Abstract
Background
A modified 13C-mixed triglyceride breath test (13C -MTGT) detects moderate pancreatic exocrine insufficiency noninvasively and reliably, but it requires prolonged breath sampling (6 hours (hr)).
Objective
We aimed to investigate whether 13C -MTGT can be abbreviated, to optimize clinical usability.
Methods
We analyzed the 13C-MTGT of 200 consecutive patients, retrospectively. Cumulative 1–5 hr 13C-exhalation values were compared with the standard parameter (6-hr cumulative 13C-exhalation). We determined the sensitivity and specificity of shortened breath sampling periods, by comparison with the normal values from 10 healthy volunteers, whom also underwent a secretin test to quantitate pancreatic secretion. Moreover, we evaluated the influence of gastric emptying (GE), using a 13C-octanoic acid breath test in a subset (N = 117).
Results
The 1–5 hr cumulative 13C-exhalation tests correlated highly and significantly with the standard parameter (p < 0.0001). Sensitivity for detection of impaired lipolysis was high (≥77%), but the specificity was low (≥38%) for the early measurements. Both parameters were high after 4 hrs (88% and 94%, respectively) and 5 hrs (98% and 91%, respectively). Multivariate linear correlation analysis confirmed that GE strongly influenced early postprandial 13C-exhalation during the 13C-MTGT.
Conclusion
Shortening of the 13C -MTGT from 6 to 4 hrs of duration was associated with similar diagnostic accuracy, yet increased clinical usability. The influence of GE on early postprandial results of the 13C-MTGT precluded further abbreviation of the test.
Keywords: Breath test, diagnostic testing, gastric emptying, lipolysis, mixed triglyceride breath test, optimization, pancreatic disease, pancreatic function
Introduction
At present, clinicians almost exclusively use the fecal elastase-1 test for measurement of pancreatic exocrine function, although this test has limited sensitivity for mild-to-moderate pancreatic exocrine insufficiency, and despite its limited specificity in patients with diarrhea.1,2 The lack of clinically available alternatives prompted creation of a research project supported by the United European Gastroenterology (UEG) National Societies LINK Award Programme, Harmonising Diagnosis and Therapy of Pancreatitis across Europe (HaPanEU), which explicitly aims to standardize the use of diagnostic criteria and test tools for pancreatic exocrine insufficiency. In particular, this research project aims to define the optimal way to perform and analyze alternative pancreatic function tests, such as secretin-enhanced magnetic resonance cholangiopancreatography (MRCP) and 13C-breath tests, under clinical conditions.
We have shown previously that a modified version of the 13C-mixed triglyceride breath test (13C-MTGT) detects moderate pancreatic exocrine insufficiency noninvasively and reliably.3 Compared with other versions of the 13C-MTGT, our modified test uses higher lipid loads, in order to exceed lipolytic capacity in individuals with moderately decreased enzyme secretion and employs strict limitation of physical activity, to reduce the variability of endogenous carbon dioxide production. The 13C-MTGT is generally based on the principle that intestinal triglyceride absorption requires prior hydrolysis by pancreatic lipase to produce free fatty acids and mono-acyl-glycerol. These metabolites are absorbed and transported to the liver. Hepatic metabolism subsequently leads to formation of 13CO2 that is absorbed into the bloodstream, transported to the lung and exhaled. It has been shown that the increase in 13CO2-concentration in breath correlates with pancreatic lipase secretion.3–5
The optimal parameter for evaluation of pancreatic exocrine function by 13C-MTGT, reported by various groups including ours, is cumulative 13CO2-exhalation (in a percentage of dose administered) over 5–8 hours (hrs)3–6; thus, although the 13C-MTGT is a rather convenient, noninvasive indirect pancreatic function test, its long period of breath sampling and patient immobilization is a major drawback for clinical application. Therefore, as part of the UEG National Societies’ LINK Award Programme HaPanEU, we aimed to study whether it is possible to shorten the 13C-MTGT for clinical purposes. Because digestion of dietary lipids by pancreatic lipase cannot occur before the meal has entered the duodenum, we additionally aimed to investigate whether gastric emptying velocity influences the results of the 13C-MTGT.
To achieve these goals, we retrospectively analyzed 200 consecutive 13C-MTGT performed at our institution and we compared the cumulative 13C-exhalation over 1- to 5-hr periods with the standard parameter, 6 hr of cumulative 13C-exhalation. These patients’ data were compared to those of healthy volunteers, in whom pancreatic exocrine function was quantified using both the secretin-test as the ‘gold standard’ for pancreatic function testing and the 13C-MTGT. Furthermore, in a subset of 117 patients whom also received a 13C-octanoic acid breath test (13C-OAT) for measurement of gastric emptying velocity, we tested the influence of gastric emptying on the results of the 13C-MTGT test.
Materials and methods
Participants
We identified and retrospectively analyzed data from 200 consecutive patients whom underwent a 13C-MTGT at our institution for clinical reasons, between January 2010 and February 2011. These patients had presented with symptoms suggestive of pancreatic disease and/or pancreatic exocrine insufficiency, such as upper abdominal pain, bloating, diarrhea/steatorrhea and weight loss.
Data were compared to those of 10 healthy subjects (mean age 28 ± 1 years, mean body mass index (BMI) of 23.1 ± 0.8 kg/m2 (six were women)), in whom a secretin test was performed. Such direct tests using hormone stimulation are the most sensitive and specific tests for assessing the pancreatic exocrine reserve; and therefore, are accepted as reference standards.7,8 All of our healthy volunteers also received a 13C-MTGT and a gastric emptying test, 13C-OAT. The data from these subjects have been partially published before.3
Ethics
The study protocol for evaluation of pancreatic exocrine function in healthy volunteers was approved by the local ethics committee (Ethik-Kommission der Ärztekammer Hamburg, reference number 1822) and all subjects gave written informed consent prior to any study-related procedures.
Secretin test, a direct pancreatic function test
The tip of a double lumen Lagerlöf tube was placed into the distal duodenum, for constant collection of gastric and duodenal juice on ice, during a 30 min basal period and then during 60 min with receipt of an intravenous infusion of 1U/kg*hr secretin (Secrelux®, Goldham Bioglan Pharma GmbH, Zusmarshausen, Germany). This duodenal juice was fractionated in 10-min intervals and we performed analyses of pH, volume, bicarbonate and enzyme outputs, as described previously.9–12
13C-mixed triglyceride breath test, an indirect test of pancreatic function
All the subjects evaluated in this study received a standardized test meal,3 consisting of two slices of white bread, 20 g butter and 30 g chocolate cream (Nutella®, Ferrero Rocher, Germany). The latter was carefully mixed with 250 mg 13C-MTG (2-octanoyl(1-13C)-1,3 distearoyl glycerol, Euriso-top, Saarbrücken, Germany, catalog number INC650P), a triglyceride with 13C-octanoic acid labeled with one 13C-atom bound to the Sn-2 position and unmarked long-chain fatty acids bound to the Sn-1 and Sn-3 positions. The meal was ingested within 10 min, together with 200 ml of water. Its total caloric value was 420 kcal (1770 kJ). All subjects were instructed to remain seated during study procedures, and we collected breath samples before ingestion of the test meal and every 30 minutes, for the 6 hrs thereafter. We determined the 13CO2/12CO2 isotope ratio in the subject’s breath by using isotope-selective non-dispersive infrared spectrometry (IRIS®, Kibion/Wagner Analysen Technik, Bremen, Germany).13 Our results were analyzed as delta values and expressed as cumulative 13C-exhalation, in the percentages of dose recovered over the 1–6 hr intervals. Our tests were performed by three nurses highly experienced in pancreatic function testing and finally, evaluated by a gastroenterologist.
Gastric emptying breath test
A subset of 117 patients additionally underwent a 13C-OAT for measurement of gastric emptying of solids.14–16 Their test meal consisted of 200 ml orange juice, two slices of white bread, 10 g butter, 50 g ham and one scrambled egg, with the yolk doped with 91 mg 13C-octanoic acid (Euriso-top, Saarbrücken, Germany) and was to be ingested within 10 min. We collected breath samples before the test meal and at 15-min intervals for 4 hrs, postprandially. Our subjects were instructed to remain seated throughout the test procedures. The 13CO2/12CO2 isotope ratio in the subjects’ breath was determined by using IRIS.13 Results were analyzed as delta values and expressed as cumulative 13C-exhalation in the percent of dose recovered over the 1–4 hr intervals.16 We evaluated the half time of gastric emptying (T ½) and lag time (T lag), according to methods in Ghoos et al.14 Tests were performed by three nurses whom were highly experienced in pancreatic function testing and finally, they were evaluated by a gastroenterologist. We excluded 13C-OAT data from further analysis if the subjects had received a modified (vegetarian) test meal and/or if the time interval between both breath tests exceeded 4 weeks.
Definitions and statistical methods
We have shown before that cumulative 13C-exhalation over 6 hrs (cut-off: 26.8% of the dose administered) is the best parameter for evaluation of the 13C-MTGT and has an excellent sensitivity (100%) and specificity (92%) for detection of impaired lipase secretion in patients with pancreatic afflictions, when compared with the secretin test as the reference standard.3 Thus, we used cumulative 13C-exhalation over 6 hrs as the internal reference parameter for evaluating both sensitivity and specificity of shorter patient breath-sampling periods. We did this by testing for whether the established parameter and breath test data obtained over shorter periods of time gave compatible results, indicating either normal or reduced pancreatic exocrine function. Furthermore, we divided patients with decreased exocrine function, according to the standard parameter, as having exhalation that was: severely impaired (cumulative 13C-exhalation over 6 hrs <8.9% of dose), moderately impaired (cumulative 13C-exhalation over 6 hrs 8.9–17.9% of dose) or mildly impaired (cumulative 13C-exhalation over 6 hrs that was 17.9% to <26.8% of the original dose given) pancreatic exocrine function. We determined the normal values for the abbreviated breath sampling periods from the data obtained in healthy volunteers (n = 10, defined as the mean value of healthy controls – SD).
We used 2-tailed Student's t-tests for paired and unpaired data, for statistical analyses and univariate or multivariate linear regression analyses, applying them as appropriate. For the association between cumulative 13C-exhalation over 6 hrs and cumulative 13C-exhalation over shorter periods of time, we additionally used the Deming regression (http://peltiertech.com/WordPress/deming-regression-utility/), as both parameters can be considered to be experimental.. We expressed our study data as a mean ± SD, unless indicated otherwise. We performed statistical analyses using the JMP® version 6.0.3 (SAS Institute, Cary, NC, USA).
Results
Of the 200 patients investigated, 19 had incomplete data, so they were excluded from further analysis (Figure 1). Among the remaining 181 patients there were 116 women, the patients’ mean age was 52.0 ± 17.7 years and their mean body mass index (BMI) was 23.4 ± 4.8 kg/m2. Breath tests were generally tolerated well by all subjects and no relevant adverse events occurred. We compared the cumulative 13C-exhalation (in percent of dose administered) over the 1- to 6-hr intervals with normal values in these subjects.
Figure 1.
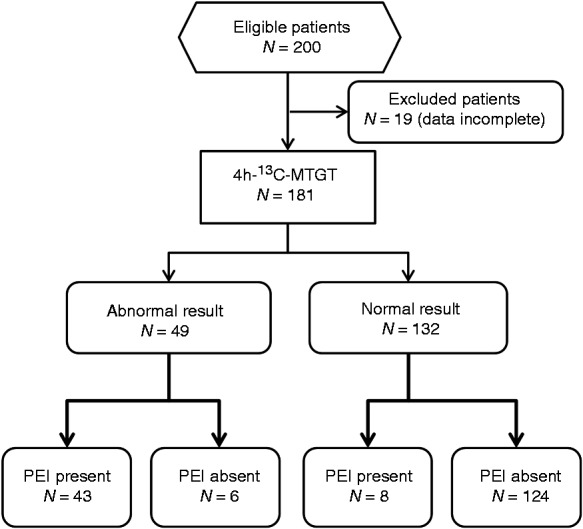
Patient flow.
13C-MTGT: 13C-mixed triglyceride breath test; PEI: pancreatic exocrine insufficiency.
In 130 patients, our standard parameter for measurement of pancreatic exocrine function (that is, 6-hr cumulative 13C-exhalation) was equal to or exceeded 26.8% of the dose administered and was therefore regarded as normal,3 but 51 patients had pathologically decreased 6-h cumulative 13C-exhalation that was compatible with impaired lipolysis and pancreatic exocrine insufficiency.
Cumulative 13C-exhalation over 1–5 hrs correlated in a highly significant manner with the standard parameter, i.e. 6-hr cumulative 13C-exhalation, as is shown in Table 1. Individual data for cumulative 13C-exhalation over 4 and 5 hrs are presented in Figure 2. Conventional linear regression and Deming regression gave almost identical results for both correlation lines (4 h: linear: y = 0.68x −3.59, R = 0.94 versus Deming: y = 0.68x −3.67, R = 0.97; and 5 h: linear: y = 0.86x −2.22, R = 0.99 versus Deming: y = 0.86x −2.23, R = 0.99).
Table 1.
Normal values for the 13C-exhalation over 1–5 h, as obtained in healthy volunteers, and the correlation in patients between cumulative 13C-exhalation over 1–5 h and the standard 6-h parameter
| Normal value (% of dose) | Correlation coefficient | Sensitivity | Specificity | |
|---|---|---|---|---|
| 1 h | ≥1.6% | 0.66a | 97% | 38% |
| 2 h | ≥4.2% | 0.82a | 93% | 71% |
| 3 h | ≥7.9% | 0.89a | 77% | 88% |
| 4 h | ≥13.8% | 0.94a | 88% | 94% |
| 5 h | ≥20.9% | 0.98a | 98% | 91% |
p < 0.0001.
h: hours.
Figure 2.
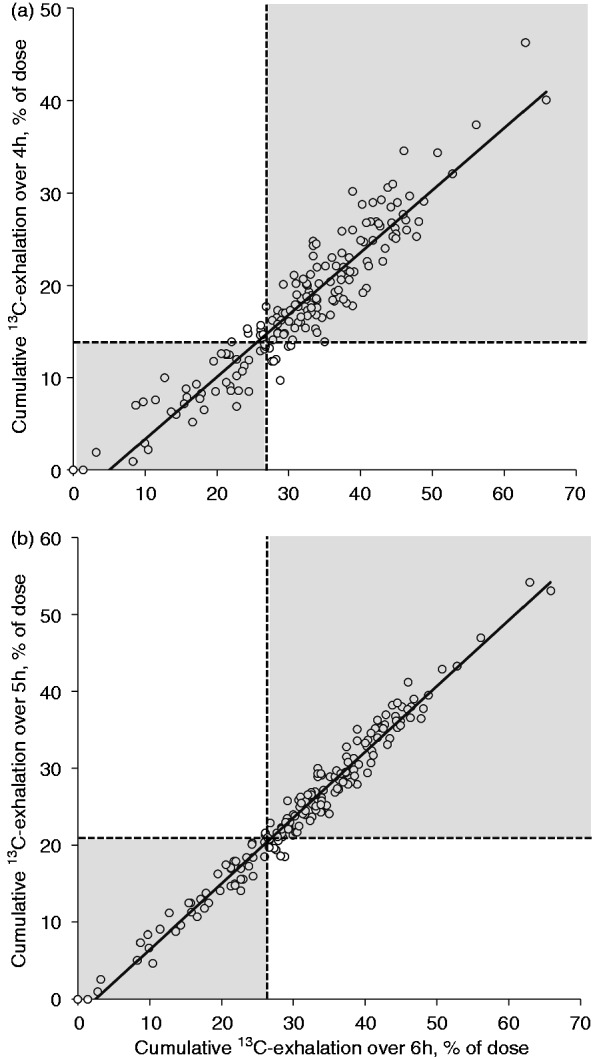
Cumulative 13C-exhalation (in percent of dose) over (a) 4 hrs and (b) 5 hrs postprandially, in association with the standard parameter (cumulative 13C-exhalation over 6 hrs). Investigations were performed in 181 patients whom received a 13C-MTGT for clinical reasons. Cumulative 13C-exhalation over 4 and 5 hrs correlated highly significantly with the standard parameter (R = 0.94 and R = 0.98, respectively; p < 0.0001). Dotted lines mark the lower level of normal; and thus, grey areas mark tests that give compatible results using the standard parameter and the abbreviated test.
hrs: hours; 13C-MTGT: mixed triglyceride breath test.
When taking normal values obtained in healthy volunteers into account (Table 1), sensitivity was already high for early postprandial measurements (1 and 2 hrs postprandially); however, specificity was low at these time points. Cumulative 13C-exhalation over 4 hrs was the first measurement that showed satisfactory results for both sensitivity and specificity (Table 1 and Figure 2, cut off: 13.8% of 13C-dose exhaled cumulatively). Importantly, all subjects with more than mildly impaired lipolysis according to the standard parameter were also detected using the 4 hr value, whereas 8 out of 30 patients with mildly impaired lipolysis had normal 4-hr 13C-exhalation (Figure 3). Sensitivity and specificity of 5-hr cumulative 13C-exhalation versus the standard parameter were high (Table 1 and Figure 2).
Figure 3.
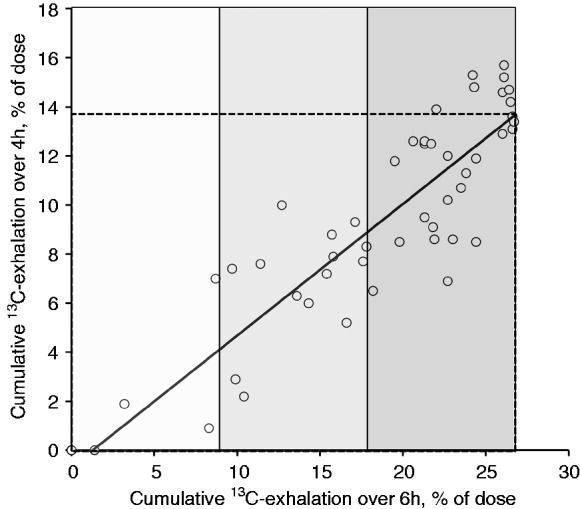
Cumulative 13C-exhalation (in percent of dose) over 4 hrs postprandially, in association with cumulative 13C-exhalation over 6 hrs in 51 subjects with impaired lipolysis, according to the standard parameter. The areas marked in different shades of grey represent severely, moderately and mildly impaired intestinal lipolysis. Broken lines give the lower level of normal for the standard parameter and the result of the abbreviated test. Remarkably, all subjects with more than mildly impaired lipolysis according to the standard parameter were also detected by the abbreviated test. Only 8 out of 30 subjects with mildly decreased cumulative 13C-exhalation over 6 hrs had normal 13C-exhalation over 4 hrs.
hrs: hours.
Impact of gastric emptying on the 13C-MTGT results
A total of 117 patients with evaluable data for the 13C-MTGT also received a 13C-OAT, for measurement of gastric emptying. Of these, 19 patients had to be excluded, because they received a different (vegetarian) test meal or because the time interval between both tests exceeded 4 weeks.
Among the remaining 98 patients, there were 64 women. The mean age of all of these patients was 52.0 ± 17.4 years and their mean BMI was 23.7 ± 4.8 kg/m2. Of these, 68 patients had normal gastric emptying of solids, with a gastric emptying half time (t ½) <200 min16,17; whereas in 30 patients, the gastric emptying t ½ exceeded 200 min, indicating delayed gastric emptying. Seven out of these patients had impaired intestinal lipolysis, as their 6-h 13C-exhalation was below 26.8% of the dose. Thus, in patients with delayed gastric emptying, the percentage of patients with pathological lipolysis, according to the standard parameter of the 13C-MTGT, was not higher than in the total patient group (23% versus 28%).
Univariate linear regression analysis revealed that cumulative 13C-exhalation rates over 1–4 hrs during the gastric emptying and the pancreatic function test were always significantly correlated (n = 98; R ≥ 0.238; p ≤ 0.018). Multivariate linear regression models confirmed there was a consistent association between 13C-exhalation rates during both tests: Cumulative 1-hr to 4-hr 13C-exhalation during the pancreatic function test was significantly predicted by cumulative 13C-exhalation during the gastric emptying test, when adjusted for age, gender and BMI; however, the influence of gastric emptying on the 1-hr cumulative 13C-exhalation during the 13C-MTGT was considerably higher (R2 = 0.159; p < 0.001) than on the 4-hr cumulative 13C-exhalation (R2 = 0.073; p = 0.037).
These findings indicate there was an association between gastric emptying velocity and the results of the pancreatic function test, particularly if evaluating early postprandial measurements of the 13C-MTGT; however, cumulative 6-hr 13C-exhalation during the 13C-MTGT was similar, for patients with normal or delayed gastric emptying (31.0 ± 12.6% versus 31.0 ± 10.9%, p > 0.5). The same was true for cumulative 4-hr 13C-recovery during the 13C-MTGT (17.3 ± 8.6% versus 16.6 ± 7.8%; p > 0.5).
Discussion
Our data showed that in a large patient population, the standard parameter used for evaluation of our modified version of the 13C-MTGT, that is cumulative 13C-exhalation over 6 hrs following application of the test meal, correlates significantly with cumulative 13C-exhalation over shorter periods of time (1–5 hrs, postprandially); however, early postprandial 13C-exhalation rates (1–3 hrs) during the 13C-MTGT appeared to be strongly influenced by gastric emptying; and thus, have insufficient specificity for detection of pancreatic exocrine insufficiency. By contrast, with cumulative 4-hr and 5-hr 13C-exhalation rates, both sensitivity and specificity nearly reached or exceeded 90%. Importantly, even the 4-hr value detected all patients with moderate or severe exocrine insufficiency and more than two-thirds of the patients with mildly impaired intestinal lipolysis.
We had shown previously that in contrast to other noninvasive pancreatic function tests,1 a modified version of the 13C-MTGT detects moderate pancreatic exocrine insufficiency reliably.3 The standard parameter for evaluation of pancreatic exocrine function using this test is the cumulative 13C-exhalation over the 6 hrs following a test meal. To avoid an increase in overall CO2-production that also influences 13CO2-exhalation, subjects need to remain seated during the test procedures18; thus, although the 13C-MTGT is a rather convenient, noninvasive indirect pancreatic function test, the long period of breath sampling and immobilization is a major drawback for clinical application, so shorter test versions would be highly desirable. The clinical need for such a test is underlined by the fact that current multinational research agendas explicitly aim to simplify the 13C-MTGT and so this study has been performed as part of the HaPanEU project (Harmonizing diagnosis and therapy of pancreatitis across Europe).
The findings of our present study suggest that for clinical purposes the breath sampling period may be shortened to 4 hours. Comparison of the results of 181 13C-MTGT revealed that cumulative 13C-exhalation over 4 hours postprandially had 88% sensitivity and 94% specificity for detection of pancreatic exocrine insufficiency when compared to the standard parameter. Cumulative 13C-exhalation over 5 hours gave almost identical results compared with the standard parameter (figure 2); however, it would only allow a minor abbreviation of the test. Moreover, even cumulative 13C-exhalation over 4 hours detected all patients with moderate or severe exocrine insufficiency and more than 2/3 of the patients with mildly impaired intestinal lipolysis. Accordingly, an abbreviated version of the 13C-MTGT with breath sampling over 4 instead of 6 hours appears to be sufficient for clinical purposes.
A further reduction of the breath sampling period would still be attractive and our data show indeed that even cumulative 1 h-13C-exhalation does not only correlate tightly with the standard parameter (R2 = 0.66, p < 0.0001) but also detects almost all patients with impaired 13C-exhalation according to the standard parameter (97% sensitivity, Table 1). However, specificity of cumulative 1 h-13C-exhalation was only 38%. Since digestion of dietary lipids by pancreatic lipase cannot occur before the meal has entered the duodenum, we tested the influence of gastric emptying on results of the 13C-MTGT in a subset of 98 patients who received both, the 13C-MTGT for measurement of pancreatic exocrine function and a 13C-OAT for measurement of gastric emptying. Multivariate linear regression analyses revealed a significant association between the parameters of the 13C-MTGT and gastric emptying velocity that was particularly strong early postprandially. Accordingly, the influence of gastric emptying on results of the 13C-MTGT probably precludes a further abbreviation of the breath sampling period to less than 4 hours.
Whether the modified 13C-MTGT may give false positive results in patients with lipid malabsorption due to other etiologies than pancreatic exocrine insufficiency19 deserves further studies.
In conclusion, abbreviation of the breath sampling period to 4 hrs is possible for clinical investigation of pancreatic exocrine function when using our modified version of the 13C-MTGT. The influence of gastric emptying on early postprandial results of the 13C-MTGT precludes a further reduction of the breath sampling period. Importantly, the abbreviated test still detects all patients with moderate or severe exocrine insufficiency and more than two-thirds of the patients with mildly impaired intestinal lipolysis. Thus, in line with current multinational research agendas such as the HaPanEU project, we showed that the 13C-MTGT can be abbreviated and still represents a reliable, noninvasive pancreatic function test.
Acknowledgements
The authors thank Karola Schmidt, Sven Scherzberg and Daniela Menge for expert technical assistance.
Funding
This work was supported by Abbott (formerly Solvay Pharmaceuticals) of Hannover, Germany (grant number PH-MGE/FH/ju 2039, for unrestricted investigation in healthy volunteers). Also, the work of J Keller and P Layer was supported by the Esther-Christiansen Foundation.
Conflict of interest
None declared.
References
- 1.Siegmund E, Lohr JM, Schuff-Werner P. Die diagnostische Validitat nichtinvasiver Pankreasfunktionstests: Eine Metaanalyse. [The diagnostic validity of non-invasive pancreatic function tests: A meta-analysis]. Z Gastroenterol 2004; 42: 1117–1128. [DOI] [PubMed] [Google Scholar]
- 2.Loehr M, Oliver M, Frulloni L. Synopsis of recent guidelines on pancreatic exocrine insufficiency. Unit Europ Gastroenterol J 2013; 1: 79–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller J, Bruckel S, Jahr C, et al. A modified 13C-mixed triglyceride breath test detects moderate pancreatic exocrine insufficiency. Pancreas 2011; 40: 1201–1205. [DOI] [PubMed] [Google Scholar]
- 4.Vantrappen GR, Rutgeerts PJ, Ghoos YF, et al. Mixed triglyceride breath test: A noninvasive test of pancreatic lipase activity in the duodenum. Gastroenterology 1989; 96: 1126–1134. [DOI] [PubMed] [Google Scholar]
- 5.Loser C, Brauer C, Aygen S, et al. Comparative clinical evaluation of the 13C-mixed triglyceride breath test as an indirect pancreatic function test. Scand J Gastroenterol 1998; 33: 327–334. [DOI] [PubMed] [Google Scholar]
- 6.Dominguez-Munoz JE, Iglesias-Garcia J, Vilarino-Insua M, et al. 13C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clin Gastroenterol Hepatol 2007; 5: 484–488. [DOI] [PubMed] [Google Scholar]
- 7.Pandol SJ. Pancreatic physiology and secretory testing. In: Feldman M, Friedman LS, Sleisenger MH. (eds). Sleisenger and Fordtran’s gastrointestinal and liver disease, 7th ed Philadelphia: Saunders, 2002, pp. 871–880. [Google Scholar]
- 8.Hoffmeister A, Mayerle J, Beglinger C, et al. S3-Leitlinie chronische pankreatitis: Definition, atiologie, diagnostik, konservative, interventionell endoskopische und operative therapie der chronischen pankreatitis. Leitlinie der Deutschen Gesellschaft fur Verdauungs und Stoffwechselkrankheiten (DGVS). [S3-Consensus guidelines on definition, etiology, diagnosis and medical, endoscopic and surgical management of chronic pancreatitis. Guidelines by the German Society of Digestive and Metabolic Diseases (DGVS)]. Z Gastroenterol 2012; 50: 1176–1224. [DOI] [PubMed] [Google Scholar]
- 9.Rauscher E, Neumann U, Schaich E, et al. Optimized conditions for determining activity concentration of alpha-amylase in serum, with 1,4-alpha-D-4-nitrophenylmaltoheptaoside as substrate. Clin Chem 1985; 31: 14–19. [PubMed] [Google Scholar]
- 10.Kruse-Jarres JD, Kaiser C, Hafkenscheid JC, et al. Evaluation of a new alpha-amylase assay using 4.6-ethylidene-(G7)-1-4-nitrophenyl-(G1)-alpha-D-maltoheptaoside as substrate. J Clin Chem Clin Biochem 1989; 27: 103–113. [PubMed] [Google Scholar]
- 11.Neumann U, Ziegenhorn J, Siest G, et al. Determination of serum lipase with automated systems. In: 4eme Colloque de Pont a Mousson, Paris, France, 1979, pp. 627–634.
- 12.Hummel B. A modified spectrophotometric determination of trypsin, chymotrypsin and thrombin. Can J Biochem 1955; 37: 1393–1397. [PubMed] [Google Scholar]
- 13.Boedeker C, Goetze O, Pfaffenbach B, et al. 13C mixed-triglyceride breath test: Isotope selective non-dispersive infrared spectrometry in comparison with isotope ratio mass spectrometry in volunteers and patients with chronic pancreatitis. Scand J Gastroenterol 1999; 34: 1153–1156. [DOI] [PubMed] [Google Scholar]
- 14.Ghoos YF, Maes BD, Geypens BJ, et al. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993; 104: 1640–1647. [DOI] [PubMed] [Google Scholar]
- 15.Delbende B, Perri F, Couturier O, et al. 13C-octanoic acid breath test for gastric emptying measurement. Eur J Gastroenterol Hepatol 2000; 12: 85–91. [DOI] [PubMed] [Google Scholar]
- 16.Keller J, Andresen V, Wolter J, et al. Influence of clinical parameters on the results of C-octanoic acid breath tests: Examination of different mathematical models in a large patient cohort. Neurogastroenterol Motil 2009; 21 1039–e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller J, Fliegner-Baia M, Layer P. Physical activity alters normal values of the ‘European standard’ 13C-octanoic acid breath test. Gut 2002; 51: A136–A136. [Google Scholar]
- 18.Kalivianakis M, Verkade HJ, Stellaard F, et al. The 13C-mixed triglyceride breath test in healthy adults: Determinants of the 13CO2 response. Eur J Clin Invest 1997; 27: 434–442. [DOI] [PubMed] [Google Scholar]
- 19.Keller J, Layer P, Brückel S, et al. 13C-mixed triglyceride breath test for evaluation of pancreatic exocrine function in diabetes mellitus. Pancreas 2014 [Epub ahead of print]. [DOI] [PubMed]


