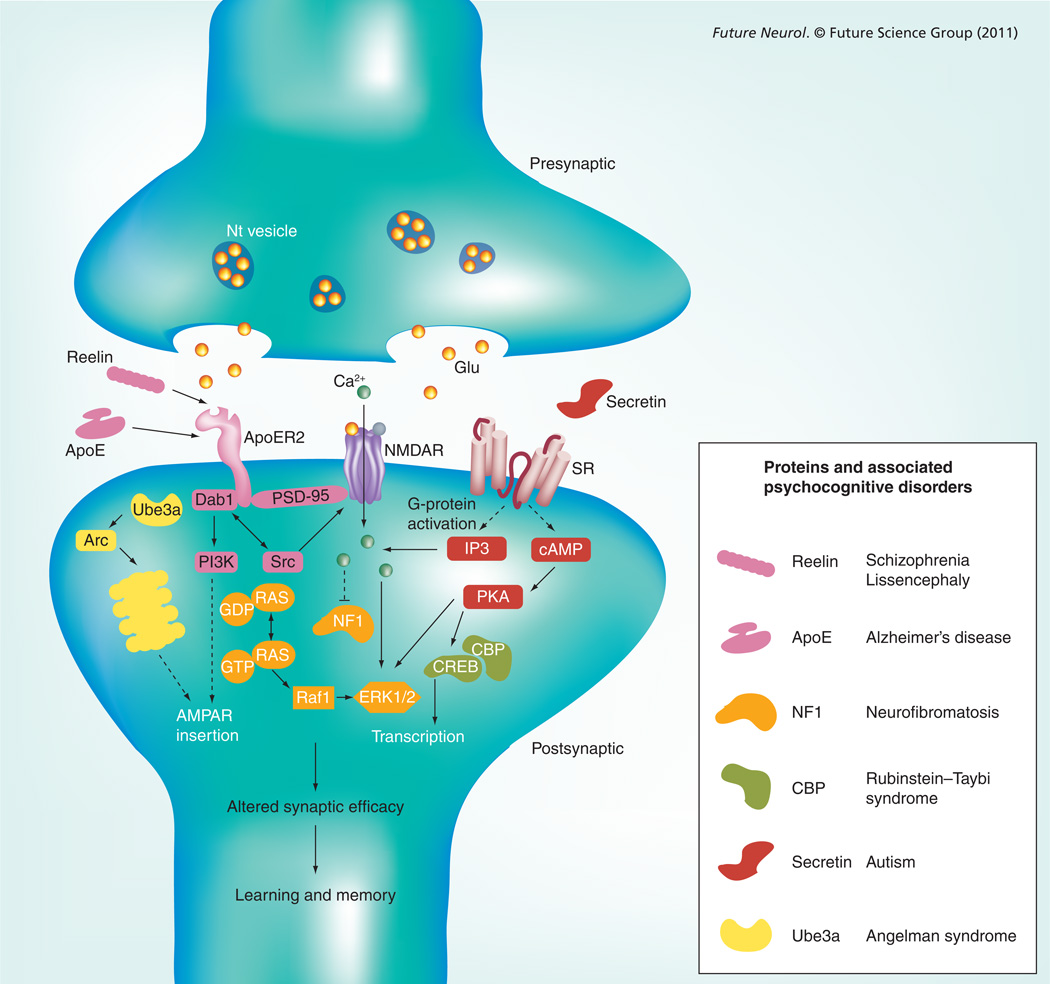
Figure 2. Model of Reelin, apoE, neurofibromatosis 1, CBP, secretin and Ube3a at the synapse.
The molecular machinery associated with signaling of Reelin, apoE, NF1, CBP, secretin and Ube3a are shown in the postsynaptic cell. Dotted lines are used to indicated transition through other signaling machinery not included (e.g., G-protein signaling). Individual pathways are color-coded and cross-talk is indicated by solid lines. Signaling through all six pathways ultimately affects synaptic function by the regulation of ion channels and/or by altering transcription, thereby modulating learning and memory. Genes, protein products and their associated cognitive disorders as discussed in the text are shown.
AMPAR: AMPA receptor; ApoER2: ApoE receptor 2; CBP: CREB binding protein; Glu: Glutamate; NF1: Neurofibromatosis 1; NMDAR: N-methyl-D-aspartate receptor; Nt: Neurotransmitter; SR: Secretin receptor.
Using mice to better understand the specific role of apoE in synaptic function in humans is difficult owing to the presence of three different APOE alleles in humans, whereas clonal mice express only a single apoE allele. The production of targeted replacement apoE isoform-expressing mice (apoE TR) under the control of the endogenous murine promoters represents a near-perfect model to address these questions [34]. The high degree of conservation between murine and human apoE receptors allows for these mice to be used as a general mammalian model for direct comparison of the actions of human apoE isoforms [35,36]. Interestingly, apoE isoform effects on LTP are region-specific. For example, perforant path LTP reveals that apoE3 TR mice show greater LTP induction than apoE2 TR mice, while apoE4 TR mice show the lowest (E3 > E2 > E4) [37]. In addition, in the perforant path, differential susceptibility to LTP inhibition by oligomeric amyloid-β is seen in a hierarchy that mirrors AD risk: apoE4 TR > apoE3 TR > apoE2 TR [37]. In contrast to perforant path LTP induction, CA1 LTP shows that there is an age-dependent enhancement of CA1 LTP in young apoE4 TR animals compared with strain- and age-matched wild-type mice [38]. A recent examination of all three genotypes in a single study using young adult mice demonstrated significantly greater LTP induction in apoE4 TR mice, less LTP induction in apoE2 TR mice and no difference compared with wild-type mice in apoE3 TR mice. Importantly, NMDAR-independent LTP shows no significant differences in any of the TR mice, suggesting an apoE isoform-dependent action on NMDAR function that is sufficient to influence stimulation-induced LTP induction. It should be noted that a similar LTP induction profile (apoE4 > apoE3 > apoE2) is seen in apoE-knockout hippocampal slices perfused with human recombinant apoE isoforms [39]. Taken together, these studies suggest that specific apoE isoforms act as signaling molecules in a way that is sufficient to affect synaptic plasticity in a subregion-specific manner. There have been a number of studies to behaviorally characterize apoE TR mice; however, results from multiple laboratories have been somewhat contradictory. In general, it can be stated that apoE4-expressing mice exhibit memory defects and increased anxiety, and that these behavioral phenotypes appear to affect females to a greater extent [40–42].
Reelin: lissencephaly & schizophrenia
Reelin is a large extracellular matrix protein that is secreted by Cajal–Retzius cells located in the marginal zone and serves as a molecular guiding cue for the subventricular migrating neurons during embryonic development of the CNS [43]. In the adult brain, Reelin is secreted by GABAergic interneurons after which it binds to two apoE-binding members of the lipoprotein receptor family, apoER2 and VLDLR (Figure 2). When bound by Reelin, signaling through these receptors results in phosphorylation of Dab1 and activation of the Src family of protein tyrosine kinases [30,44]. Disruption of Reelin signaling has been associated with the human developmental disorder lissencephaly [45]. Lissencephaly (smooth brain) is a rare genetic disorder characterized by the absence of the normal convolutions found in the cerebral cortex and is a prime example of the necessary role of Reelin signaling during embryonic development. Type 1 or ‘classic’ lissencephaly can occur through disruption of the NUDE and LIS1 genes, whose products function to stabilize microtubules and are downstream of Reelin–lipoprotein receptor activation [46]. Another form of lissencephaly that manifests with cerebellar hypoplasia, also known as Norman–Roberts syndrome, is caused by mutations in the Reelin gene (RELN) itself; therefore, Reelin may represent a critical link that connects the extracellular matrix with regulation of the cytoskeleton. Individuals with lissencephaly that manifests with cerebellar hypoplasia are severely ataxic, mentally retarded and suffer from epilepsy [47].
The human RELN gene is the ortholog of the extensively studied RELN gene in mice, which is mutated in the naturally occurring neurological mutant reeler [42,48]. Reeler mice, so named after their reeling gait, show abnormal cellular layering in the neocortex, cerebellum and hippocampus, and mice expressing a single mutant allele exhibit impaired learning and memory [28,49]. Although the histology of affected humans has not been characterized, the existence of pachygyria in these people strongly suggests that they have layering abnormalities. Thus, humans who are deficient in Reelin signaling seem to share several phenotypic features of reeler mice. While there is no current treatment for lissencephaly, the identification of the signaling defects in these patients provided a backdrop for further investigation into potentially important Reelin signaling in the adult brain beyond its role in developmental processes.
The known role for Reelin during development of the CNS was the basis for the ‘two hit hypothesis’ presented by Costa et al. for schizophrenia [50]. Similar to the ‘two hit hypothesis’ of other complex disorders, Costa’s hypothesis postulated that a prenatal ‘hit’, in this case genetic or environmental alterations, is paired with a ‘hit’ later in life, resulting in the disorder. A possible candidate for the first ‘hit’ was Reelin. Costa and others found that post-mortem brains of schizophrenic patients had an approximately 50% reduction in Reelin protein and mRNA [50]. Subsequent to these findings, a Reelin haploinsufficient mouse model was made. Further investigation with genetically unaltered wild-type mice found that perfusion of Reelin onto acute hippocampal slices significantly elevated the magnitude of LTP induced by high-frequency stimulation (HFS) [29]. Using whole-cell and field recording techniques, a possible mechanism for this phenomenon was found, with the identification of increased Ca2+ conductance following a brief application of Reelin [51,52]. This increase was associated with an increase in tyrosine phosphorylation of the NMDAR subunit NR2A [51]. Longer exposure of Reelin showed increased AMPA receptor insertion and a significant decrease in silent synapses (those synapses containing only NMDARs) [53]. The culmination of these synaptic changes suggests that alterations in adult Reelin signaling could affect hippocampal plasticity and cognitive ability. Moreover, in unpublished studies, our laboratory has found that wild-type mice treated with Reelin via bilateral ventricle cannulation show enhanced spatial and associative learning with a single injection. Taken together, the Reelin signaling pathway may provide a number of therapeutic targets for treatment of neuropsychiatric and neurodegenerative disorders associated with cognitive loss.
Neurofibromin: neurofibromatosis 1
Neurofibromatosis type 1 (NF1), formerly known as von Reklinghousen disease after the researcher who first documented the disorder, is one of the most common single-gene disorders and is associated with a predisposition to nerve sheath tumors, cognitive impairment and learning disability. The cognitive domains commonly affected include attention, executive function, language and visual perception [54]. The responsible gene, NF1, is located on chromosome 17 and encodes a 2818-amino acid protein named neurofibromin, which is expressed in highest abundance in neuronal tissue [55]. Pathological mutations range from single nucleotide substitutions to large-scale genomic deletions dispersed throughout the gene.
Neurofibromin has multiple functions including negative regulation of p21RAS, control of adenylyl cyclase activity and modulation of mammalian target of rapamycin (Figure 2). Each of these signaling molecules has repeatedly been demonstrated to be critical for synaptic plasticity and learning and memory [56], and could therefore contribute to the disruption in cognitive ability observed in individuals with NF1 The first attempt to model the disease in mice was made in 1994, whereby homozygous elimination of the NF1 gene proved to be lethal to embryos owing to developmental abnormalities in the heart and various neural crest-derived tissues [57]. Success followed a few years later when Silva et al. engineered a mouse carrying a heterozygous null (NF1+/−) that recapitulated the human symptoms of increased predisposition to tumors and compromised cognitive ability [58]. Specifically, the NF1+/− mice showed deficits in spatial learning in the hidden platform water maze and contextual fear conditioning.
NF1+/− mice have a decrease in the input–output function of extracellularly recorded excitatory postsynaptic potentials at the Schaffer collateral/CA1 synapse. They also show theta-burst stimulation (TBS)-induced LTP deficits, while a variety of presynaptic measures and short-term plasticity appear unaltered [59]. Interestingly, the LTP deficits of NF1+/− mice were revealed by TBS, but not HFS stimulation. In the hippocampus, GABAergic interneurons provide both feed forward and feedback inhibitory synaptic input onto pyramidal cells to time action potentials and exert control over network excitability [60]. LTP induced by TBS is more sensitive to changes in GABA-mediated inhibition than LTP induced by HFS [61]. This research found that evoked inhibitory postsynaptic potentials are increased in NF1+/− mice and that picrotoxin, a GABA-receptor antagonist, rescued the deficit in TBS-induced LTP [59]. Therefore, neurofibromin may be involved in the tuning of GABAergic transmission and inhibitory tone homeostasis in the hippocampus.
Neurofibromin contains a GTPase-activating protein domain, known to inhibit p21RAS-mediated signal transduction [62] that, when genetically compromised, selectively impacts cognitive ability but does not alter development or increase tumor susceptibility [59]. Therefore, it was postulated that learning impairments may arise distinctly from developmental abnormalities or increased tumor predisposition in NF1 Both the synaptic plasticity deficit and cognitive impairment in the NF1+/− mice have been subsequently linked with excessive RAS activity, a feature also observed in the human condition [63]. The learning problems, synaptic plasticity and the GABA inhibition demonstrated by the NF1+/− mice are all reversed by genetic and pharmacological reduction in RAS activity [59], suggesting that the human cognitive symptoms may be similarly improved by selective intervention at the site of p21RAS. The cholesterol-lowering drug lovastatin inhibits p21RAS by substrate depletion for post-translational protein farnesylation, a process that is necessary for localization of RAS to the cell membrane, where it performs the majority of its effector interactions. Silva’s group has recently demonstrated that the drug reverses synaptic plasticity, learning and attention deficits in the NF1+/− mice [64]. Currently, lovastatin is being investigated as a potential treatment for cognitive impairment in children with NF1.
Ube3A: Angelman syndrome
Angelman syndrome (AS) is a rare neurological disorder of maternal imprinting and disrupted ubiquitin ligase function characterized by developmental delay, severe intellectual disability, absent speech, motor impairment and epilepsy. AS is caused by various abnormalities of chromosome 15, resulting in the disruption of maternal UBE3A expression. The UBE3A gene has a spatially restricted imprinted expression pattern, showing imprinted expression in the brain, but biallelic expression in other tissues. Although the causative gene was identified in 1997 [65], the underlying pathophysiology of AS is still a matter of speculation. The gene product, Ube3A (also known as E6-AP), acts as an E3 ubiquitin–protein ligase within the ubiquitin proteasome pathway, wherein it marks specific target proteins for degradation.
Two mouse models were developed, each with a different strategy to disrupt UBE3A. The first mouse line, created by Gabriel and colleagues, utilized an Epstein–Barr virus latent membrane protein 2A transgenic insertion, which in effect deleted the entire equivalent AS critical region [66,67]. This is essentially what is observed in humans that exhibit the most common genetic defect leading to AS; an approximately 4–6 megabase deletion in the 15q11–13 region. Alternatively, the mouse model developed by Jiang and colleagues utilized a null mutation in the UBE3A exon 2 via a single gene knockout [68]. While both of these mouse models effectively disrupt UBE3A, there is some controversy over whether UBE3A deficiency in and of itself is responsible for the manifestation of AS or whether the full phenotype is due to the combined effect of disrupting several genes in the human chromosome 15q11–13 critical region [69]. Nevertheless, the UBE3A maternal deficient model (i.e., maternal-negative, paternal-positive UBE3A) created by Jiang and colleagues remains the best-characterized animal model of the disorder, and the following studies relate directly to this model [68].
Maternal-negative, paternal-positive UBE3A mice display behavioral phenotypes that emulate the human condition, including audiogenic-induced seizures, motor coordination deficits and reduced associative and spatial learning ability [70]. In addition, the mouse model shows normal synaptic transmission in the hippocampus, but it also shows a threshold-dependent LTP defect that can be overcome with multiple trains of HFS [71]. Interestingly, neither the mouse model nor human AS patients display gross neuroanatomical defects, suggesting that disruption of cognitive function in AS is due to altered synaptic plasticity and cellu-lar signaling. Two major findings in the mouse model indicate potential changes at a molecular level that may explain the overt phenotypes. First, UBE3A maternal deficiency results in a significant increase in hippocampal CaMKII, specifically at sites Thr286 (activating) and Thr305 (inhibiting), resulting in an overall reduction in phospho-CaMKII responsiveness to calcium–calmodulin activation. Importantly, the major phenotypes of the maternal-negative, paternal-positive UBE3A mouse can be rescued with the addition of mutations at the Thr305 inhibitory phosphorylation site [72]. Second, a target of UBE3A is the synaptic protein activity-related cytoskeleton-related protein, Arc (Figure 2) [73]. This well-investigated protein is shown to dramatically influence synaptic activity through the membrane insertion of AMPA-type receptors [74,75]. This suggests a link between Ube3a maternal deficiency, alterations in AMPA-dependent synaptic function, and calcium-dependent changes in CaMKII phosphorylation. While there is no current treatment for AS, these recent findings represent at least two major sites for potential therapeutic intervention.
CREB binding protein: Rubinstein-Taybi syndrome
Rubinstein–Taybi syndrome (RTS) was first described by Rubinstein and Taybi in 1963 and is characterized by severe cognitive disruption, growth retardation and a particular dysmorphology [76]. Several studies have shown that RTS patients have a variety of mutations in CREB and CREBBP, which encode CREB and CREB binding protein (CBP), respectively [77]. However, mutations in CREBBP account for less than half of all RTS cases [78], suggesting that at least one other gene is involved. Indeed, mutations causing RTS have also been found in the EP300 gene, which encodes p300 [79]. Both CBP and p300 are ubiquitously expressed, homologous proteins that act as transcriptional coactivators (Figure 2). Both proteins form a physical bridge between the DNA-binding transcription factors and the RNA polymerase II complex. Apart from this bridging or scaffolding function, CBP and p300 contain intrinsic histone acetyltransferase (HAT) activity in the carboxy-terminal domain that mediates acetylation of histone proteins (for review, see [80]). Acetylation neutralizes the positively charged lysine residues in histones and disrupts the interaction between histones and DNA, increasing DNA accessibility for transcription factors to activate gene expression [81].
To better understand the consequences of loss of these transcriptional coactivators on cognition, several mouse models have been generated that have complete or partial loss of CBP or p300 function. Unfortunately, p300 and CBP play a major role in development, making straight-forward interpretation of changes in synaptic function and memory formation in animals with life-long, global genetic disruption difficult. To avoid deleterious effects on development, two laboratories independently developed models of inducible CBP–HAT deficiency that was accomplished by coupling a CREBBP-dominant negative allele to an inducible forebrain-specific promoter (CBPHAT-) [82], and coupling a truncated form of CREBBP to an inducible forebrain-specific promoter (CBPA1) [83]. Both of these CBP mouse models exhibit significant deficits in the spatial water maze task and contextual fear conditioning [82]. Moreover, the CBPHAT- mice also exhibited deficits in novel object recognition [82].
To evaluate whether p300 plays a similar role in long-term memory formation, genetically modified mice expressing an inducible truncated form of p300 (p300Δl) that lacks the HAT domain were generated [84]. Similar to various CBP-deficient mouse models, p300Δl mice demonstrated compromised contextual fear conditioning and recognition memory. By contrast, these mice exhibited normal spatial learning in the hidden platform water maze task [84]. Taken together, these results suggest that derangement of histone acetylation has serious consequences on the formation of long-term memory and probably contributes to the mental impairments exhibited by individuals with RTS. This suggests the intriguing hypothesis that inhibitors of histone deacetylase activity, which would increase overall levels of histone acetylation and presumably compensate for the lack of CBP–HAT or p300–HAT activity in RTS, may ameliorate some or all cognitive impairment symptoms. Indeed, inhibition of histone deacetylase activity with various compounds restored normal hippocampus-dependent long-term memory formation in CBPHAT- and CBP+/− mice [85].
When synaptic physiology was examined at the Schaffer collateral/CAl synapse in the CBPΔ1 mouse, baseline electrophysiological properties and short-term plasticity were reported to be normal [83]. Curiously, a form of LTP enhancement achieved by a single 1-s, 100-Hz train delivered in the presence of a Dl dopaminergic agonist is impaired in the CBPΔ1 mice, but other forms of LTP induced by single or multiple 1-s, 100-Hz train(s) are normal. Similar plasticity phenotypes were observed in transgenic mice expressing KCREB, a dominant negative form of CREB [86], suggesting that while multiple forms of transcription-dependent potentiation can be experimentally induced at the Schaffer collateral/CAl synapse, some bypass the requirement for CBP-CREB mediated activation, raising the intriguing question of which transcriptional mechanisms mediate LTP in response to multiple tetanic trains.
Secretin: autism
One of the most interesting examples of disease investigation expanding our knowledge of synaptic function involves the secretin signaling system. Secretin is a 27-amino acid protein that is a member of the glucagon hormone family [87]. Originally isolated from the duodenum, secretin stimulates secretion of pancreatic fluids, and exposure to purified secretin is used as a test for diagnosis of digestive disorders and gastrointestinal function [88]. In 1998, Horvath et al. reported that autistic children receiving a purified porcine secretin showed improved overall digestive ability, as well as improvements in behavior, use of language and eye contact [89]. This study prompted the hypothesis of a ‘gut–brain’ connection in autism that links gastrointestinal disorders often observed in autistic children with brain dysfunctions. Unfortunately, further studies exploring the use of secretin as a possible treatment for autism have been disappointing, with only a few treatment studies showing any significant behavioral improvements [90,91]. As a result of this ambiguity, several studies have explored a possible role for secretin and the secretin receptor in synaptic plasticity and memory formation.
Secretin is expressed in many regions of the CNS, including the hippocampus [92]. Its receptor is a type II G-protein-coupled receptor that is positively coupled with intracellular cAMP, phospholipase and mitogen-activated protein kinase activity (Figure 2) [93–97]. In the absence of secretin or its receptor, mice undergo normal neuronal development and do not display gross changes in brain structure or inflammation. However, secretin receptor-knockout mice have specific deficits in social recognition [98], which are remarkably similar to social phobias that are common to autistic patients and mice that express Rett syndrome-associated, MeCP2 mutations [99]. Moreover, although secretin receptor-knockout mice have normal spatial learning in the hidden platform water maze, they have impaired reversal training [98]. This behavioral change is consistent with the repetitive behavioral phenotype common to autistic mouse models and characteristic of individuals with autism [100,101]. Mice that are deficient in either secretin or its receptor have impaired synaptic transmission between CA3 and CA1 of the hippocampus [98,102] and a concurrent reduced induction and maintenance of LTP in CA1 [98]. It is unclear whether the reduced LTP is due to altered synaptic connectivity or disruption in signal transduction, but perhaps the two are not mutually exclusive.
While secretin supplementation does not appear to be the ‘magic bullet’ for the treatment of autism that many hoped it would be, it may represent a single target in a multiple-target therapeutic strategy. However, it is clear that the secretin receptor system has been co-opted to serve functions in both the gastrointestinal system and the brain. Interestingly, this same apparent evolutionary strategy is seen for the family of lipoprotein receptors discussed earlier and may represent a common weak link for proteins that serve multiple roles in specific tissues throughout the body.
Conclusion
Advances in the past decade regarding the molecular basis of memory have not only led to a better understanding of how a typical brain works, but have also shed new light on our understanding of many pathologies of the nervous system, including diverse syndromes involving mental impairment. The multi-disciplinary analysis of various mouse models for human cognitive disorders has shown the power of animal models to produce an important leap forward in our understanding of complex mental diseases while simultaneously opening new avenues for their treatment. These studies also suggest that some of the cognitive and physiological deficits observed in mental impairment syndromes may not simply be caused by defects originating during development, but may result from the continued requirement of specific signal transduction cascades throughout life. The biochemical entities participating in signal transduction cascades that ultimately lead to functional and structural changes in the synapses are numerous. In this article, signaling molecules associated with various cognitive disorders have been considered; understanding their role in synaptic physiology and their contribution to learning and memory offers interesting and promising targets to address disorders of cognitive ability.
Future perspecitve
Over the past several years, tremendous progress has been made in developing technologies that can manipulate the murine genome. Soon, researchers will have an arsenal of tools that will allow them to define temporal and spatial properties of gene expression and specifically how genetic variation, such as that associated with disease, may lead to altered nervous system function. New knock-in strategies have been developed that allow investigators to introduce clinically relevant genetic variation. These new techniques will allow some of the most relevant questions facing neuroscience research to be addressed through the ability to: have a detailed map of the connectome; monitor changes in connectivity and neuron function in vivo during learning and memory; manipulate gene structure as a way to control gene expression; map the function of specific cell types and circuits and modulate them in a physiologically relevant manner; and track gene expression during development through to the aged brain. Combined with new genetic analyses of human disease states and disorders, the murine mouse model will continue to be used as the cornerstone for basic neuroscience research.
Executive summary.
Background
-
▪
Mouse models are particularly useful for studying single gene disorders.
-
▪
Models for cognitive disorders can shed light on the mechanisms of specific gene products.
Hippocampus & the measurement of synaptic plasticity
-
▪
The hippocampus is a region involved in memory formation.
-
▪
Electrophysiology of acute or cultured hippocampal slices can be used to measure synaptic transmission and plasticity.
-
▪
Mouse models for human cognitive disorders almost always have measurable defects in hippocampal synaptic function.
ApoE & lipoprotein receptors: Alzheimer's disease
-
▪
ApoE binds to a family of highly conserved lipoprotein receptors.
-
▪
Lipoprotein receptors are expressed in the CNS and have recently been shown to be important in learning and memory.
-
▪
Allelic variation of the apoE isoform is correlated with Alzheimer's disease risk.
-
▪
Current mouse models include a targeted replacement mouse model that expresses one of the three human isoforms of apoE under the endogenous mouse promoter.
-
▪
Mouse models using the apoE targeted replacement mouse models show that allelic variation has a differential effect on synaptic function.
Reelin: lissencephaly & schizophrenia
-
▪
Reelin is a large extracellular matrix protein.
-
▪
Reelin is involved in developmental neuronal migration, especially in the hippocampus, cerebellum and cortex.
-
▪
Reelin absence during development leads to lissencephaly (smooth brain), and postnatal reductions may be involved in memory disruption and schizophrenia.
-
▪
The link between Reelin deficiency in human cognitive disorders is unknown, but Reelin may represent a target for treatment in a number of human disorders.
Neurofibromin: neurofibromatosis 1
-
▪
Patients with neurofibromatosis type 1 (NF1) exhibit noncancerous fibroid tumors and cognitive disruption.
-
▪
NF1 negatively regulates p21-RAS.
-
▪
Reduction of RAS activity through a single gene mutation rescued the cognitive disruption in the mouse model.
-
▪
Current clinical trials are investigating the use of RAS inhibitors to treat NF1.
Ube3a: Angelman syndrome
-
▪
Angelman syndrome (AS) is due to the maternal disruption of the imprinted UBE3A gene.
-
▪
UBE3A codes for an E3 ubiquitin ligase.
-
▪
Maternal Ube3a deficiency presents with decreased CaMKII activity and altered regulatory phosphorylation of CaMKII.
-
▪
A site mutation of the autoinhibitory phosphorylation site of CaMKII rescued the AS mouse phenotype.
-
▪
The connection between Ube3a and CaMKII is unknown, but AS appears to be a biochemical defect, similarly to NF1, and not a development disorder.
CREB binding protein: Rubinstein-Taybi syndrome
-
▪
Rubinstein-Taybi syndrome shows a variety of mutations in CREBBP or in the p300 gene.
-
▪
CREB binding protein and p300 act as transcriptional coactivators.
-
▪
Mouse models for Rubinstein-Taybi syndrome show that synaptic plasticity defects can be overcome with certain forms of high-frequency stimulation.
-
▪
Some forms of synaptic plasticity are transcriptionally independent.
Secretin: autism
-
▪
Originally identified in the gastrointenstinal system, secretin has been found in the CNS.
-
▪
Mice deficient in secretin ligand or secretin receptor have impaired synaptic plasticity and learning.
-
▪
Secretin supplementation has not proven to be an effective treatment, but the secretin signaling system may represent a target for future autism therapeutics.
Conclusion
-
▪
Animal models of human cognitive disorders have limitations.
-
▪
Mouse models for human cognitive disorders can be useful in understanding the molecular mechanisms of specific gene disruption, and provide a model for the development of human therapeutics.
Acknowledgments
This work was supported by NIH Grant No. 1-R01-AG022574.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
Papers of special note have been highlighted as:
▪ of interest
▪▪ of considerable interest
- 1.Aboitiz F, Morales D, Montiel J. The evolutionary origin of the mammalian isocortex: towards an integrated developmental and functional approach. Behav. Brain Sci. 2003;26(5):535–552. doi: 10.1017/s0140525x03000128. discussion 52–85. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed OJ, Mehta MR. The hippocampal rate code: anatomy, physiology and theory. Trends Neurosci. 2009;32(6):329–338. doi: 10.1016/j.tins.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerr KM, Agster KL, Furtak SC, Burwell RD. Functional neuroanatomy of the parahippocampal region: the lateral and medial entorhinal areas. Hippocampus. 2007;17(9):697–708. doi: 10.1002/hipo.20315. [DOI] [PubMed] [Google Scholar]
- 4.Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347(6292):477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- 5.Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J. Neurosci. 1998;18(9):3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J. Neurosci. 1996;16(17):5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teyler TJ. Long-term potentiation and memory. Int. J. Neurol. 1987;21–22:163–171. [PubMed] [Google Scholar]
- 8.Teyler TJ, DiScenna P. Long-term potentiation. Annu. Rev. Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- 9.Teyler TJ, Fountain SB. Neuronal plasticity in the mammalian brain: relevance to behavioral learning and memory. Child Dev. 1987;58(3):698–712. [PubMed] [Google Scholar]
- 10.Racine RJ, Milgram NW, Hafner S. Long-term potentiation phenomena in the rat limbic forebrain. Brain Res. 1983;260(2):217–231. doi: 10.1016/0006-8993(83)90676-5. [DOI] [PubMed] [Google Scholar]
- 11.Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Res. 1987;435(1–2):227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- 12.Harris EW, Ganong AH, Cotman CW. Long-term potentiation in the hippocampus involves activation of N-methyl-D-aspartate receptors. Brain Res. 1984;323(1):132–137. doi: 10.1016/0006-8993(84)90275-0. [DOI] [PubMed] [Google Scholar]
- 13.Lu W, Man H, Ju W, Trimble WS, MacDonald JF, Wang YT. Activation of synaptic NMDA receptors induces membrane insertion of new AMPA receptors and LTP in cultured hippocampal neurons. Neuron. 2001;29(1):243–254. doi: 10.1016/s0896-6273(01)00194-5. [DOI] [PubMed] [Google Scholar]
- 14.Pickard L, Noel J, Duckworth JK, et al. Transient synaptic activation of NMDA receptors leads to the insertion of native AMPA receptors at hippocampal neuronal plasma membranes. Neuropharmacology. 2001;41(6):700–713. doi: 10.1016/s0028-3908(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 15.Grosshans DR, Clayton DA, Coultrap SJ, Browning MD. LTP leads to rapid surface expression of NMDA but not AMPA receptors in adult rat CA1. Nat. Neurosci. 2002;5(1):27–33. doi: 10.1038/nn779. [DOI] [PubMed] [Google Scholar]
- 16.Duguid I, Sjostrom PJ. Novel presynaptic mechanisms for coincidence detection in synaptic plasticity. Curr. Opin. Neurohiol. 2006;16(3):312–322. doi: 10.1016/j.conb.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Harris EW, Cotman CW. Long-term potentiation of guinea pig mossy fiber responses is not blocked by N-methyl D-aspartate antagonists. Neurosci. Lett. 1986;70(1):132–137. doi: 10.1016/0304-3940(86)90451-9. [DOI] [PubMed] [Google Scholar]
- 18.Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, Ap5. Nature. 1986;319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- 19.Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the ‘spatial mapping’ and ‘working memory’ theories of hippocampal function. Q. J. Exp. Psychol. B. 1986;38(4):365–395. [PubMed] [Google Scholar]
- 20.Abraham WC, Mason SE. Effects of the NMDA receptor/channel antagonists CPP and MK801 on hippocampal field potentials and long-term potentiation in anesthetized rats. Brain Res. 1988;462(1):40–46. doi: 10.1016/0006-8993(88)90582-3. [DOI] [PubMed] [Google Scholar]
- 21.Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281(5385):2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- 22. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. ▪ First report that a specific apoE isoform in humans increased the risk for Alzheimer’s disease.
- 23.Rebeck GW, Reiter JS, Strickland DK, Hyman BT. Apolipoprotein E in sporadic Alzheimer’s disease: allelic variation and receptor interactions. Neuron. 1993;11(4):575–580. doi: 10.1016/0896-6273(93)90070-8. [DOI] [PubMed] [Google Scholar]
- 24.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: high-avidity binding to β-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc. Natl Acad. Sci. USA. 1993;90(5):1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beffert U, Stolt PC, Herz J. Functions of lipoprotein receptors in neurons. J. Lipid Res. 2004;45(3):403–409. doi: 10.1194/jlr.R300017-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Koch S, Strasser V, Hauser C, et al. A secreted soluble form of apoE receptor 2 acts as a dominant-negative receptor and inhibits Reelin signaling. EMBO J. 2002;21(22):5996–6004. doi: 10.1093/emboj/cdf599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima K, Mikoshiba K, Miyata T, Kudo C, Ogawa M. Disruption of hippocampal development in vivo by CR-50 mAb against Reelin. Proc. Natl Acad. Sci. USA. 1997;94(15):8196–8201. doi: 10.1073/pnas.94.15.8196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qiu S, Korwek KM, Pratt-Davis AR, Peters M, Bergman MY, Weeber EJ. Cognitive disruption and altered hippocampus synaptic function in Reelin haploinsufficient mice. Neurohiol. Learn. Mem. 2006;85(3):228–242. doi: 10.1016/j.nlm.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 29.Weeber EJ, Beffert U, Jones C, et al. Reelin and apoE receptors cooperate to enhance hippocampal synaptic plasticity and learning. J. Biol. Chem. 2002;277(42):39944–39952. doi: 10.1074/jbc.M205147200. [DOI] [PubMed] [Google Scholar]
- 30.Trommsdorff M, Gotthardt M, Hiesberger T, et al. Reeler/disabled-like disruption of neuronal migration in knockout mice lacking the VLDL receptor and apoE receptor 2. Cell. 1999;97(6):689–701. doi: 10.1016/s0092-8674(00)80782-5. [DOI] [PubMed] [Google Scholar]
- 31.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr. Biol. 2003;13(1):18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 32.Beffert U, Morfini G, Bock HH, Reyna H, Brady ST, Herz J. Reelin-mediated signaling locally regulates protein kinase B/Akt and glycogen synthase kinase 3β. J. Biol. Chem. 2002;277(51):49958–49964. doi: 10.1074/jbc.M209205200. [DOI] [PubMed] [Google Scholar]
- 33.Beffert U, Weeber EJ, Morfini G, et al. Reelin and cyclin-dependent kinase 5-dependent signals cooperate in regulating neuronal migration and synaptic transmission. J. Neurosci. 2004;24(8):1897–1906. doi: 10.1523/JNEUROSCI.4084-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sullivan PM, Mezdour H, Aratani Y, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 1997;272(29):17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Iijima H, Goto K, et al. Human apolipoprotein E receptor 2. A novel lipoprotein receptor of the low density lipoprotein receptor family predominantly expressed in brain. J. Biol. Chem. 1996;271(14):8373–8380. doi: 10.1074/jbc.271.14.8373. [DOI] [PubMed] [Google Scholar]
- 36.Nimpf J, Schneider WJ. The VLDL receptor: an LDL receptor relative with eight ligand binding repeats, LR8. Atherosclerosis. 1998;141(2):191–202. doi: 10.1016/s0021-9150(98)00172-5. [DOI] [PubMed] [Google Scholar]
- 37.Trommer BL, Shah C, Yun SH, et al. ApoE isoform affects LTP in human targeted replacement mice. Neuroreport. 2004;15(17):2655–2658. doi: 10.1097/00001756-200412030-00020. [DOI] [PubMed] [Google Scholar]
- 38.Kitamura HW, Hamanaka H, Watanabe M, et al. Age-dependent enhancement of hippocampal long-term potentiation in knock-in mice expressing human apolipoprotein E4 instead of mouse apolipoprotein E. Neurosci. Lett. 2004;369(3):173–178. doi: 10.1016/j.neulet.2004.07.084. [DOI] [PubMed] [Google Scholar]
- 39.Korwek KM, Trotter JH, Ladu MJ, Sullivan PM, Weeber EJ. ApoE isoform-dependent changes in hippocampal synaptic function. Mol. Neurodegener. 2009;4:21. doi: 10.1186/1750-1326-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grootendorst J, Bour A, Vogel E, et al. Human apoE targeted replacement mouse lines: h-apoE4 and h-apoE3 mice differ on spatial memory performance and avoidance behavior. Behav. Brain Res. 2005;159(1):1–14. doi: 10.1016/j.bbr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 41.Bour A, Grootendorst J, Vogel E, et al. Middle-aged human apoE4 targeted-replacement mice show retention deficits on a wide range of spatial memory tasks. Behav. Brain Res. 2008;193(2):174–182. doi: 10.1016/j.bbr.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 42.Siegel JA, Haley GE, Raber J. Apolipoprotein E isoform-dependent effects on anxiety and cognition in female TR mice. Neurohiol. Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374(6524):719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 44.D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24(2):471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 45.Hong SE, Shugart YY, Huang DT, et al. Autosomal recessive l issencephaly with cerebellar hypoplasia is associated with human RELN mutations. Nat. Genet. 2000;26(1):93–96. doi: 10.1038/79246. [DOI] [PubMed] [Google Scholar]
- 46.Ross ME, Swanson K, Dobyns WB. Lissencephaly with cerebellar hypoplasia (LCH): a heterogeneous group of cortical malformations. Neuropediatrics. 2001;32(5):256–263. doi: 10.1055/s-2001-19120. [DOI] [PubMed] [Google Scholar]
- 47.Hourihane JO, Bennett CP, Chaudhuri R, Robb SA, Martin ND. A sibship with a neuronal migration defect, cerebellar hypoplasia and congenital lymphedema. Neuropediatrics. 1993;24(1):43–46. doi: 10.1055/s-2008-1071511. [DOI] [PubMed] [Google Scholar]
- 48.Hirotsune S, Takahara T, Sasaki N, et al. The reeler gene encodes a protein with an EGF-like motif expressed by pioneer neurons. Nat. Genet. 1995;10(1):77–83. doi: 10.1038/ng0595-77. [DOI] [PubMed] [Google Scholar]
- 49.Hamburgh M. Analysis of the postnatal developmental effects of’ Reeler’, a neurological mutation in mice. A study in developmental genetics. Dev. Biol. 1963;19:165–185. doi: 10.1016/0012-1606(63)90040-x. [DOI] [PubMed] [Google Scholar]
- 50. Impagnatiello F, Guidotti AR, Pesold C, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc. NatlAcad. Sci. USA. 1998;95(26):15718–15723. doi: 10.1073/pnas.95.26.15718. ▪▪ First report of the protein reelin as a possible biochemical component of the two-hit hypothesis.
- 51.Beffert U, Weeber EJ, Durudas A, et al. Modulation of synaptic plasticity and memory by Reelin involves differential splicing of the lipoprotein receptor apoER2. Neuron. 2005;47(4):567–579. doi: 10.1016/j.neuron.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 52.Chen Y, Beffert U, Ertunc M, et al. Reelin modulates NMDA receptor activity in cortical neurons. J. Neurosci. 2005;25(36):8209–8216. doi: 10.1523/JNEUROSCI.1951-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qiu S, Zhao LF, Korwek KM, Weeber EJ. Differential Reelin-induced enhancement of NMDA and AMPA receptor activity in the adult hippocampus. J. Neurosci. 2006;26(50):12943–12955. doi: 10.1523/JNEUROSCI.2561-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.North KN, Riccardi V, Samango-Sprouse C, et al. Cognitive function and academic performance in neurofibromatosis. 1: consensus statement from the NF1 Cognitive Disorders Task Force. Neurology. 1997;48(4):1121–1127. doi: 10.1212/wnl.48.4.1121. [DOI] [PubMed] [Google Scholar]
- 55.Daston MM, Scrable H, Nordlund M, Sturbaum K, Nissen LM, Ratner N. The protein product of the neurofibromatosis type 1 gene is expressed at highest abundance in neurons, Schwann cells, and oligodendrocytes. Neuron. 1992;8(3):415–428. doi: 10.1016/0896-6273(92)90270-n. [DOI] [PubMed] [Google Scholar]
- 56.Mazzucchelli C, Brambilla R. Ras-related and MAPK signalling in neuronal plasticity and memory formation. Cell. Mol. Life Sci. 2000;57(4):604–611. doi: 10.1007/PL00000722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brannan CI, Perkins AS, Vogel KS, et al. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8(9):1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- 58. Silva AJ, Frankland PW, Marowitz Z, et al. A mouse model for the learning and memory deficits associated with neurofibromatosis type I. Nat. Genet. 1997;(3):281–284. doi: 10.1038/ng0397-281. ▪▪ Model for neurofibromatosis-1 demonstrating that a specific signal transduction pathway was potentially involved in a human cognitive disruption disorder.
- 59.Costa RM, Federov NB, Kogan JH, et al. Mechanism for the learning deficits in a mouse model of neurofibromatosis type 1. Nature. 2002;415(6871):526–530. doi: 10.1038/nature711. [DOI] [PubMed] [Google Scholar]
- 60.Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293(5532):1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- 61.Chapman CA, Perez Y, Lacaille JC. Effects of GABA inhibition on the expression of long-term potentiation in CAl pyramidal cells are dependent on tetanization parameters. Hippocampus. 1998;8(3):289–298. doi: 10.1002/(SICI)1098-1063(1998)8:3<289::AID-HIPO10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 62.Martin GA, Viskochil D, Bollag G, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with Ras p21. Cell. 1990;63(4):843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- 63.Klose A, Ahmadian MR, Schuelke M, et al. Selective disactivation of neurofibromin GAP activity in neurofibromatosis type 1. Hum. Mol. Genet. 1998;7(8):1261–1268. doi: 10.1093/hmg/7.8.1261. [DOI] [PubMed] [Google Scholar]
- 64.Li W, Cui Y, Kushner SA, et al. The HMG-CoA reductase inhibitor lovastatin reverses the learning and attention deficits in a mouse model of neurofibromatosis type 1. Curr. Biol. 2005;15(21):1961–1967. doi: 10.1016/j.cub.2005.09.043. [DOI] [PubMed] [Google Scholar]
- 65.Kishino T, Lalande M, Wagstaff J. UBE3A/E6-AP mutations cause Angelman syndrome. Nat. Genet. 1997;15(1):70–73. doi: 10.1038/ng0197-70. [DOI] [PubMed] [Google Scholar]
- 66.Albrecht U, Sutcliffe JS, Cattanach BM, et al. Imprinted expression of the murine Angelman syndrome gene, Ube3a, in hippocampal and Purkinje neurons. Nat. Genet. 1997;17(1):75–78. doi: 10.1038/ng0997-75. [DOI] [PubMed] [Google Scholar]
- 67.Gabriel JM, Merchant M, Ohta T, et al. A transgene insertion creating a heritable chromosome deletion mouse model of Prader-Willi and Angelman syndromes. Proc. Nat lAcad. Sci. USA. 1999;96(16):9258–9263. doi: 10.1073/pnas.96.16.9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Jiang YH, Armstrong D, Albrecht U, et al. Mutation of the Angelman ubiqukin ligase in mice causes increased cytoplasmic p53 and deficits of contextual learning and long-term potentiation. Neuron. 1998;21(4):799–811. doi: 10.1016/s0896-6273(00)80596-6. [DOI] [PubMed] [Google Scholar]
- 69.Lee S, Wevrick R. Identification of novel imprinted transcripts in the Prader-Willi syndrome and Angelman syndrome deletion region: further evidence for regional imprinting control. Am. J. Hum. Genet. 2000;66(3):848–858. doi: 10.1086/302817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miura K, Kishino T, Li E, et al. Neurobehavioral and electroencephalographic abnormalities in Ube3a maternal-deficient mice. Neurobiol. Dis. 2002;9(2):149–159. doi: 10.1006/nbdi.2001.0463. [DOI] [PubMed] [Google Scholar]
- 71.Weeber EJ, Jiang YH, Elgersma Y, et al. Derangements of hippocampal calcium/ calmodulin-dependent protein kinase II in a mouse model for Angelman mental retardation syndrome. J. Neurosci. 2003;23(7):2634–2644. doi: 10.1523/JNEUROSCI.23-07-02634.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. van Woerden GM, Harris KD, Hojjati MR, et al. Rescue of neurological deficits in a mouse model for Angelman syndrome by reduction of α CaMKII inhibitory phosphorylation. Nat. Neurosci. 2007;10(3):280–282. doi: 10.1038/nn1845. ▪▪ Reports a complete recovery of the mouse model phenotype using a genetic rescue technique.
- 73.Greer PL, Hanayama R, Bloodgood BL, et al. The Angelman syndrome protein Ube3A regulates synapse development by ubiquitinating arc. Cell. 2010;140(5):704–716. doi: 10.1016/j.cell.2010.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shepherd JD, Rumbaugh G, Wu J, et al. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52(3):475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chowdhury S, Shepherd JD, Okuno H, et al. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52(3):445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rubinstein JH, Taybi H. Broad thumbs and toes and facial abnormalities. A possible mental retardation syndrome. Am. J. Dis. Child. 1963;105:588–608. doi: 10.1001/archpedi.1963.02080040590010. [DOI] [PubMed] [Google Scholar]
- 77.Blough RI, Petrij F, Dauwerse JG, et al. Variation in microdeletions of the cyclic AMP-responsive element-binding protein gene at chromosome band I6pl3.3 in the Rubinstein-Taybi syndrome. Am. J. Med. Genet. 2000;90(1):29–34. doi: 10.1002/(sici)1096-8628(20000103)90:1<29::aid-ajmg6>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 78.Bartsch O, Schmidt S, Richter M, et al. DNA sequencing of CREBBP demonstrates mutations in 56% of patients with Rubinstein–Taybi syndrome (RSTS) and in another patient with incomplete RSTS. Hum. Genet. 2005;117(5):485–493. doi: 10.1007/s00439-005-1331-y. [DOI] [PubMed] [Google Scholar]
- 79.Roelfsema JH, White SJ, Ariyurek Y, et al. Genetic heterogeneity in Rubinstein-Taybi syndrome: mutations in both the CBP and EP300 genes cause disease. Am. J. Hum. Genet. 2005;76(4):572–580. doi: 10.1086/429130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chan HM, Krstic-Demonacos M, Smith L, Demonacos C, La Thangue NB. Acetylation control of the retinoblastoma tumour-suppressor protein. Nat. Cell Biol. 2001;3(7):667–674. doi: 10.1038/35083062. [DOI] [PubMed] [Google Scholar]
- 81.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389(6649):349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 82.Korzus E, Rosenfeld MG, Mayford M. CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron. 2004;42(6):961–972. doi: 10.1016/j.neuron.2004.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wood MA, Kaplan MP, Park A, et al. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn. Mem. 2005;12(2):111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oliveira AM, Wood MA, McDonough CB, Abel T. Transgenic mice expressing an inhibitory truncated form of p300 exhibit long-term memory deficits. Learn. Mem. 2007;14(9):564–572. doi: 10.1101/lm.656907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Alarcon JM, Malleret G, Touzani K, et al. Chromatin acetylation, memory, and LTP are impaired in CBP+/− mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron. 2004;42(6):947–959. doi: 10.1016/j.neuron.2004.05.021. ▪▪ First report that genetic modulation through histone acetylation is a mechanism underlying cognitive disruption in Rubinstein–Taybi syndrome.
- 86.Pittenger C, Huang YY, Paletzki RF, et al. Reversible inhibition of CREB/ATF transcription factors in region CAl of the dorsal hippocampus disrupts hippocampus-dependent spatial memory. Neuron. 2002;34(3):447–462. doi: 10.1016/s0896-6273(02)00684-0. [DOI] [PubMed] [Google Scholar]
- 87.Bayliss WM, Starling EH. The mechanism of pancreatic secretion. J. Physiol. 1902;28(5):325–353. doi: 10.1113/jphysiol.1902.sp000920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chey WY, Chang TM. Secretin, 100 years later. J. Gastroenterol. 2003;38(11):1025–1035. doi: 10.1007/s00535-003-1235-3. [DOI] [PubMed] [Google Scholar]
- 89. Horvath K, Stefanatos G, Sokolski KN, Wachtel R, Nabors L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. J. Assoc. Acad. Minor. Phys. 1998;9(1):9–15. ▪ Reports the use of the secretin protein for the treatment of autism.
- 90.Kern JK, Van Miller S, Evans PA, Trivedi MH. Efficacy of porcine secretin in children with autism and pervasive developmental disorder. J. Autism Dev. Disord. 2002;32(3):153–160. doi: 10.1023/a:1015441428154. [DOI] [PubMed] [Google Scholar]
- 91.Esch BE, Carr JE. Secretin as a treatment for autism: a review of the evidence. J. Autism Dev. Disord. 2004;34(5):543–556. doi: 10.1007/s10803-004-2549-6. [DOI] [PubMed] [Google Scholar]
- 92.Tay J, Goulet M, Rusche J, Boismenu R. Age-related and regional differences in secretin and secretin receptor mRNA levels in the rat brain. Neurosci. Lett. 2004;366(2):176–181. doi: 10.1016/j.neulet.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 93.Dong M, Miller LJ. Molecular pharmacology of the secretin receptor. Receptors Channels. 2002;8(3–4):189–200. [PubMed] [Google Scholar]
- 94.Gardner JD, Conlon TP, Adams TD. Cyclic AMP in pancreatic acinar cells: effects of gastrointestinal hormones. Gastroenterology. 1976;70(1):29–35. [PubMed] [Google Scholar]
- 95.Vilardaga JP, Ciccarelli E, Dubeaux C, De Neef P, Bollen A, Robberecht P. Properties and regulation of the coupling to adenylate cyclase of secretin receptors stably transfected in Chinese hamster ovary cells. Mol. Pharmacol. 1994;45(5):1022–1028. [PubMed] [Google Scholar]
- 96.Mayo KE, Miller LJ, Bataille D, et al. International Union of Pharmacology. XXXV. The glucagon receptor family. Pharmacol. Rev. 2003;55(1):167–194. doi: 10.1124/pr.55.1.6. [DOI] [PubMed] [Google Scholar]
- 97.Van Rampelbergh J, Poloczek P, Francoys I, et al. The pituitary adenylate cyclase activating polypeptide (PACAP I) and VIP (PACAP II VIPl) receptors stimulate inositol phosphate synthesis in transfected CHO cells through interaction with different G proteins. Biochim. Biophys. Acta. 1997;1357(2):249–255. doi: 10.1016/s0167-4889(97)00028-1. [DOI] [PubMed] [Google Scholar]
- 98. Nishijima I, Yamagata T, Spencer CM, et al. Secretin receptor-deficient mice exhibit impaired synaptic plasticity and social behavior. Hum. Mol. Genet. 2006;15(21):3241–3250. doi: 10.1093/hmg/ddl402. ▪ Discusses a mouse model used to determine the role of secretin in neuronal function and memory formation.
- 99.Shahbazian M, Young J, Yuva-Paylor L, et al. Mice with truncated MeCP2 recapitulate many Rett syndrome features and display hyperacetylation of histone H3. Neuron. 2002;35(2):243–254. doi: 10.1016/s0896-6273(02)00768-7. [DOI] [PubMed] [Google Scholar]
- 100.Hollander E, King A, Delaney K, Smith CJ, Silverman JM. Obsessive-compulsive behaviors in parents of multiplex autism families. Psychiatry Res. 2003;117(1):11–16. doi: 10.1016/s0165-1781(02)00304-9. [DOI] [PubMed] [Google Scholar]
- 101.Crawley JN. Designing mouse behavioral tasks relevant to autistic-like behaviors. Ment. Retard. Dev. Disahil. Res. Rev. 2004;10(4):248–258. doi: 10.1002/mrdd.20039. [DOI] [PubMed] [Google Scholar]
- 102.Yamagata T, Urano H, Weeber EJ, Nelson DL, Nishijima I. Impaired hippocampal synaptic function in secretin deficient mice. Neuroscience. 2008;154(4):1417–1422. doi: 10.1016/j.neuroscience.2008.04.037. [DOI] [PubMed] [Google Scholar]