Abstract
Background
Hepatocellular carcinoma (HCC) is the leading cause of death amongst cirrhotic patients. Its diagnosis and discrimination from non-HCC malignant lesions in cirrhosis includes contrast enhanced computed tomography (CECT), contrast enhanced magnetic resonance imaging (CEMRI), or, in selected cases, liver biopsy. The role of contrast-enhanced ultrasonography (CEUS) is still controversial.
Aims
To evaluate whether, by selecting an appropriate ‘time to wash-out’ cut-off value, CEUS capability of discriminating between HCC and non-HCC malignancies in cirrhotic patients may be enhanced.
Methods
We enrolled 282 cirrhotic patients who underwent CEUS at our institute, from January 2008 to January 2012, for focal liver lesions (FLLs) detected at ultrasound (US). We used liver biopsy and subsequent histological evaluation as the gold standard for correct classification of FLLs. We calculated the area under receiver operator characteristic curves for CEUS to distinguish patients with HCC from those with non-HCC malignancies. The best ‘time to wash-out’ cut-off values were selected.
Results
Histological diagnosis of FLLs was as follows: 34 benign lesions (i.e. 25 regenerative nodules and 9 dysplastic nodules) and 248 malignant lesions (223 well-to-moderately differentiated HCCs; 7 poorly-differentiated HCCs; 5 intrahepatic colangiocellular carcinomas (ICCs); 5 primary non-Hodgkin B-cell lymphomas (NHBLs); and 8 metastatic liver tumors). A time to wash-out > 55 s identified patients with HCC with the highest level of accuracy (92.7%). Similarly, a time to wash-out ≤ 55 s correctly identified the vast majority of the non-HCC malignancies (100% sensitivity, 98.2% specificity and diagnostic accuracy of 98.3%).
Conclusions
CEUS is an accurate and safe procedure for discriminating FLLs in cirrhotic patients, especially when a cut-off time to wash-out of 55 s is chosen as a reference value.
Keywords: Biopsy, cirrhosis, cholangiocellular carcinoma, contrast enhancement, focal liver lesions, hepatocellular carcinoma, liver, ultrasound
Introduction
Hepatocellular carcinoma (HCC) is the leading cause of death amongst cirrhotic liver patients.1 A close surveillance of these patients for its early detection (based on biannual ultrasonography (US))2,3 is required, and in this setting, focal liver lesions (FLLs) other than HCC may be detected.4 Until 10 years ago, diagnosis of FLLs in cirrhotic patients only included contrast enhanced computed tomography (CECT), contrast enhanced magnetic resonance imaging (CEMRI), or, in selected cases, liver biopsy. The improvement of US, its low cost and safety strongly support its role as a first line technique for characterization of FLLs with good sensitivity and high specificity3; however, B-mode US presents low accuracy (20–40%) in characterizing liver nodules, particularly in cirrhotic patients.5
The development of contrast-enhanced US (CEUS) expanded the role of US by a real-time observation of contrast enhancement during arterial (15–30 s), portal venous (from 30 s up to 2 min) and late phase (4–6 min after) contrast injection6,7 with a sensitivity and specificity ranging from 85% to 90% and from 80% to 99%, respectively.8 According to the more recent European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) guidelines,9,10 FLLs can be easily classified when a typical enhancement pattern is detected. The role of CEUS as a first line procedure for diagnosis of HCC still remains controversial. CEUS, in fact, is definitely accepted by the Asian Pacific Association for the Study of the Liver (APASL)11 and the Japanese Society of Hepatology (JSH),12 while its diagnostic validity has been questioned by the American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (EASL), and European Organization for Research and Treatment of Cancer (EORTC).13,14 The main reason is that CEUS may not be able to accurately discriminate between HCC and intrahepatic cholangiocellular carcinoma (ICC).15 Recent guidelines from the Italian Association for the Study of the Liver (AISF)16 suggest that CEUS may be used for non-invasive diagnosis of HCC, with a low risk of misdiagnosis of ICC as HCC (less than 2%), with a very early wash-out (i.e. < 60 sec) being highly suggestive for ICC. This retrospective study was designed:
To assess the optimal ‘time to wash-out’ cut-off value of contrast agents, for better discriminating between HCC and malignant lesions other-than-HCC in cirrhotic patients;
To define the diagnostic accuracy of CEUS, in comparison to the gold standard (percutaneous biopsy).
Materials and methods
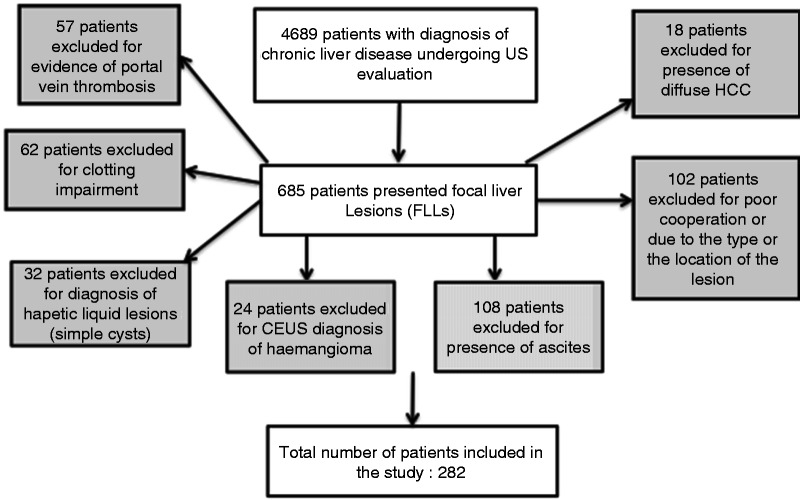
We conducted a retrospective study including 4689 cirrhotic patients undergoing US evaluation at the Interventional Ultrasonography Section in our Department of Hepatogastroenterology, over a 4-year period (January 2008 - January 2012). We detected FLLs in 685 patients. We excluded 57 patients for evidence of portal vein thrombosis, 108 for large ascites, 18 for diffuse HCC, 102 for poor cooperation or due to the type (i.e. pedunculated) or to the location of the lesion (i.e. subcapsular or close by vascular structures and/or gallbladder) that made it unsafe to perform the biopsy; 62 for a clotting impairment (platelet count < 50,000/mmc and prothrombin time ≤ 40%), 32 for diagnosis of simple cysts, 24 for a diagnosis (based on CEUS and subsequent follow-up) of hemangioma (i.e. peripheral globular pattern during arterial phase, followed by a centripetal fill-in during portal and late phase) (Figure 1).
Figure 1.
Flow diagram of the study.
CEUS: contrast-enhanced ultrasonography; FLL: focal liver lesions; US: ultrasonography.
Study population
We documented the demographic and clinical characteristics for each patient. We enrolled a total of 282 patients (197 M, 85 F; mean age, 67 ± 7 years; range, 28–79 years) with a ‘de novo’ diagnosis of single (n = 165; 58%) and multiple (n = 117) FLLs. The underlying etiology of cirrhosis was: hepatitis C (n = 220; 78%), hepatitis B (n = 31; 11%), alcoholic cirrhosis (n = 11; 4%), alcoholic plus hepatitis C (n = 7; 2.5%), hepatitis C plus hepatitis B (n = 10; 3.5%), cryptogenetic cirrhosis (n = 2) and primary biliary cirrhosis (n = 1) (Table 1).
Table 1.
Characteristics of studied population
| Total patients (n = 282) | M: n = 197 (70%) and F: n = 85 (30%) |
| Age (M ± SD; range) | M: 66 ± 7 yr; 36–79 yr and F: 67 ± 7 yr; 28–79 yr |
| Etiology of cirrhosis | Hepatitis C virus: n = 220 (78%) |
| Hepatitis B virus: n = 31 (11%) | |
| Alcoholic cirrhosis: n = 11 (4%) | |
| Alcoholic + hepatitis C virus: n = 7 (2.5%) | |
| Hepatitis C + Hepatitis B virus: n = 10 (3.5%) | |
| Other (cryptogenetic, primary biliary cirrhosis): n = 3 (1%) | |
| FLLs | Single: n = 165 (58%) |
| Multiple: n = 117 (42%) |
F: female gender; FLL: focal liver lesions; M: male gender; yr: years.
Imaging technique
US examinations were performed by experienced ultrasonographers with an Esaote Technos MPX and Esaote Twice (from October 2011 to January 2012), both equipped with real-time contrast imaging software. A convex array transducer of 3.5 MHz was used. All patients underwent both conventional B-mode ultrasound examination and enhanced sonography. The patient’s liver was scanned thoroughly with gray scale US, to identify the target lesion. The scanner was then switched to a software with set low Mechanical Index (MI) < 0.3 (0.08 Kpa). CEUS examination started and continued for 5 min after injection of US contrast agent SonoVue® (Bracco SpA, Milan, Italy), a sulfur hexafluoride-filled microbubble covered by a phospholipid shell. We conducted the contrast injection procedure and observation of its hemodynamic behavior during arterial, portal and late vascular phases as previously described.17 CEUS examination was considered conclusive if the studied lesion showed a contrastographic pattern so typical as to be classified as HCC or as a lesion other than HCC, according to EFSUMB guidelines.11 The wash-out was defined as ‘early’ when it started within 60 s, according to AISF guidelines.16
Diagnostic performance
All sonographic examinations and CEUS clips were digitally recorded. The imaging review was performed by three operators, with at least 5 years’ experience in liver CEUS, all blinded to the patients’ clinical histories and final histopathological diagnoses. The start of the wash-out was just assessed by visual inspection (no dedicated software was adopted). The k statistic was used to assess inter-observer agreement. Agreement was graded as poor (k < 0.20), moderate (k = 0.20 to < 0.40), fair (k = 0.40 to < 0.60), good (k = 0.60 to < 0.80) and very good (k = 0.80 to 1.00).
Pathological analysis
For all lesions (primarily studied at CEUS) a confirmation of diagnosis was obtained by US-guided percutaneous biopsy (adopted as the gold standard). If the patient presented multiple focal lesions, those showing a typical enhancement pattern at CEUS were considered optimal to obtain a sample for pathology. We used a real-time scanner (Esaote Technos MPX and Esaote Twice) with a side adapter (20–30° and 15–25–35° variable angle, respectively) and a multifrequency convex probe (2.5–5 MHz). All patients gave informed consent. We used large cutting needles (Biomol 18 G HS) for histological examination, as previously described.18 Histological specimens were fixed in formalin, embedded in paraffin and then stained with hematoxylin-eosin. Further immunohistochemical analysis of material was possible for specific indications. The final diagnosis was confirmed by histopathological examination, according to a modified Edmondson-Steiner grading system for HCC.19 For the diagnosis of regenerative nodule (RN), dysplastic nodule (DN) and ICC, we referred to criteria reported elsewhere.20,21
Statistical analysis
Adopting modified Edmondson-Steiner grades I-II-III HCCs as well-to-moderately differentiated HCC, and grade IV as poorly differentiated HCCs, the lesions were divided into two groups. The diagnostic performance of CEUS in differentiating HCC and non-HCC malignancy was assessed by using Receiver Operating Characteristic (ROC) analysis. A subject was identified as having HCC or non-HCC malignancy according to whether the ‘time to wash-out’ was greater than or less than the cut-off value of interest. The area under the ROC (AUROC) was used as an index of diagnostic accuracy, with an AUROC nearest to the upper left corner of the ROC graph indicating high accuracy. The optimal cut-off values were selected, based on AUROCs. Descriptive statistics such as frequencies and cross-tabulations, mean values and standard deviations (mean ± SD), sensitivity, specificity, and positive and negative predictive values (PPV, NPV) were calculated. Statistical significance was determined at P-value < 0.05. Statistical analyses were performed using a computer software package (NCSS 2007).
Results
The histological evaluation of biopsy specimens obtained from the 282 FLLs led to the following diagnoses: (a) 34 were benign lesions: 25 RN (maximum transverse diameter, mean ± SD: 19 ± 10.4 mm) and 9 DN (19 ± 4.1 mm);
(b) 248 were malignancies: 223 well-to-moderately differentiated HCC (35 ± 11.5 mm), 7 poorly-differentiated HCC (29 ± 5.6 mm), 5 ICC (36 ± 8.3 mm), 5 primary non-Hodgkin B-cell lymphoma (NHBL, 43 ± 9.1 mm) and 8 metastatic liver tumors (3 colon cancers, 2 gastric cancers, 1 neuroendocrine cancer, 1 lung cancer and 1 sarcoma; 50 ± 28.2 mm). The maximum transverse diameter (mean ± SD) of liver lesions was 33 ± 14.3 mm for single lesions (range, 12–115) and 33 ± 11.5 mm (range, 10–84) for multiple ones, as seen in Table 2.
Table 2.
Histological diagnosis of FLLs (n = 228) and their relative maximum transverse diameter
| Histological diagnosis | Maximum transverse diameter (M ± SD) | N |
|---|---|---|
| RN | 19 ± 10.4 mm (range, 10–58 mm) | 25 |
| DN | 19 ± 4.1 mm (range, 15–28 mm) | 9 |
| Well-moderately differentiated HCC | 35 ± 11.5 mm (range, 18–85 mm) | 223 |
| Poorly-differentiated HCC | 29 ± 5.6 mm (range, 22–35 mm) | 7 |
| ICC | 36 ± 8.3 mm (range, 28–48 mm) | 5 |
| NHBL | 43 ± 9.1 mm (range, 33–55 mm) | 5 |
| Mtx | 50 ± 28.2 mm (range, 28–115 mm) | 8 |
| Total n = 282 |
DN: Dysplastic nodules; FLL: focal liver lesions; HCC: hepatocellular carcinoma; ICC: intrahepatic cholangiocellular carcinoma; M + /– SD: mean plus standard deviation; Mtx: metastasis; NHBL: primary non-Hodgkin B-cell lymphoma; RN: regenerative nodules.
All malignant lesions (both HCC and non-HCC) were shown to be hypervascular during the arterial phase, so that ‘time to wash-out’ during the portal and late phases was considered for final diagnosis.
Mean ‘time to wash-out’ of all HCC (including both well-to-moderately and poorly-differentiated) resulted in being 136 ± 30 s, while non-HCC malignancies showed a mean ‘time to wash-out’ of 39 ± 8.7 s (ICC: 38 ± 6 s; primary NHBL: 31 ± 6.1 s; and metastases: 45 ± 7.7 s). Mean ‘time to wash-out’ between HCC and non-HCC malignant lesions was statistically significant (p < 0.000). In contrast, we observed no significant differences between non-HCC malignancies of different origin.
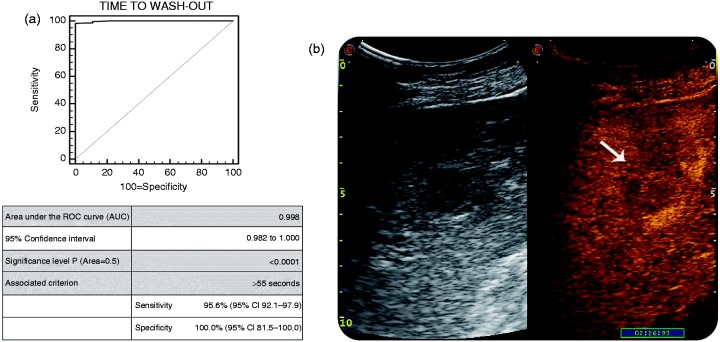
By using a cut-off value of ‘time to wash-out’ > 55 s (Figure 2; AUC 0.998; p < .0001), CEUS correctly diagnosed 217 (97.3%) of the 223 histologically proven, well-differentiated HCC, with a mean time to wash-out of 139 ± 26 s; and 3 (42.9%) of the 7 histologically proven poorly-differentiated HCC, with a mean time to wash-out of 57 ± 1 s (Figure 2(b)). The remaining 6/223 (2.7%) histologically proven well-to-moderately differentiated HCC behaved as benign lesions (isovascular during the arterial phase), even though the lack of wash-out does never qualify for a benign lesion in cirrhosis, especially if the lesion is hypervascular. On the other hand, 4/7 (57.1%) of the histologically-proven, poorly-differentiated HCC presented an early wash-out (i.e. 53 ± 4 s), thus leading to an erroneous classification of non-HCC malignancies. The sensitivity was of 95.6% and the specificity of 100.0%. PPV and NPV were 100.0% and 64.2%, respectively, with an accuracy of 92.7% in diagnosing HCC.
Figure 2.
(a) AUROC for CEUS in detecting HCC within cirrhotic liver, in comparison with the gold standard (liver biopsy). (b): B-mode US (on left) and CEUS examination (on right) of FLL in cirrhosis. The typical late wash-out (i.e. 2 min and 26 s) was suggestive for diagnosis of HCC.
AUC: Area under the curve; AUROC: area under the receiver operating characteristics curve; CEUS: contrast-enhanced ultrasonography; FLL: focal liver lesions; HCC: hepatocellular carcinoma; ROC: receiver operating characteristics; US: ultrasonography
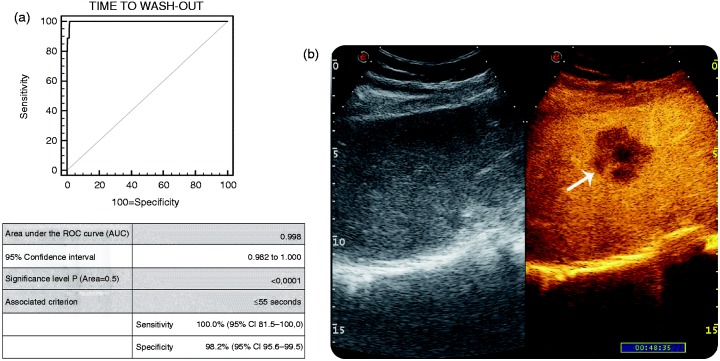
Similarly, according to the AUROC analysis (Figure 3; AUC 0.998; p < .0001), a cut-off value of ‘time to wash-out’ ≤ 55 s was selected to diagnose non-HCC malignancy. This non-invasive biomarker was in agreement with liver biopsy in all of the 18 histologically-proven non-HCC malignant lesions, including ICC, NHBL and metastatic lesions, which all showed an early wash-out (i.e. 39 ± 8.7 s), as seen in Figure 3(b). Sensitivity was 100.0%, specificity 98.2%, PPV 82%, NPV 100.0% and accuracy 98.3% in diagnosing non-HCC malignancy (Table 3 and Table 4).
Figure 3.
(a) AUROC for CEUS in detecting non-HCC malignancy within cirrhotic liver in comparison with the gold standard (liver biopsy). (b): B-mode US (on left) and CEUS examination (on right) of FLL in cirrhosis. The early wash-out (i.e. 48 s) was suggestive for non-HCC malignancy (pathologically confirmed to be an ICC).
AUROC: Area under the curve; CEUS: contrast-enhanced ultrasonography; FLL: focal liver lesions; HCC: hepatocellular carcinoma; ICC: intrahepatic colangiocellular carcinoma; US: ultrasonography.
Table 3.
Comparison of ‘time to wash-out’ at CEUS and histological diagnosis of all studied lesions
| Time to wash-out (seconds) M ± SD | N | Diagnosis on biopsy: n |
|---|---|---|
| NO wash-out | 40 | RN: n = 25 |
| DN: n = 9 | ||
| Well-to-moderately differentiated HCC: n = 6 | ||
| > 55 s (M ± SD: 136 ± 30 s) | 220 | Well-to-moderately differentiated HCC: n = 217 |
| Poorly-differentiated HCC: n = 3 | ||
≤ 55 s Overall M ± SD: 43 ± 10 s:
|
22 | Poorly-differentiated HCC: n = 4 |
| Mtx: n = 8 ICC: n = 5 NHBL: n = 5 |
CEUS: contrast-enhanced ultrasonography; M ± SD: mean ± standard deviation; DN: dysplastic nodules; HCC: hepatocellular carcinoma; ICC: intrahepatic cholangiocellular carcinoma; Mtx: metastasis; NHBL: primary non-Hodgkin B-cell lymphoma; RN: regenerative nodules.
Table 4.
Diagnostic accuracy of the ‘time to wash-out’ cut-off values above and below 55 s, for diagnosis of HCC and non-HCC malignant lesions, respectively
| Cut-off time to wash-out of contrast agent | ||
|---|---|---|
| For HCC diagnosis | > 55 s | Sensitivity: 95.6% (95% CI 92–98%) |
| Specificity: 100.0% (95% CI 81.5–100.0%) | ||
| PPV: 100.0% (95% CI 98.3–100.0%) | ||
| NPV: 64.2% (95% CI 44–81.3%) | ||
| Diagnostic accuracy: 92.7% | ||
| For diagnosis of malignant lesions other than HCC | ≤ 55 s | Sensitivity: 100% (95% CI 81.4–100.0%) |
| Specificity: 98.2% (95% CI 95.6–99.5%) | ||
| PPV: 82% (95% CI 59.7–94.8%) | ||
| NPV: 100.0% (95% CI 93.3–100.0%) | ||
| Diagnostic accuracy: 98.3% | ||
CI: confidence interval; HCC: hepatocellular carcinoma; NPV: negative predictive value; PPV: positive predictive value.
All of the 34 benign lesions, both RN and DN, retained the contrast agent during all three vascular phases and were therefore correctly diagnosed by CEUS. The interobserver agreement in reviewing CEUS clips was k = 0.780 and the 95% CI (0.724–0.849). We observed no side-effects after i.v. injection of the contrast agent SonoVue® nor biopsy-related complications in any of the patients.
Discussion
HCC represents the most frequent focal lesion detected in cirrhotic liver patients. Biannual US is the main screening method for detection at a stage when curative treatments can still be performed.22,23 Un-enhanced US presents limited accuracy in FLL characterization, because both benign and malignant lesions may reveal similar echo patterns.24 The administration of a contrast agent gives additional information for FLL characterization. The diagnostic value of CEUS has been previously confirmed by multicenter studies,25–27 including the DEGUM (Deutsche Gesellschaft für Ultraschall in der Medizin) trial and the French Multicenter Study.
The first study25 included 1349 patients: The overall diagnostic accuracy of CEUS in characterizing FLLs, when compared with a diagnostic gold standard (biopsy in more than 75% of the lesions, spiral contrast computed tomography (CT) and contrast magnetic resonance imaging (MRI) in the rest of cases) was 90.3%; the sensitivity and specificity were 95.8% and 83.1%, respectively. The positive predictive value for the classification of malignant lesions was 95.0% and the negative predictive value was 95.9%. The authors concluded that CEUS, CT and MRI should be considered similar, with regard to tumor characterization. The French study26 evaluated whether the characterization of 134 FLLs could be improved by using CEUS, as compared to un-enhanced-US, CECT and CEMRI. CEUS emerged as the most sensitive (95.5%), most specific (75.0%); and thus, most accurate imaging modality for FLL characterization, when compared to the other imaging techniques. Quaia et al.27 report that the diagnostic accuracy for FLL characterization increased from 49% at baseline US to 85% after CEUS (with sensitivity and specificity increasing from 53% to 83%, and to 41% to 95%, respectively).
In 2010, the AASLD practice guidelines removed CEUS from the diagnostic flow-chart for HCC because of CEUS being not reliable in discriminating between ICC and HCC.13 The rationale for the AASLD guidelines was based on a retrospective study by Vilana et al.,15 which reported that ICC may display a vascular pattern indistinguishable from HCC at CEUS, so that it should not be used as the sole diagnostic tool for noninvasive diagnosis of HCC. The same conclusions were reached by EASL-EORTC guidelines.14 In 2012, the Italian AISF stated that a ‘cut-off time to wash-out’ < 60 s for the characterization of FLLs in cirrhosis might be helpful to discriminate HCC from ICC, even if studies aimed at confirming its diagnostic value were still lacking.16
In the current study, basing on ROC curves, a ‘time to wash-out’ cut-off level of 55 s showed it possessed the greatest accuracy in differentiating HCC and non-HCC malignancies. Particularly, two ‘cut-off times to wash-out’ were selected: > 55 s for diagnosis of HCC and ≤ 55 s for non-HCC malignancies. CEUS correctly diagnosed 220 out of 230 HCC (217 well-to-moderately differentiated and 3 poorly-differentiated HCC) with a sensitivity of 95.6%, while it misdiagnosed as benign lesions six well-differentiated HCC that did not wash-out up to 5 min after injection of contrast agent. This might be accounted for by the fact that echogenicity in portal and late phases correlates with HCC cellular differentiation with a significant likelihood that well-differentiated lesions retain contrast,28 whereas poorly-differentiated lesions wash out contrast in the portal or late phase.29 Isovascularity of six HCCs may be partially accounted for by the sensitivity of the software that was used for the analysis of CEUS imaging or, more likely, because the lesions had not yet developed neovascularization (the so-called vaguely nodular HCC). No false positive diagnoses of well-to-moderate HCC was observed. On the other hand, a ‘cut-off time to wash-out’ ≤ 55 s correctly identified all of the 18 non-HCC malignant lesions; however, similarly four of the poorly-differentiated HCC showed an early wash-out and were erroneously classified as non-HCC FFLs.
The diagnostic accuracy of a ‘cut-off time to wash-out’ ≤ 55 s for the diagnosis of non-HCC malignancies was 98.3%, with a sensitivity of 100.0% and a specificity of 98.2%. Benign FLLs (i.e. RN and DN) appear mainly to have hypoenhancing in the arterial phase and isoenhancing in the portal venous phase, even though the same pattern may also be observed in well-to-moderately differentiated HCC.30 As expected, in our study all 34 RN and DN did not wash out the contrast agent during the portal venous and late phases; however, a similar wash-out pattern was also observed in 6 out of the 223 well-differentiated HCCs (five of them measuring > 20 mm).
The apparent discrepancy between our results and those reported by Vilana et al. might be partially due to various factors: the small number of enrolled patients (21 patients observed over a 6-year period) and the high prevalence of small lesions (median size 32 mm), which are known to have higher probability of misdiagnosis with HCC.31 In partial support of our data, Chen et al.32 studied the CEUS enhancement pattern in 50 patients with pathologically proven ICC and 50 patients with pathologically proven HCC: The occurrence of a pattern resembling that of HCC (i.e. homogeneous enhancement) was observed in only 6% of ICC cases (median size 72 mm), which is lower than that reported by Vilana et al. (i.e. 47.6%). An AISF expert panel16 has recently reported that a contrastographic pattern typical for HCC at CEUS has a positive predictive value > 95.0%; ICC accounts for 1–2% of all new nodules detected in cirrhosis, with one-half of them showing the typical HCC pattern at CEUS.
Safety and cost are important issues to consider when dealing with diagnostic procedures. We did not record any adverse events related to CEUS, confirming previous evidence.33,34 Compared with CETC or CEMRI, we showed CEUS to be a more cost-effective option as a first-line procedure for characterization of FLLs in cirrhotic patients.17,35 The limitations of this study are:
The absence of direct comparison between CEUS data and those deriving from another imaging technique (CECT or CEMRI);
The use of a visual estimation, without the help of specialized software, in measuring the ‘time to wash-out’.
Moreover, because some of the patients were referred to our unit from other institutions, not all of the patients were under a biannual US screening for HCC, so that some of their lesions were of a larger diameter than might be expected if the patients had been checked every 6 months. The observation of a RN with a diameter of 58 mm is unusual and this might be partially explained as a sampling error; however, the RN was followed up, showing no increase in size and then the diagnosis was confirmed by a second biopsy.
In conclusion, CEUS was highly accurate for classifying FLLs in cirrhotic liver patients. In particular, HCC and ICC were best differentiated by wash-out features, rather than the arterial enhancement pattern, with ICC having the tendency to wash out more thoroughly and more quickly (i.e. ≤ 55 sec) than HCC in the portal and delayed phases. In our study population, the clinical impact of ICC on the false-positive rate for HCC turned out to be very low (1.7%).
When a liver lesion is first detected in a cirrhotic patient during a surveillance program, CEUS may provide relevant diagnostic information, mainly based on ‘time to wash-out’ of the contrast agent. In particular, when a FLL shows a global ‘wash-in’, followed by a very rapid (≤ 55 s) and marked ‘wash-out’, a sample for histology should be obtained, because this pattern is a potential indicator of ICC or other malignancies. On the contrary, when a ‘cut-off time to wash-out’ > 55 s is recognized (which in our study, had a specificity and a PPV for HCC diagnosis equal to 100.0%), no further evaluation is needed. In conclusion, because of its high safety profile, low cost and easy repeatability, CEUS should maintain a prominent role as a first-line examination in the characterization of FLLs in patients with cirrhosis and should be combined with other imaging modalities (CECT or CEMRI) or histological confirmation only in equivocal cases.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors declare that there are no conflicts of interest.
References
- 1.Llovet JM, Bruix J. Novel advancements in the management of hepatocellular carcinoma in 2008. J Hepatol 2008; 48: S20–S37. [DOI] [PubMed] [Google Scholar]
- 2.Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 European Association for the Study of the Liver (EASL) conference. J Hepatol 2001; 35: 421–430. [DOI] [PubMed] [Google Scholar]
- 3.Singal A, Volk ML, Waljee A, et al. Meta-analysis: Surveillance with ultrasound for early-stage hepatocellular carcinoma in patients with cirrhosis. Alimentary Pharmacol Therapeut 2009; 30: 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caturelli E, Bartolucci F, Biasini E, et al. Diagnosis of liver nodules observed in chronic liver disease patients during ultrasound screening for early detection of hepatocellular carcinoma. Am J Gastroenterol 2002; 97: 397–405. [DOI] [PubMed] [Google Scholar]
- 5.Zardi EM, Uwechie V, Picardi A, et al. Liver focal lesions and hepatocellular carcinoma in cirrhotic patients: From screening to diagnosis. Clin Ter 2001; 152: 185–188. [PubMed] [Google Scholar]
- 6.Jang HJ, Yu H, Kim TK. Imaging of focal liver lesions. Semin Roentgenol 2009; 44: 266–282. [DOI] [PubMed] [Google Scholar]
- 7.Bolondi L, Correas JM, Lencioni R, et al. New perspectives for the use of contrast-enhanced liver ultrasound in clinical practice. Dig Liver Dis 2007; 39: 187–195. [DOI] [PubMed] [Google Scholar]
- 8.Von Herbay A, Westendorff J, Gregor M. Contrast-enhanced ultrasound with SonoVue: Differentiation between benign and malignant focal liver lesions in 317 patients. J Clin Ultrasound 2010; 38: 1–9. [DOI] [PubMed] [Google Scholar]
- 9.Claudon M, Cosgrove D, Albrecht T, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) - update 2008. Ultraschall Med 2008; 29: 28–44. [DOI] [PubMed] [Google Scholar]
- 10.Claudon M, Dietrich CF, Choi BI, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver - update 2012: A WFUMB-EFSUMB initiative in cooperation with representatives of AFSUMB, AIUM, ASUM, FLAUS and ICUS. Ultraschall Med 2013; 34: 11–29. [DOI] [PubMed] [Google Scholar]
- 11.Omata M, Lesmana LA, Tateishi R, et al. Asian Pacific Association for the Study of the Liver: Consensus reccomendations on hepatocellular carcinoma. Hepatol Int 2010; 4: 439–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: Consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis 2011; 29: 339–364. [DOI] [PubMed] [Google Scholar]
- 13.Bruix J, Sherman M. Management of hepatocellular carcinoma: An update. Hepatology 2011; 53: 1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56(4): 908–943. [DOI] [PubMed]
- 15.Vilana R, Forner A, Bianchi L, et al. Intrahepatic peripheral cholangiocarcinoma in cirrhosis patients may display a vascular pattern similar to hepatocellular carcinoma on contrast-enhanced ultrasound. Hepatology 2010; 51: 2020–2029. [DOI] [PubMed] [Google Scholar]
- 16.AISF, AISF Expert Panel, AISF Coordinating Committee, et al. Position paper of the Italian Association for the Study of the Liver (AISF): The multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis 2013; 45: 712–723. [DOI] [PubMed] [Google Scholar]
- 17.Sirli R, Sporea I, Martie A, et al. Contrast enhanced ultrasound in focal liver lesions: A cost efficiency study. Med Ultrason 2010; 12: 280–285. [PubMed] [Google Scholar]
- 18.de Sio I, Funaro A, Vitale LM, et al. Ultrasound-guided percutaneous biopsy for diagnosis of gastrointestinal lesions. Dig Liver Dis 2013; 45(10): 816–819. [DOI] [PubMed]
- 19.Edmondson HA, Steiner PE. Primary carcinoma of the liver: A study of 100 cases among 48,900 necropsies. Cancer 1954; 7: 462–503. [DOI] [PubMed] [Google Scholar]
- 20.Caturelli E, Solmi L, Anti M, et al. Ultrasound guided fine needle biopsy of early hepatocellular carcinoma complicating liver cirrhosis: A multicentre study. Gut 2004; 53: 1356–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim JH, Park CK. Pathology of cholangiocarcinoma. Abdom Imaging 2004; 29: 540–547. [DOI] [PubMed] [Google Scholar]
- 22.Bruix J, Llovet JM. Prognostic prediction and treatment strategy in hepatocellular carcinoma. Hepatology 2002; 35: 519–524. [DOI] [PubMed] [Google Scholar]
- 23.Santi V, Trevisani F, Gramenzi A, et al. Semiannual surveillance is superior to annual surveillance for the detection of early hepatocellular carcinoma and patient survival. J Hepatol 2010; 53(2): 291–297. [DOI] [PubMed] [Google Scholar]
- 24.Reinhold C, Hammers L, Taylor CR, et al. Characterization of focal hepatic lesions with duplex sonography: Findings in 198 patients. Am J Roentgenol 1995; 164: 1131–1135. [DOI] [PubMed] [Google Scholar]
- 25.Strobel D, Seitz K, Blank W, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions: Diagnostic accuracy in clinical practice (DEGUM multicenter trial). Ultraschall Med 2008; 29: 499–505. [DOI] [PubMed] [Google Scholar]
- 26.Tranquart F, Correas JM, Ladam Marcus V, et al. Real-time contrast-enhanced ultrasound in the evaluation of focal liver lesions: Diagnostic efficacy and economical issues from a French multicentric study. J Radiol 2009; 90: 109–122. [DOI] [PubMed] [Google Scholar]
- 27.Quaia E, Lorusso A, Grisi G, et al. The role of CEUS in the characterization of hepatocellular nodules detected during the US surveillance program: Current practices in Europe. Ultraschall Med 2012; 33: S48–56. [DOI] [PubMed] [Google Scholar]
- 28.Nicolau C, Catalá V, Vilana R, et al. Evaluation of hepatocellular carcinoma using SonoVue, a second generation ultrasound contrast agent: Correlation with cellular differentiation. Eur Radiol 2004; 14: 1092–1099. [DOI] [PubMed] [Google Scholar]
- 29.Boozari B, Soudah B, Rifai K, et al. Grading of hypervascular hepatocellular carcinoma using the late phase of contrast enhanced sonography: A prospective study. Dig Liver Dis 2011; 43: 484–490. [DOI] [PubMed] [Google Scholar]
- 30.Braun B. Focal liver processes: ‘Better is the enemy of good’. CEUS in the fast lane. Ultraschall Med 2009; 30: 329–332. [DOI] [PubMed] [Google Scholar]
- 31.Dietrich CF, Cui XW, Boozari B, et al. Contrast-enhanced ultrasound (CEUS) in the diagnostic algorithm of hepatocellular and cholangiocellular carcinoma, comments on the AASLD guidelines. Ultraschall Med 2012; 33: S57–66. [DOI] [PubMed] [Google Scholar]
- 32.Chen LD, Xu HX, Xie XY, et al. Intrahepatic cholangiocarcinoma and hepatocellular carcinoma: Differential diagnosis with contrast-enhanced ultrasound. Eur Radiol 2010; 20: 743–753. [DOI] [PubMed] [Google Scholar]
- 33.Bokor D, Chambers JB, Rees PJ, et al. Clinical safety of SonoVue, a new contrast agent for ultrasound imaging, in healthy volunteers and in patients with chronic obstructive pulmonary disease. Invest Radiol 2001; 36: 104–109. [DOI] [PubMed] [Google Scholar]
- 34.Piscaglia F, Bolondi L and Italian Society for Ultrasound in Medicine and Biology (SIUMB) Study Group on Ultrasound Contrast Agents. The safety of Sonovue in abdominal applications: Retrospective analysis of 23188 investigations.Ultrasound Med Biol 2006; 32: 1369–1375. [DOI] [PubMed]
- 35.Giesel FL, Delorme S, Sibbel R, et al. Contrast-enhanced ultrasound for the characterization of incidental liver lesions - An economical evaluation in comparison with multi-phase computed tomography. Ultraschall Med 2009; 30: 259–268. [DOI] [PubMed] [Google Scholar]