Abstract
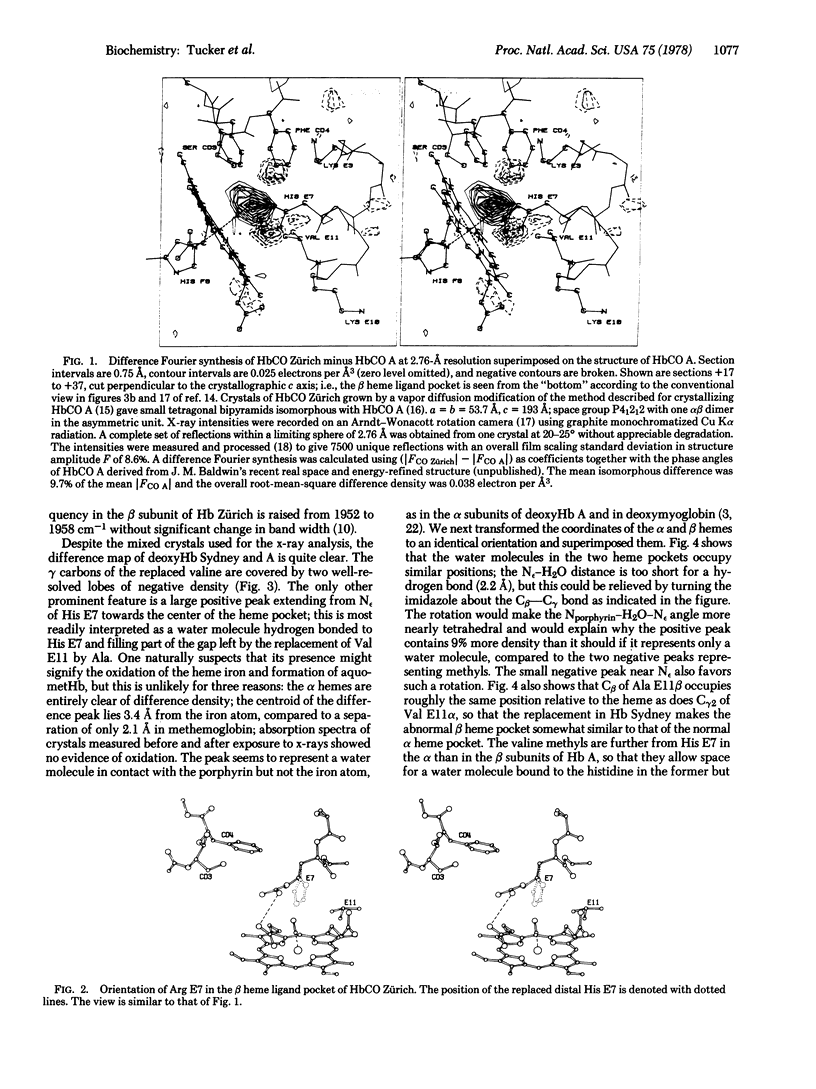
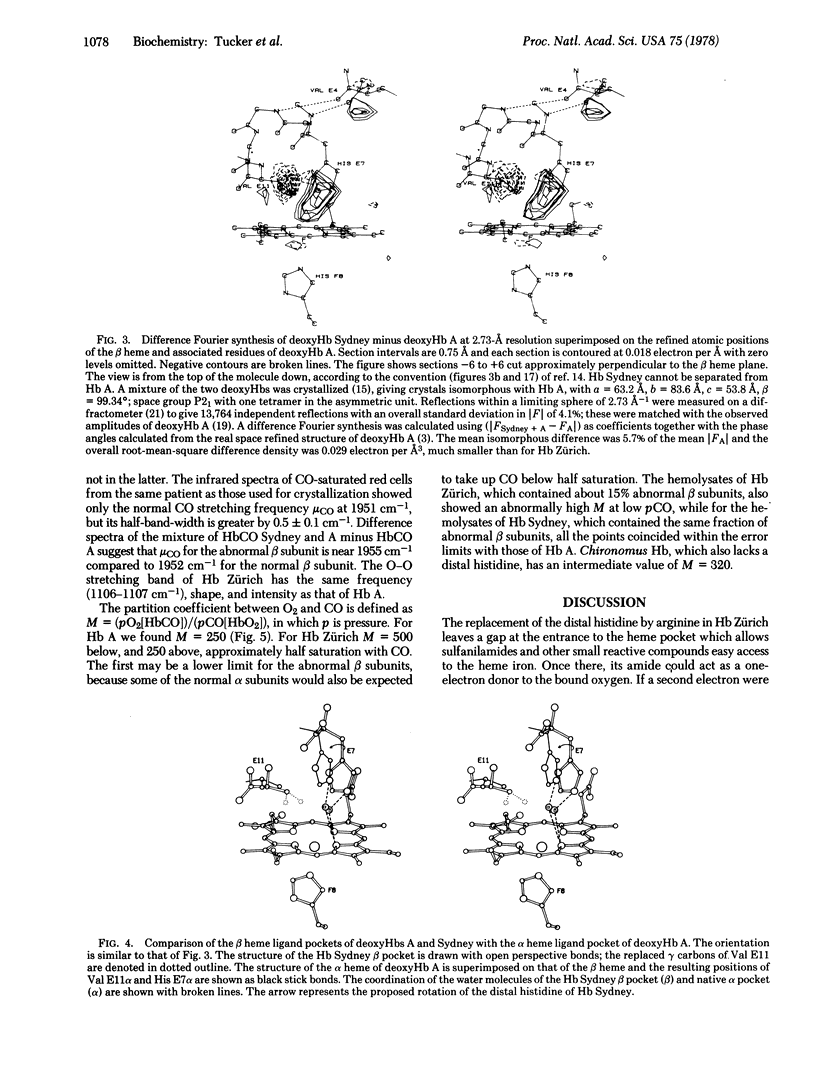
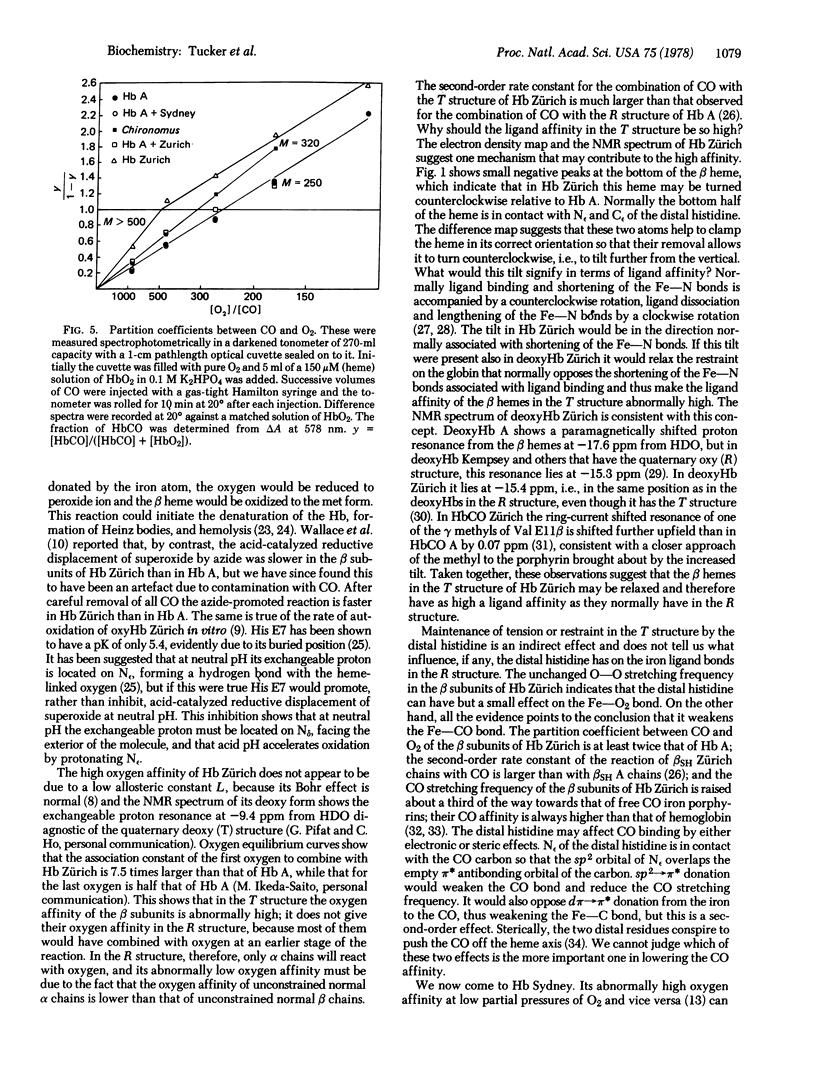
In hemoglobin Zürich the side chain of the distal arginine attaches itself to the propionate of the heme, leaving the heme pocket wide open, allowing sulfanilamides easy access to the iron, and doubling the partition coefficient between CO and O2. The replacement of the distal valine by alanine in hemoglobin Sydney leaves a large gap inside the heme pocket, which is partly filled by a water molecule bonded to the distal histidine. Hemoglobin Sydney has the same partition coefficient between CO and O2 as hemoglobin A.Replacement of the distal histidine increases the stretching frequency of CO linked to the beta heme by 6 cm-1, but replacement of the distal valine increases it by only 3 cm-1, but replacement of the distal histidine leaves the O-O stretching frequency unchanged.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. Intermediate structure of normal human haemoglobin: methaemoglobin in the deoxy quaternary conformation. J Mol Biol. 1973 Sep 25;79(3):495–506. doi: 10.1016/0022-2836(73)90401-4. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Carrell R. W., Lehmann H., Lorkin P. A., Raik E., Hunter E. Haemoglobin sydney: Beta-67 (E11) valine modified to alanine: an emerging pattern of unstable haemoglobins. Nature. 1967 Aug 5;215(5101):626–628. doi: 10.1038/215626a0. [DOI] [PubMed] [Google Scholar]
- Casey R., Kynoch P. A., Lang A., Lehmann H., Nozari G., Shinton N. K. Double heterozygosity for two unstable haemoglobins: Hb Sydney (beta67[E11] Val leads to Ala) and Hb Coventry (beta141[H19] Leu deleted). Br J Haematol. 1978 Feb;38(2):195–209. doi: 10.1111/j.1365-2141.1978.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Caughey W. S. Carbon monoxide bonding in hemeproteins. Ann N Y Acad Sci. 1970 Oct 5;174(1):148–153. doi: 10.1111/j.1749-6632.1970.tb49781.x. [DOI] [PubMed] [Google Scholar]
- Collman J. P., Brauman J. I., Halbert T. R., Suslick K. S. Nature of O2 and CO binding to metalloporphyrins and heme proteins. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3333–3337. doi: 10.1073/pnas.73.10.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis D. G., Mock N. H., Lindstrom T. R., Charache S., Ho C. Nuclear magnetic resonance studies of hemoglobiss. V. The heme proton spectra of human deoxyhemoglobins A, F, Zurich, and Chesapeake. Biochem Biophys Res Commun. 1970 Jul 27;40(2):343–349. doi: 10.1016/0006-291x(70)91015-6. [DOI] [PubMed] [Google Scholar]
- FRICK P. G., HITZIG W. H., BETKE K. Hemoglobin Zurich. I. A new hemoglobin anomaly associated with acute hemolytic episodes with inclusion bodies after sulfonamide therapy. Blood. 1962 Sep;20:261–271. [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Giacometti G. M., Di Iorio E. E., Antonini E., Brunori M., Winterhalter K. H. Binding of carbon monoxide to hemoglobin Zürich. Proposal for a kinetic model. Eur J Biochem. 1977 May 2;75(1):267–273. doi: 10.1111/j.1432-1033.1977.tb11526.x. [DOI] [PubMed] [Google Scholar]
- HITZIG W. H., FRICK P. G., BETKE K., HUISMAN T. H. [Hemoglobin Zuerich: a new hemoglobin anomaly with sulfonamide-induced inclusion body anemia]. Helv Paediatr Acta. 1960 Dec;15:499–514. [PubMed] [Google Scholar]
- Heidner E. J., Ladner R. C., Perutz M. F. Structure of horse carbonmonoxyhaemoglobin. J Mol Biol. 1976 Jul 5;104(3):707–722. doi: 10.1016/0022-2836(76)90130-3. [DOI] [PubMed] [Google Scholar]
- Ikeda-Saito M., Iizuka T., Yamamoto H., Kayne F. J., Yonetani T. Studies on cobalt myoglobins and hemoglobins. Interaction of sperm whale myoglobin and Glycera hemoglobin with molecular oxygen. J Biol Chem. 1977 Jul 25;252(14):4882–4887. [PubMed] [Google Scholar]
- Itano H. A. Phenyldiimide, hemoglobin, and Heinz bodies. Proc Natl Acad Sci U S A. 1970 Oct;67(2):485–492. doi: 10.1073/pnas.67.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob H., Winterhalter K. Unstable hemoglobins: the role of heme loss in Heinz body formation. Proc Natl Acad Sci U S A. 1970 Mar;65(3):697–701. doi: 10.1073/pnas.65.3.697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner R. C., Heidner E. J., Perutz M. F. The structure of horse methaemoglobin at 2-0 A resolution. J Mol Biol. 1977 Aug 15;114(3):385–414. doi: 10.1016/0022-2836(77)90256-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom T. R., Norén I. B., Charache S., Lehmann H., Ho C. Nuclear magnetic resonance studies of hemoglobins. VII. Tertiary structure around ligand binding site in carbonmonoxyhemoglobin. Biochemistry. 1972 Apr 25;11(9):1677–1681. doi: 10.1021/bi00759a023. [DOI] [PubMed] [Google Scholar]
- MULLER C. J., KINGMA S. Haemoglobin Zurich: alpha 2A beta 2-63 Arg. Biochim Biophys Acta. 1961 Jul 8;50:595–595. doi: 10.1016/0006-3002(61)90028-2. [DOI] [PubMed] [Google Scholar]
- Mallett J. F., Champness J. N., Faruqi A. R., Gossling T. H. A new automatic flat-bed microdensitometer for use in x-ray crystallography. J Phys E. 1977 Apr;10(4):351–358. doi: 10.1088/0022-3735/10/4/010. [DOI] [PubMed] [Google Scholar]
- PERUTZ R. R., LIQUORI A. M., EIRICH F. X-ray and solubility studies of the haemoglobin of sickle-cell anaemia patients. Nature. 1951 Jun 9;167(4258):929–931. doi: 10.1038/167929a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Ladner J. E., Simon S. R., Ho C. Influence of globin structure on the state of the heme. I. Human deoxyhemoglobin. Biochemistry. 1974 May 7;13(10):2163–2173. doi: 10.1021/bi00707a026. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., Lehmann H. Molecular pathology of human haemoglobin. Nature. 1968 Aug 31;219(5157):902–909. doi: 10.1038/219902a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F., TenEyck L. F. Stereochemistry of cooperative effects in hemoglobin. Cold Spring Harb Symp Quant Biol. 1972;36:295–310. doi: 10.1101/sqb.1972.036.01.040. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. The Croonian Lecture, 1968. The haemoglobin molecule. Proc R Soc Lond B Biol Sci. 1969 May 20;173(1031):113–140. doi: 10.1098/rspb.1969.0043. [DOI] [PubMed] [Google Scholar]
- Richards F. M. The matching of physical models to three-dimensional electron-density maps: a simple optical device. J Mol Biol. 1968 Oct 14;37(1):225–230. doi: 10.1016/0022-2836(68)90085-5. [DOI] [PubMed] [Google Scholar]
- Takano T. Structure of myoglobin refined at 2-0 A resolution. II. Structure of deoxymyoglobin from sperm whale. J Mol Biol. 1977 Mar 5;110(3):569–584. doi: 10.1016/s0022-2836(77)80112-5. [DOI] [PubMed] [Google Scholar]
- TenEyck L. F., Arnone A. Three-dimensional Fourier synthesis of human deoxyhemoglobin at 2-5 A resolution I. X-ray analysis. J Mol Biol. 1976 Jan 5;100(1):3–11. doi: 10.1016/s0022-2836(76)80029-0. [DOI] [PubMed] [Google Scholar]
- Tucker P. W., Perutz M. F. Mechanism of charge compensation and impairment of co-operative functions in haemoglobin Tacoma (Arg B12(30)beta leads to Ser). J Mol Biol. 1977 Aug 15;114(3):415–420. doi: 10.1016/0022-2836(77)90258-3. [DOI] [PubMed] [Google Scholar]
- Wallace W. J., Caughey W. S. Mechanism for the autoxidation of hemoglobin by phenols, nitrite and "oxidant" drugs. Peroxide formation by one electron donation to bound dioxygen. Biochem Biophys Res Commun. 1975 Feb 3;62(3):561–567. doi: 10.1016/0006-291x(75)90435-0. [DOI] [PubMed] [Google Scholar]
- Wallace W. J., Volpe J. A., Maxwell J. C., Caughey W. S. Properties of hemoglobin A and hemoglobin Zurich (beta63 histidine replaced by arginine): quantitative evaluation of functional abnormalities in hemoglobins. Biochem Biophys Res Commun. 1976 Feb 23;68(4):1379–1386. doi: 10.1016/0006-291x(76)90348-x. [DOI] [PubMed] [Google Scholar]
- Winterhalter K. H., Anderson N. M., Amiconi G., Antonini E., Brunori M. Functional properties of hemoglobin Zürich. Eur J Biochem. 1969 Dec;11(3):435–440. doi: 10.1111/j.1432-1033.1969.tb00793.x. [DOI] [PubMed] [Google Scholar]


