Abstract
The kinase RIP1 wears a coat of many colors during TNF receptor signaling and can regulate both activation of pro-survival NFκB and programmed cell death pathways. In this review, we outline how coating RIP1 with K63-linked ubiquitin chains forms a protective layer that prevents RIP1 from binding apoptotic regulators and serves as an early guard against cell death. Further on, binding of NFκB signaling components to the ubiquitin coat of RIP1 activates long-term pro-survival signaling and forms a more impenetrable suit of armor against cell death. If RIP1 is not decorated with ubiquitin chains it becomes an unstoppable harbinger of bad news: programmed cell death.
Keywords: TNF, RIP, IAP, TRAF, apoptosis, necrosis, NFκB, ubiquitin
Ligation of TNF Receptor 1 (TNFR1) can lead to either cell survival or cell death, often within a single cell type.1 The mechanism that determines entry into either of the two diametrically opposing effects remains incompletely understood. The prevailing model in the last dozen years has been that activation of the NFκB transcription factors by TNF and the subsequent induction of pro-survival genes inhibits the cell death pathway that may be concurrently activated.2–4 In this model, NFκB-mediated gene transcription therefore functions as a cell death checkpoint: a productive NFκB response leads to survival whereas an absence of NFκB leads to death.5 When most cell types are treated with TNF alone, very little cell death is observed unless a protein synthesis inhibitor (e.g., cycloheximide) is also present, most likely because cycloheximide is blocking the synthesis of NFκB-regulated survival genes. This well-documented effect of cycloheximide suggests (1) the survival response is generally dominant; and (2) the death signaling machinery is pre-existing and does not require new protein synthesis. If NFκB-mediated gene transcription is the sole cell death checkpoint in TNFR1 signaling as the current model suggests, why is the survival response the dominant response when it is dependent on intracellular signaling, gene transcription and protein synthesis? Why is the death response not the dominant response when it is ready to go and does not require new protein synthesis? We have termed this conundrum the “NFκB paradox” and it suggests that there may be additional cell death checkpoints.
Once the verdict to die has been delivered, the manner by which the death sentence is to be carried out has to be decided. TNF can induce caspase-dependent apoptosis and the sequelae of signaling events that drive this process have been studied to death. For the purpose of this discussion, we have defined apoptosis as cell death that is strictly dependent on caspase activity. However, gathering reports in the literature suggest that there is much more to death than apoptosis. If caspase activity is devoid during death receptor signaling, many cell types can switch to a caspase-independent necrotic cell death pathway. Instead of the neat partitioning of the dying cell into the apoptotic bodies characteristic of caspase-driven cell death, cells dying by programmed necrosis lose their plasma membrane integrity.6,7 Various pathophysiological situations involve necrosis as a mediator of tissue damage such as stroke,8,9 heart attack10 and acute pancreatitis.11 Pro-inflammatory cytokines, especially TNF, are implicated in the tissue damage that occurs.12–14 Like apoptosis, specific signaling events control this necrotic cell death program. Top billing in this program goes to the kinase RIP1,6,7,15–17 a dual-function regulator of cell viability after TNF stimulation.
RIP1 is absolutely essential for activation of NFκB-mediated gene transcription by TNFR1,18,19 and some other receptors of the innate immune system, such as TLR3.20 NFκB drives expression of genes that prevent caspase activation, such as cFLIP,3,21 thus RIP1 transmits a pro-survival signal. TNFR1 can trigger apoptosis in the absence of RIP1, but if the ubiquitination of RIP1 is blocked, RIP1 binds Caspase 8 and enhances apoptosis. The kinase activity of RIP1 is dispensable both for its function as a pro-survival NFκB signaling molecule and its apoptosis-enhancing activity. In contrast, when Caspase 8 is inactive, RIP1 kinase activity is crucial for programmed necrosis to occur in response to many stimuli.15,16,22 Necrostatin 1, a specific inhibitor of the kinase activity of RIP1,23 protects the heart and brain from ischaemia-reperfusion injury in vivo.9,10 Although some of the core components of the signaling machinery required for programmed necrosis have recently been identified,24 very few details of the signaling events that control programmed necrosis and the function of this cell death pathway in vivo have been discovered. In this perspective, we will give an overview of the recent studies of RIP1 signaling mechanisms and discuss the cell death checkpoints that determine whether RIP1 triggers apoptosis or necrosis.
The RIP family comprises seven members with a kinase domain related to RIP1.25 RIP kinases are distinguished by the additional effector or protein interaction domains they contain, which enable them to plug into different signaling pathways. These domains have disparate functions, for example, the CARD domain in RIP2 is required for innate immune responses to intracellular bacteria recognized by the NOD-like receptors.26 RIP1 is unique as it contains a death domain that can bind death domains in other signaling molecules, for example FADD and TRADD, utilized by death receptors which trigger caspase activation.27
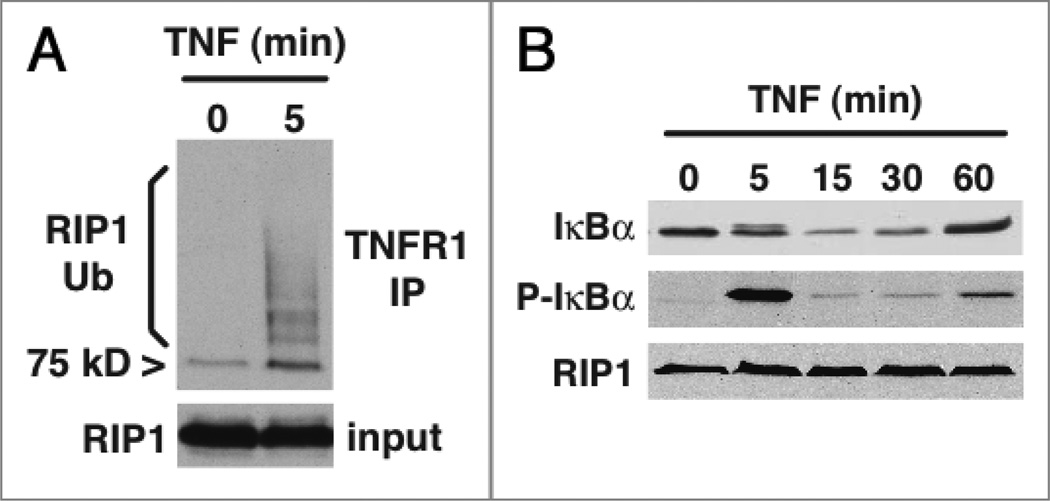
Within minutes of TNFR1 stimulation, the RIP1 protein recruited to the receptor undergoes a specialized type of nondegradative ubiquitination (Fig. 1A).28 The ubiquitin E3 ligases TRAF2, cIAP1 and cIAP2 catalyse the attachment of K63- linked polyubiquitin chains29–32 to lysine 377 of RIP1.33,34 These polyubiquitin chains serve as a scaffold for the binding of adaptor molecules that contain ubiquitin recognition domains.35 The NFκB essential modifier protein NEMO is one of the adaptor molecules that specifically docks with ubiquitinated RIP1.33,36 NEMO binding mediates recruitment of the IκBα kinase complex and subsequent activation of NFκB transcription factors. Various anti-apoptotic proteins are synthesized as part of this NFκB transcriptional program, comprising a well-characterized cell survival strategy. Not surprisingly, mutation of lysine 377 of RIP1 to arginine (RIP1-K377R) enhances apoptosis due to abrogated activation of the NFκB pathway. However, it takes a certain amount of time for pro-survival gene expression pathways to come into effect. For example, if we look at the time-course of re-expression of IκBα after TNF treatment, as an example of an immediate early NFκB-inducible gene, the protein is not detectable until at least one-hour post stimulation (Fig. 1B). During this first hour of receptor ligation, the potentially pro-death signaling molecule RIP1 is bound to TNFR1. So what prevents RIP1 from engaging cell death pathways via this early TNFR1 complex?4 We have recently shown that the K63-linked ubiquitination of RIP1 provides a pro-survival signal early after TNFR1 stimulation that does not require NFκBdriven transcription.37 Expression of RIP1-K377R sensitizes T cells to TNF-induced apoptosis at early time-points, before the protective effect of NFκB activation is evident. Loss of the E3 ligases that ubiquitinate RIP1 has the same pro-death effect,31,38 supporting the hypothesis that ubiquitinated RIP1 is unable to engage apoptotic pathways. RIP1-K377R rapidly forms a complex with Caspase 8 after TNFR1 ligation and this indicates that if the ubiquitination of RIP1 is blocked, RIP1 becomes a pro-death signaling molecule that turns on Caspase 8 and apoptosis signaling. So what prevents ubiquitinated RIP1 from binding to Caspase 8? Our subsequent studies have shown that interaction of the adaptor molecule NEMO with ubiquitinated RIP1 prevents RIP1 from binding Caspase 8 and enhancing apoptosis.39 NEMO-deficient T cells undergo apoptosis very rapidly in response to TNF, more so than NEMO-sufficient T cells in which all NFκB-mediated pro-survival signaling has been blocked by co-expression of the non-degradable IκBαSR. Therefore, the protective effect of NEMO on cell survival does not totally depend on NFκB-mediated gene transcription, revealing an entirely new function for the NEMO signaling adaptor. NEMO mutants that are unable to recognize ubiquitinated RIP1,33,36 are unable to protect against apoptosis.39 These studies revealed the biochemical basis for RIP1’s dual function in cell survival and cell death. K63-linked polyubiquitination of RIP1 provides two barriers to apoptosis. At early time-points, this modification prevents RIP1 from binding to and activating Caspase 8; at later time-points ubiquitination results in pro-survival NFκB activity. If RIP1 is not ubiquitinated, it rapidly forms a complex with Caspase 8 and becomes a potent trigger of apoptosis.
Figure 1.
TNF induces rapid ubiquitination of RIP1. (A) Jurkat T cells were stimulated with 100 ng/ml human TNF for 5 minutes and TNFR1 was immunoprecipitated from the cytoplasmic protein fraction. The TNFR1 complex and a sample of the cytoplasmic lysate were probed by western blot for RIP1. The TNFR1 complex recruits the 75 kD RIP1 protein but the majority of the receptor-associated RIP1 undergoes modification consistent with ubiquitination (RIP1 Ub). (B) Jurkat T cells were stimulated with 100 ng/ml human TNF and cytoplasmic protein samples were probed by western blot for phosphorylated IκBα, total I㮫α and RIP1 as a loading control. Evidence of IKK activity is seen 5 minutes post-stimulation by the appearance of phosphorylated IκBα, co-incident with the polyubiquitination of receptor-associated RIP1 shown in (A). IκBα is then degraded but re-expression of this immediate early NFκB-inducible gene is not observable at the protein level until approximately one hour post stimulation. Therefore, the potentially pro-death RIP1 protein is bound to TNFR1 during the first hour of TNF stimulation before the appearance of NFκB-dependent gene products.
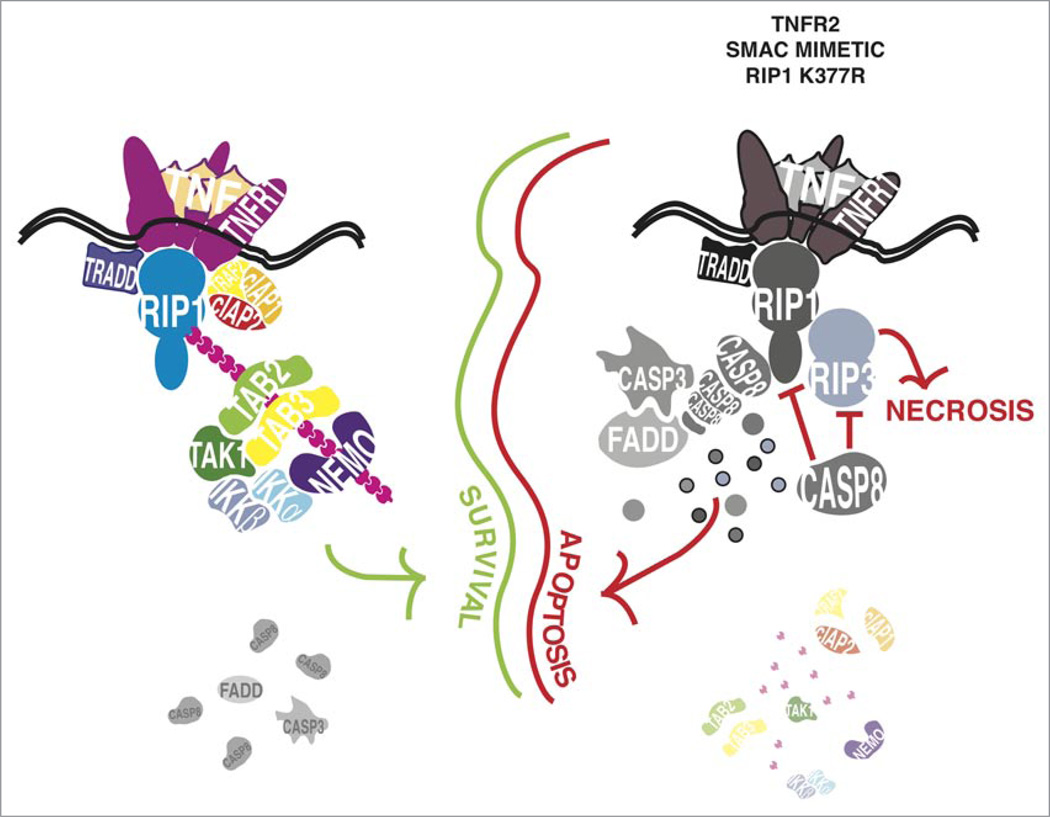
The above observations led us to propose a new model that resolves the ‘NFκB paradox’. This new model posits that there are two sequential cell death checkpoints during TNF signaling. The first checkpoint involves the ubiquitination of RIP1, followed by its sequestration by NEMO thus keeping RIP1 away from Caspase 8 (Fig. 2). This initial checkpoint is transcription alter independent, likely to be transient in nature, and when it fails, RIP1 initiates the death signaling cascade. If the initial checkpoint is intact, i.e., RIP1 is prevented from interacting with Caspase 8, then the association of ubiquitinated RIP1 with the TAK1 and IKK kinase complexes leads to NFκB-mediated transcription. The second cell death checkpoint is dependent on NFκB-mediated induction of pro-survival genes and this provides a long-lasting protection from death. If this second checkpoint fails, death also ensues but in this case, the death signaling cascade is not initiated by RIP1. Rather, the lack of NFκB-mediated induction of pro-survival genes such as c-FLIP, concurrent with the clustering of Caspase 8 by other signaling adapters, leads to apoptosis. Thus, the function of the first cell death checkpoint is to hold the pre-existing death signaling machinery in check, allowing time for the NFκB-dependent pro-survival genes to come into effect to provide a more permanent protection from death.
Figure 2.
Ubiquitination of RIP1 is an early pro-survival checkpoint in TNF signaling. TNFR1 ligation triggers formation of a complex of TRADD, RIP1, TRAF2, cIAP1 and cIAP2 and the latter three enzymes catalyse the formation of K63-linked polyubiquitin chains on lysine 377 of RIP1. The K63-linked polyubiquitin chains form a docking site for proteins that contain a ubiquitin recognition motif such as TAB2, TAB3 and NEMO. These adaptor proteins recruit and activate the TAK1 and IKK complexes, which lead to phosphorylation and degradation of IκB. Formation of this complex on the ubiquitinated RIP1 prevents RIP1 from interacting with Caspase 8 and activating the apoptotic machinery. If the lysine 377 ubiquitin acceptor site of RIP1 is mutated or the E3 ligase enzymes cIAP1 and cIAP2 are degraded by treatment with SMAC mimetic or through TNFR2 ligation, non-ubiquitinated RIP1 rapidly forms a complex with Caspase 8 and turns on the apoptotic machinery. RIP1 and RIP3 are themselves substrates for Caspase 8, which may prevent them from triggering the alternate death pathway of programmed necrosis. If the activity of Caspase 8 is inhibited, RIP1 forms a complex with RIP3 and triggers cell death by necrosis, which is dependent on the kinase activity of RIP1 and RIP3. While binding of NEMO to ubiquitinated RIP1 is an important factor in this early pro-survival event, it remains unclear what contribution the binding of TAB2 and TAB3 or other proteins with a ubiquitin recognition domain might make in this pro-survival step. Similarly, it remains to be seen whether the TAK1 and IKK kinase complexes participate in a transcription-independent manner in this early pro-survival checkpoint, prior to long-term anti-death gene expression programs coming into effect.
The first cell death checkpoint also provides an instance in which the decision to live or die can be rapidly made. This can be achieved through the attachment or removal of ubiquitin chains from RIP1 dependent on the activity of E3 ligases and deubiquitinases, respectively (Fig. 2). Expression levels of E3 ligases and deubiquitinases can be regulated transcriptionally and post-translationally by signals emanating from other receptors. So what are the physiological triggers that could alter the ubiquitination status of RIP1 and permit RIP1 to enhance apoptosis? TNFR2 is another receptor that can bind TNF and is induced in T cells by TCR activation40 or expressed by other cell types in response to viral infection.41,42 Ligation of TNFR2 results in degradation of the E3 ligases TRAF2, cIAP1 and cIAP2;43,44 this correlates with TNFR2’s ability to enhance apoptosis.45 Therefore, we would predict that TNFR2 signaling enhances apoptosis by abrogating the ubiquitination of RIP1. Similarly, other members of the TNF receptor superfamily have also been reported to trigger degradation of TRAF2 and alter expression of the cIAPs,46,47 and are very likely to affect the first cell death checkpoint, for example, CD30 sensitizes cells to TNF-induced apoptosis.
Can this early cell death checkpoint be manipulated pharmacologically? Several groups have investigated the effect of apoptosis-inducing SMAC mimetics on various cancer cell lines.48,49,32 SMAC mimetics are tetrapeptides,50 or related chemical structures, derived from the interaction motif required for the SMAC protein to bind to members of the inhibitor of apoptosis family of proteins (IAPs), which includes the cIAP1 and cIAP2 E3 ligases that ubiquitinate RIP1. SMAC mimetics were originally designed to trigger cell death in cancer cells by preventing the IAPs from binding to SMAC, thus freeing the pro-apoptotic SMAC protein to activate caspases and trigger apoptosis.48,49 However, these SMAC mimetics have been found to trigger the autoubiquitination and degradation of the cIAPs.32 SMAC mimetics provoke the interaction of RIP1 with Caspase 8,29 and in this complex RIP1 actively enhances apoptosis without requiring loss of the NFκBinducible pro-survival gene cFLIP.51 In contrast, a different death complex containing Caspase 8 triggers apoptosis in the presence of a protein synthesis inhibitor; the activation of Caspase 8 in this complex does not require RIP1. Therefore, like the RIP1-K377R mutation, SMAC mimetics enhance an early pro-apoptotic pathway that is not subject to regulation by the synthesis of pro-survival factors. SMAC mimetics prevent ubiquitination of RIP1 and as a result, RIP1 directly binds Caspase 8 and can trigger apoptosis. The observation that SMAC mimetics unveil the apoptosis-enhancing properties of RIP1 is in agreement with our earlier studies and supports our original hypothesis that the first cell death checkpoint in the TNF pathway is the ubiquitination of RIP1, which transmits an NFκB-independent pro-survival signal. The importance of the first pro-survival checkpoint and the ability of ubiquitination to restrain RIP1’s pro-death activity is underlined by the fact that the SMAC mimetic treatment, like TNFR2 stimulation, is a potent inducer of NFκB activity.32,45,52 Therefore, non-ubiquitinated RIP1 activates Caspase 8 in SMAC-mimetic treated cells at time-points when expression of NFκB genes can be detected, suggesting that the new synthesis of pro-survival proteins is not sufficient to overcome the loss of the early transcription-independent pro-survival signal.
In addition to caspase-dependent apoptosis, TNF can also trigger programmed necrosis,16 Since programmed necrosis requires TNFR2 ligation, which degrades TRAF2, cIAP1 and cIAP2, we hypothesized that the ubiquitination of RIP1 and recruitment of NEMO might also serve as a pro-survival checkpoint in the necrotic signaling pathway. Cell death in T cells that express RIP1-K377R, or are deficient in NEMO, is only partially blocked by the pan-caspase inhibitor zVAD in both instances (manuscript in preparation). The cell death that occurs in the presence of zVAD and TNF is completely blocked by Necrostatin 1, confirming that ubiquitination of RIP1 and recruitment of NEMO prevents programmed necrosis. Necrostatin 1 has no effect on the cell death of NEMO-deficient T cells treated with TNF alone, indicating that T cells will switch from apoptosis to programmed necrosis only when caspases are blocked. This is consistent with the hypothesis that apoptosis is the default cell death mechanism and programmed necrosis serves as a back-up cell death pathway only when caspase activity is lost, for example during infection with viruses that encode caspase inhibitors. Therefore, ubiquitination of RIP1 protects against both the apoptotic and necrotic cell death pathways. In the presence of active caspases, non-ubiquitinated RIP1 triggers apoptosis. If caspase activity is prevented, non-ubiquitinated RIP1 triggers necrosis. This suggests that RIP1 ubiquitination is a pivotal moment in cell death signaling: RIP1 appears to be able to interact with the pro-apoptotic and pro-necrotic apparatus, activation of both pathways is inhibited by ubiquitination. Therefore, the downstream protein that binds non-ubiquitinated RIP1 ultimately determines the type of cell death pathway activated by RIP1. In the apoptosis pathway, this is likely due to non-ubiquitinated RIP1’s interaction with Caspase 8, the initiator of apoptosis. At present we do not know which mediator of programmed necrosis permits non-ubiquitinated RIP1 to activate the necrosis-signaling pathway. Three recent papers may shed some light on this cell death process by pinpointing RIP3 as a crucial mediator of necrosis.53–55
RIP1 and RIP3 are the only two RIP kinase family members that contain a RIP homotypic interaction motif or RHIM, a protein-binding domain. Through this RHIM domain, RIP1 and RIP3 can bind each other and RIP1 is phosphorylated by RIP3.56,57 Several studies in vitro suggested that RIP3 can regulate both NFκB activation and apoptosis58–60 but RIP3 knockout mice maintain normal NFκB activation and cell death responses to a number of stimuli.61 Overexpression of RIP3 can kill cells by apoptosis in a kinase-independent fashion. Even more spookily reminiscent of RIP1’s pro-death qualities, RIP3 overexpression can also initiate cell death in the absence of caspase activity, and crucially, this caspase-independent function requires RIP3’s kinase activity.62 The role of the RIP3 kinase in vivo appeared enigmatic, however, three groups have recently described a defect in programmed necrotic responses in RIP3 KO mice and point to a role for RIP3 in triggering necrosis by modulating the activity of RIP1.
Wang et al. have discovered that treatment of several cancer cell lines with caspase inhibitors is unable to block cell death during stimulation with TNF and SMAC mimetics, indicating that the SMAC mimetics can trigger programmed necrosis.53 They identified the kinase RIP3 as being essential for SMAC mimetics to trigger necrosis by using a genome-wide siRNA approach. Similarly, Chan et al. used a kinase-specific siRNA screen and identified RIP3 as an essential gene required for the activation of programmed necrosis during virus infection, which is dependent upon TNFR2 signaling.54 Both groups confirm earlier studies and showed that the RHIM of RIP1 and RIP3 is needed for these two kinases to interact and to trigger caspase-independent cell death. RIP3 coimmunoprecipitates with RIP1 only in cells undergoing necrosis; the interaction between these two proteins is not detectable in cells undergoing apoptosis with active caspases. RIP3 does not bind RIP1 in cells treated with the RIP1 kinase inhibitor Necrostatin 1 but interaction is clearly seen between kinase dead RIP3 and wildtype RIP1. Therefore, the interaction of RIP3 with RIP1 during necrosis depends upon the kinase activity of RIP1 but not that of RIP3. Surprisingly, RIP1 is a very poor kinase for RIP3 and RIP3 is probably not directly phosphorylated by RIP1 in vivo, therefore, RIP1 must phosphorylate another substrate in order to permit RIP1:RIP3 complexes to form. Although both RIP kinases can autophophorylate, RIP3 kinase activity appears to mediate phosphorylation of both RIP1 and RIP3 in the pronecrotic complex. The kinase activity of both RIP1 and RIP3 is a prerequisite for cell death by necrosis to occur, however, there is no evidence that the phosphorylation of RIP1 and RIP3 themselves is required for cell death to proceed. Indeed, it could be envisaged that phosphorylation of RIP1 and RIP3 may inhibit their function as pro-death molecules in order to establish a negative feedback loop and limit cell death. Necrosis may require phosphorylation of other downstream signaling mediators, therefore, a thorough inventory of the substrates phosphorylated by RIP1 and RIP3 is required to determine the targets of these kinases that are important for the execution phase of programmed necrosis to occur. RIP1 and RIP3 can be co-immunoprecipitated with Caspase 8 and FADD in cells undergoing necrosis. Since Caspase 8 and FADD are key components of the apoptotic machinery, it is unclear whether or not the recruitment of RIP1 and RIP3 to this complex is involved in the regulation of programmed necrosis. RIP1,63,64 and RIP3,62 are both substrates for cleavage by Caspase 8 and this has been proposed to mediate the ability of Caspase 8 to inhibit necrosis. Both groups suggest that the apoptotic machinery is transformed into a pro-necrotic complex by the binding of RIP1 and RIP3. Since RIP1 has apoptosis-enhancing activity and RIP3 overexpression triggers apoptosis it is plausible that RIP1 and RIP3 may serve as pro-apoptotic signaling molecules in the right circumstances. Caspase 8 activity blocks necrosis signaling, which suggests that at some point Caspase 8 interacts with a key mediator of necrosis, but necrosis can be triggered in Caspase 8 and FADD knockout cells.16 Therefore, it remains to be seen if RIP1 and RIP3 form a pro-necrotic complex independently of Caspase 8 and FADD. It is interesting that RIP3 recruited to RIP1 also undergoes post-translational modification suggestive of ubiquitination.54 Based on our studies of RIP1, we favour the hypothesis that RIP3 may also be a target for non-degradative polyubiquitination in order to limit its capacity to trigger necrosis. Much more work is required to elucidate the events involved in formation and activation of the pro-necrotic complex.
So how does the complex of RIP1 and RIP3 trigger he execution phase of programmed necrosis? Chan et al. show that cell death by programmed necrosis is mediated by reactive oxygen species, a well-known trigger of caspase-independent cell death.22 In the work published by Han et al. they have explored in more detail the mechanism for RIP3 to trigger production of ROS.55 In their study, they discovered that RIP3 interacts with various metabolic enzymes such as the glycogen phosphorylase PYGL, which mediates the conversion of glycogen energy stores to ATP. siRNA-mediated downregulation of some of these metabolic enzymes protected against RIP3-dependent necrosis, indicating that changes in the metabolic activity of the cell might be important for the generation of ROS and subsequent cell death. Exactly how ROS, RIP1 and RIP3 kinase activity orchestrates the execution phase is unclear. It is interesting to note that RIP3 does not co-localize with the mitochondria during programmed necrosis, which would be anticipated to be the site of ROS production by metabolic pathways, although earlier studies do suggest a mitochondrial localization for RIP3.59 The requirement for ROS to trigger cell death by necrosis constitutes a signaling step in which the second NFκB-dependent cell death checkpoint may halt death. NFκB can drive expression of enzymes such as the superoxide dismutase that counteract the effects of ROS.65 Some of the protection against necrosis mediated by ubiquitinated RIP1 is dependent on NFκB-driven gene transcription (unpublished data). Apoptosis and necrosis, therefore, share both cell death checkpoints. At the early checkpoint, ubiquitination prevents RIP1 from binding Caspase 8 and enhancing apoptosis. If caspase activity is blocked, ubiquitination of RIP1 also prevents activation of the pro-necrotic complex by RIP1, probably by altering the interaction of RIP1 with RIP3. At the later checkpoint, NFκB activity has the ability to limit both apoptosis and necrosis by driving expression of pro-survival genes.
While the second NFκB-dependent cell death checkpoint has been well studied for the last dozen years, the early cell death checkpoint dependent on the ubiquitination of RIP1 is a more recent discovery. As our understanding of this early TNFR1 cell death checkpoint increases, it is likely to prove to be an attractive pharmacological target in anti-inflammatory and anti-tumour therapies. SMAC mimetics have been shown to enhance cell death in some tumour cell lines by altering the ubiquitination status of RIP1. Thus the SMAC mimetics function by targeting the early cell death checkpoint. Additional pharmacological agents to disrupt this checkpoint are likely to be developed as our understanding of the regulation of this checkpoint increases. There are many interesting questions remaining about the regulation of cell death by RIP1 ubiquitination. We are currently investigating how ubiquitination of RIP1 and recruitment of NEMO prevents RIP1 from interacting with the pro-apoptotic and pro-necrotic complexes. Ubiquitination of RIP1 and NEMO binding may prevent other interaction partners binding RIP1 by direct steric hindrance, altering the subcellular localization of RIP1 or by regulating the kinase activity of RIP1. Moreover, ubiquitination of RIP1 regulates cell death at the pivotal point when the presence of Caspase 8 activity determines apoptosis versus necrosis; it is not clear how Caspase 8 activity prevents RIP1 and RIP3 from activating necrosis.
The discovery of an early cell death checkpoint in TNFR1 signaling regulated by the ubiquitination of RIP1 resolves the ‘NFκB paradox’. This checkpoint temporarily prevents the preexisting death signaling pathway from being activated, allowing time for the NFκB-dependent pro-survival genes to come into effect. When this checkpoint fails, RIP1-dependent death, whether by apoptosis or necrosis, rapidly ensues. Metaphorically, RIP1 behaves like a jury in a capital murder trial. It decides whether the accused is innocent and allowed to live, or guilty and therefore sentenced to death. Once a guilty verdict has been rendered, RIP1 will then decide the form of execution by which the death sentence is to be carried out.
Acknowledgements
ATT is supported by NIH grant AI052417. MAO'D is a recipient of a Research Fellowship Award from the Crohn's and Colitis Foundation of America.
Abbreviations
- TNF
tumor necrosis factor
- TNFR
tumor necrosis factor receptor
- RIP
receptor interacting protein
- IAP
inhibitor of apoptosis protein
- NFκB
nuclear factor kappaB
- K63
lysine 63 of ubiquitin
- TLR3
toll like receptor 3
- cFLIP
cellular FLICE (caspase 8) inhibitory protein
- CARD
caspase recruitment domain
- NOD
nucleotide oligomerisation domain
- FADD
FAS associated death domain
- TRADD
TNFR associated death domain
- TRAF
TNFR associated factor
- NEMO
NFκB essential modifier
- IκBαSR
inhibitor of NFκB super-repressor
- SMAC
small mitochondria-derived activator of caspases
- siRNA
small interfering RNA
- RHIM
RIP homotypic interaction motif
- ROS
reactive oxygen species
- PYGL
glycogen phosphorylase
- TAK
transforming growth factor beta activated kinase
- IKK
IκBα kinase
- TCR
T cell receptor
References
- 1.Natoli G, Costanzo A, Guido F, Moretti F, Levrero M. Apoptotic, non-apoptotic and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem Pharmacol. 1998;56:915–920. doi: 10.1016/s0006-2952(98)00154-3. [DOI] [PubMed] [Google Scholar]
- 2.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NFkappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 3.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NFkappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- 5.Liu ZG, Hsu H, Goeddel DV, Karin M. Dissection of TNF receptor 1 effector functions: JNK activation is not linked to apoptosis while NFkappaB activation prevents cell death. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 6.Kitanaka C, Kuchino Y. Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 1999;6:508–515. doi: 10.1038/sj.cdd.4400526. [DOI] [PubMed] [Google Scholar]
- 7.Kroemer G, Martin SJ. Caspase-independent cell death. Nat Med. 2005;11:725–730. doi: 10.1038/nm1263. [DOI] [PubMed] [Google Scholar]
- 8.Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. doi: 10.1038/nrn1106. [DOI] [PubMed] [Google Scholar]
- 9.Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, et al. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112–119. doi: 10.1038/nchembio711. [DOI] [PubMed] [Google Scholar]
- 10.Smith CC, Davidson SM, Lim SY, Simpkin JC, Hothersall JS, Yellon DM. Necrostatin: a potentially novel cardioprotective agent? Cardiovasc Drugs Ther. 2007;21:227–233. doi: 10.1007/s10557-007-6035-1. [DOI] [PubMed] [Google Scholar]
- 11.Bhatia M. Apoptosis versus necrosis in acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2004;286:189–196. doi: 10.1152/ajpgi.00304.2003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang M, Chen L. Status of cytokines in ischemia reperfusion induced heart injury. Cardiovasc Hematol Disord Drug Targets. 2008;8:161–172. doi: 10.2174/187152908785849116. [DOI] [PubMed] [Google Scholar]
- 13.Tuttolomondo A, Di Raimondo D, di Sciacca R, Pinto A, Licata G. Inflammatory cytokines in acute ischemic stroke. Curr Pharm Des. 2008;14:3574–3589. doi: 10.2174/138161208786848739. [DOI] [PubMed] [Google Scholar]
- 14.Malleo G, Mazzon E, Siriwardena AK, Cuzzocrea S. Role of tumor necrosis factor-alpha in acute pancreatitis: from biological basis to clinical evidence. Shock. 2007;28:130–140. doi: 10.1097/shk.0b013e3180487ba1. [DOI] [PubMed] [Google Scholar]
- 15.Holler N, Zaru R, Micheau O, Thome M, Attinger A, Valitutti S, et al. Fas triggers an alternative, caspase-8- independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 16.Chan FK, Shisler J, Bixby JG, Felices M, Zheng L, Appel M, et al. A role for tumor necrosis factor receptor-2 and receptor-interacting protein in programmed necrosis and antiviral responses. J Biol Chem. 2003;278:51613–51621. doi: 10.1074/jbc.M305633200. [DOI] [PubMed] [Google Scholar]
- 17.Li M, Beg AA. Induction of necrotic-like cell death by tumor necrosis factor alpha and caspase inhibitors: novel mechanism for killing virus-infected cells. J Virol. 2000;74:7470–7477. doi: 10.1128/jvi.74.16.7470-7477.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ting AT, Pimentel-Muinos FX, Seed B. RIP mediates tumor necrosis factor receptor 1 activation of NFkappaB but not Fas/APO-1-initiated apoptosis. EMBO J. 1996;15:6189–6196. [PMC free article] [PubMed] [Google Scholar]
- 19.Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ, Leder P. The death domain kinase RIP mediates the TNF-induced NFkappaB signal. Immunity. 1998;8:297–303. doi: 10.1016/s1074-7613(00)80535-x. [DOI] [PubMed] [Google Scholar]
- 20.Meylan E, Burns K, Hofmann K, Blancheteau V, Martinon F, Kelliher M, Tschopp J. RIP1 is an essential mediator of Toll-like receptor 3-induced NFkappaB activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 21.Yeh WC, Itie A, Elia AJ, Ng M, Shu HB, Wakeham A, et al. Requirement for Casper (c-FLIP) in regulation of death receptor-induced apoptosis and embryonic development. Immunity. 2000;12:633–642. doi: 10.1016/s1074-7613(00)80214-9. [DOI] [PubMed] [Google Scholar]
- 22.Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, et al. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- 23.Degterev A, Hitomi J, Germscheid M, Ch’en IL, Korkina O, Teng X, et al. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313–321. doi: 10.1038/nchembio.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hitomi J, Christofferson DE, Ng A, Yao J, Degterev A, Xavier RJ, Yuan J. Identification of a molecular signaling network that regulates a cellular necrotic cell death pathway. Cell. 2008;135:1311–1323. doi: 10.1016/j.cell.2008.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meylan E, Tschopp J. The RIP kinases: crucial integrators of cellular stress. Trends Biochem Sci. 2005;30:151–159. doi: 10.1016/j.tibs.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, et al. RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature. 2002;416:194–199. doi: 10.1038/416194a. [DOI] [PubMed] [Google Scholar]
- 27.Stanger BZ, Leder P, Lee TH, Kim E, Seed B. RIP: a novel protein containing a death domain that interacts with Fas/APO-1 (CD95) in yeast and causes cell death. Cell. 1995;81:513–523. doi: 10.1016/0092-8674(95)90072-1. [DOI] [PubMed] [Google Scholar]
- 28.Wertz IE, O’Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, et al. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NFkappaB signalling. Nature. 2004;430:694–699. doi: 10.1038/nature02794. [DOI] [PubMed] [Google Scholar]
- 29.Bertrand MJ, Milutinovic S, Dickson KM, Ho WC, Boudreault A, Durkin J, et al. cIAP1 and cIAP2 facilitate cancer cell survival by functioning as E3 ligases that promote RIP1 ubiquitination. Mol Cell. 2008;30:689–700. doi: 10.1016/j.molcel.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 30.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 31.Varfolomeev E, Goncharov T, Fedorova AV, Dynek JN, Zobel K, Deshayes K, et al. c-IAP1 and c-IAP2 are critical mediators of tumor necrosis factor alpha (TNFalpha)-induced NFkappaB activation. J Biol Chem. 2008;283:24295–24299. doi: 10.1074/jbc.C800128200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Varfolomeev E, Blankenship JW, Wayson SM, Fedorova AV, Kayagaki N, Garg P, et al. IAP antagonists induce autoubiquitination of c-IAPs, NFkappaB activation, and TNFalpha-dependent apoptosis. Cell. 2007;131:669–681. doi: 10.1016/j.cell.2007.10.030. [DOI] [PubMed] [Google Scholar]
- 33.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 34.Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor alpha-induced NFkappaB activation. J Biol Chem. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 35.Kanayama A, Seth RB, Sun L, Ea CK, Hong M, Shaito A, et al. TAB2 and TAB3 activate the NFkappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15:535–548. doi: 10.1016/j.molcel.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 36.Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NFkappaB activation. Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- 37.O’Donnell MA, Legarda-Addison D, Skountzos P, Yeh WC, Ting AT. Ubiquitination of RIP1 regulates an NFkappaB-independent cell-death switch in TNF signaling. Curr Biol. 2007;17:418–424. doi: 10.1016/j.cub.2007.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeh WC, Shahinian A, Speiser D, Kraunus J, Billia F, Wakeham A, et al. Early lethality, functional NFkappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- 39.Legarda-Addison D, Hase H, O’Donnell MA, Ting AT. NEMO/IKKgamma regulates an early NFkappaB-independent cell-death checkpoint during TNF signaling. Cell Death Differ. 2009;16:1279–1288. doi: 10.1038/cdd.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ware CF, Crowe PD, Vanarsdale TL, Andrews JL, Grayson MH, Jerzy R, et al. Tumor necrosis factor (TNF) receptor expression in T lymphocytes. Differential regulation of the type I TNF receptor during activation of resting and effector T cells. J Immunol. 1991;147:4229–4238. [PubMed] [Google Scholar]
- 41.Ruby J, Bluethmann H, Peschon JJ. Antiviral activity of tumor necrosis factor (TNF) is mediated via p55 and p75 TNF receptors. J Exp Med. 1997;186:1591–1596. doi: 10.1084/jem.186.9.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kalinkovich A, Engelmann H, Harpaz N, Burstein R, Barak V, Kalickman I, et al. Elevated serum levels of soluble tumour necrosis factor receptors (sTNF-R) in patients with HIV infection. Clin Exp Immunol. 1992;89:351–355. doi: 10.1111/j.1365-2249.1992.tb06961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fotin-Mleczek M, Henkler F, Samel D, Reichwein M, Hausser A, Parmryd I, et al. Apoptotic crosstalk of TNF receptors: TNF-R2-induces depletion of TRAF2 and IAP proteins and accelerates TNF-R1-dependent activation of caspase-8. J Cell Sci. 2002;115:2757–2770. doi: 10.1242/jcs.115.13.2757. [DOI] [PubMed] [Google Scholar]
- 44.Li X, Yang Y, Ashwell JD. TNF-RII and c-IAP1 mediate ubiquitination and degradation of TRAF2. Nature. 2002;416:345–347. doi: 10.1038/416345a. [DOI] [PubMed] [Google Scholar]
- 45.Chan FK, Lenardo MJ. A crucial role for p80 TNFR2 in amplifying p60 TNF-R1 apoptosis signals in T lymphocytes. Eur J Immunol. 2000;30:652–660. doi: 10.1002/1521-4141(200002)30:2<652::AID-IMMU652>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 46.Duckett CS, Thompson CB. CD30-dependent degradation of TRAF2: implications for negative regulation of TRAF signaling and the control of cell survival. Genes Dev. 1997;11:2810–2821. doi: 10.1101/gad.11.21.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown KD, Hostager BS, Bishop GA. Differential signaling and tumor necrosis factor receptor-associated factor (TRAF) degradation mediated by CD40 and the Epstein-Barr virus oncoprotein latent membrane protein 1 (LMP1) J Exp Med. 2001;193:943–954. doi: 10.1084/jem.193.8.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG. A small molecule Smac mimic potentiates TRAIL- and TNFalpha-mediated cell death. Science. 2004;305:1471–1474. doi: 10.1126/science.1098231. [DOI] [PubMed] [Google Scholar]
- 49.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, et al. IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell. 2007;131:682–693. doi: 10.1016/j.cell.2007.10.037. [DOI] [PubMed] [Google Scholar]
- 50.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y. Structural basis of IAP recognition by Smac/ DIABLO. Nature. 2000;408:1008–1012. doi: 10.1038/35050012. [DOI] [PubMed] [Google Scholar]
- 51.Wang L, Du F, Wang X. TNFalpha induces two distinct caspase-8 activation pathways. Cell. 2008;133:693–703. doi: 10.1016/j.cell.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 52.Pimentel-Muinos FX, Seed B. Regulated commitment of TNF receptor signaling: a molecular switch for death or activation. Immunity. 1999;11:783–793. doi: 10.1016/s1074-7613(00)80152-1. [DOI] [PubMed] [Google Scholar]
- 53.He S, Wang L, Miao L, Wang T, Du F, Zhao L, Wang X. Receptor interacting protein kinase-3 determines cellular necrotic response to TNFalpha. Cell. 2009;137:1100–1111. doi: 10.1016/j.cell.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 54.Cho YS, Challa S, Moquin D, Genga R, Ray TD, Guildford M, Chan FK. Phosphorylation-driven assembly of the RIP1-RIP3 complex regulates programmed necrosis and virus-induced inflammation. Cell. 2009;137:1112–1123. doi: 10.1016/j.cell.2009.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang DW, Shao J, Lin J, Zhang N, Lu BJ, Lin SC, et al. RIP3, an energy metabolism regulator that switches TNF-induced cell death from apoptosis to necrosis. Science. 2009;325:332–336. doi: 10.1126/science.1172308. [DOI] [PubMed] [Google Scholar]
- 56.Kaiser WJ, Offermann MK. Apoptosis induced by the toll-like receptor adaptor TRIF is dependent on its receptor interacting protein homotypic interaction motif. J Immunol. 2005;174:4942–4952. doi: 10.4049/jimmunol.174.8.4942. [DOI] [PubMed] [Google Scholar]
- 57.Sun X, Yin J, Starovasnik MA, Fairbrother WJ, Dixit VM. Identification of a novel homotypic interaction motif required for the phosphorylation of receptorinteracting protein (RIP) by RIP3. J Biol Chem. 2002;277:9505–9511. doi: 10.1074/jbc.M109488200. [DOI] [PubMed] [Google Scholar]
- 58.Yu PW, Huang BC, Shen M, Quast J, Chan E, Xu X, et al. Identification of RIP3, a RIP-like kinase that activates apoptosis and NFkappaB. Curr Biol. 1999;9:539–542. doi: 10.1016/s0960-9822(99)80239-5. [DOI] [PubMed] [Google Scholar]
- 59.Kasof GM, Prosser JC, Liu D, Lorenzi MV, Gomes BC. The RIP-like kinase, RIP3, induces apoptosis and NFkappaB nuclear translocation and localizes to mitochondria. FEBS Lett. 2000;473:285–291. doi: 10.1016/s0014-5793(00)01473-3. [DOI] [PubMed] [Google Scholar]
- 60.Sun X, Lee J, Navas T, Baldwin DT, Stewart TA, Dixit VM. RIP3, a novel apoptosis-inducing kinase. J Biol Chem. 1999;274:16871–16875. doi: 10.1074/jbc.274.24.16871. [DOI] [PubMed] [Google Scholar]
- 61.Newton K, Sun X, Dixit VM. Kinase RIP3 is dispensable for normal NFkappaBs, signaling by the B-cell and T-cell receptors, tumor necrosis factor receptor 1, and Toll-like receptors 2 and 4. Mol Cell Biol. 2004;24:1464–1469. doi: 10.1128/MCB.24.4.1464-1469.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng S, Yang Y, Mei Y, Ma L, Zhu DE, Hoti N, et al. Cleavage of RIP3 inactivates its caspase-independent apoptosis pathway by removal of kinase domain. Cell Signal. 2007;19:2056–2067. doi: 10.1016/j.cellsig.2007.05.016. [DOI] [PubMed] [Google Scholar]
- 63.Lin Y, Devin A, Rodriguez Y, Liu ZG. Cleavage of the death domain kinase RIP by caspase-8 prompts TNF-induced apoptosis. Genes Dev. 1999;13:2514–2526. doi: 10.1101/gad.13.19.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Martinon F, Holler N, Richard C, Tschopp J. Activation of a pro-apoptotic amplification loop through inhibition of NFkappaB-dependent survival signals by caspase-mediated inactivation of RIP. FEBS Lett. 2000;468:134–136. doi: 10.1016/s0014-5793(00)01212-6. [DOI] [PubMed] [Google Scholar]
- 65.Dopp JM, Sarafian TA, Spinella FM, Kahn MA, Shau H, de Vellis J. Expression of the p75 TNF receptor is linked to TNF-induced NFkappaB translocation and oxyradical neutralization in glial cells. Neurochem Res. 2002;27:1535–1542. doi: 10.1023/a:1021608724117. [DOI] [PubMed] [Google Scholar]