Abstract
Background
The epidemiological decrease of Helicobacter pylori (Hp) infection has been recently associated to the increase of several extra-intestinal allergic disorders.
Objective
We investigated the role of specific Hp IgG production in the development of IgE or not IgE mediated food allergy (FA) in children affected by atopic dermatitis (AD).
Methods
From January 2010 to July 2013, 290 South Italian children, aged between 26 and 142 months, were consecutively referred to the Pediatric Clinic of the Pediatric Department at Second University of Naples and were diagnosed as affected by AD. The patients were classified in two groups on the basis of diagnosis of food allergy (88 FA affected and 202 not FA affected) and further divided on the basis of the diagnosis of atopy (63 IgE mediated and 23 not IgE mediated). Hp serum IgG was detected using an enzyme linked immunosorbent assay (ELISA) kit (Wampole® Helicobactor pylori IgG ELISA II, Wampole Laboratories, Cranbury, NJ) and Hp stool antigens using enzyme immunoassay (Premier Platinum HpSa plus, Cincinnati OH).
Results
We found a statistically significant higher prevalence of Hp serology positivity in not FA vs. FA AD-affected children (p = 0.032) and a significant inverse association between FA and Hp immunization (1/OR 0.32 95% CI 0.11–0.95). Further, we identified an absolute prevalence Hp serology positivity in not-IgE-mediated rather than in IgE-mediated FA AD-affected patients (p = 0.0006).
Conclusion
We hypothesize that specific Hp IgG production could protect against the development of both FA and atopy in AD-affected children.
Keywords: Atopic dermatitis, food allergy, Helicobacter pylori, children, atopy
Background
A potential role of Helicobacter pylori (Hp) infection has been suggested in some extra-intestinal pathologies such as iron deficiency anaemia and idiopathic thrombocytopenic purpura.1 The postulated role of Hp in the pathogenesis of extra-intestinal manifestations is based on the facts that (I) local inflammation has systemic effects, (II) Hp gastric infection is a chronic process that lasts for several decades, and (III) persistent infection induces a chronic inflammatory and immune response that is able to induce lesions both locally and remote to the primary site of infection.2 Recent evidence suggests that Hp infection could play a role in the pathogenesis of a variety of skin diseases.3 The best evidence for such a link is found for chronic urticaria4 and immune thrombocytopenic purpura.5 Worthy of note, many case reports have described interesting associations between Hp infection and atopic dermatitis (AD),6 rosacea,7 aphthous stomatitis,8 alopecia areata,9 Schoenlein–Henoch purpura10 and Sjögren syndrome.11
Furthermore, an inverse association has been observed between Hp infection and many allergic diseases such as recent wheezing, allergic rhinitis, dermatitis, eczema or rash.12 On the contrary, a positive association between Hp infection and food allergy (FA) presenting with gastrointestinal symptoms has been reported in literature also.13,14
In order to evaluate a possible association between specific Hp IgG production and both atopy and FA, we describe our experience with AD-affected patients suffering from either IgE-mediated and not IgE-mediated FA.
Methods
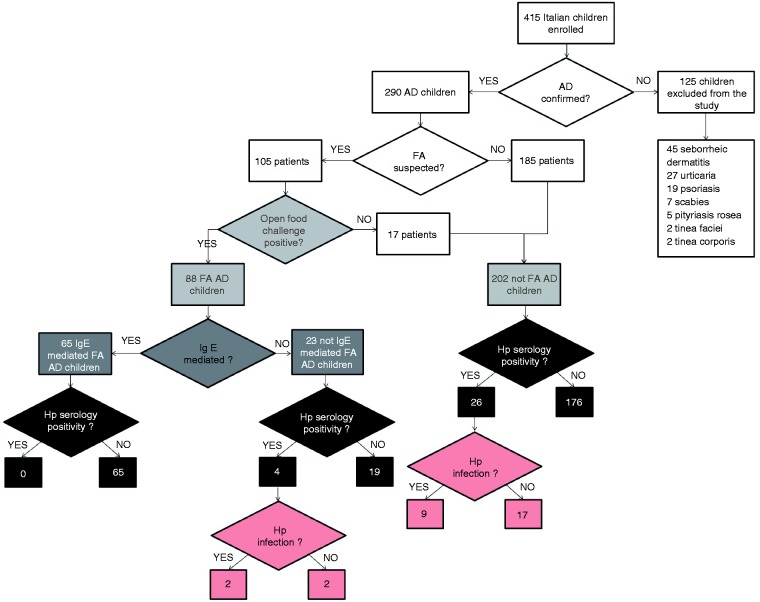
From January 2010 to July 2013, 415 South Italian children (Caucasian race), aged between 26 and 142 months, were consecutively referred to the Pediatric Clinic of the Pediatric Department at Second University of Naples for evaluation of dermatological symptoms such as pruritus, rash, papule, vesicle, dry skin, and wheal.
AD was defined according to Hanifin,15 and we included in the study 290 children in which a diagnosis of AD was confirmed by a dermatologist. In total, 125 patients were excluded because they were diagnosed as affected by seborrhoeic dermatitis (n = 45), urticaria (n = 27), psoriasis (n = 19), scabies (n = 7), pityriasis rosea (n = 5), tinea faciei (n = 2), and tinea corporis (n = 2).
The diagnosis of FA was suspected in 105 out of 290 children on the basis of family history, supportive medical history, laboratory data and 3-week elimination diet. The suspected diagnosis was confirmed by open food challenge.
Suspected foods were identified on the basis of diet records and symptom diaries as well as 3-week elimination diet. The first dose of the challenge was evaluated on the basis of patient’s case history and correlated to available data from the literature.16,17 The dose was doubled every 15 minutes. The top dose (maximal amount administered) was the normal daily intake in a serving of the food in question, adjusted for the age of the patient. A maximum dose of 5 g of lyophilized food was used, followed by open feeding.
Drugs such as antihistamines, neuroleptics, oral steroid above 5 mg per day, aspirin and other NSAIDs, ACE inhibitors and beta-blockers, which may enhance, mask, delay or prevent evaluation of a reaction or interfere with treatment of a reaction, were discontinued. Intravenous hydration therapy and supplies for resuscitation were immediately available.
The challenge was considered positive when an immediate rash causing itchiness was elicited. When the challenge was negative, the patients were considered diagnostic for ruling out FA. Therefore, we identified 88 out 105 (83.8%) FA–AD-affected children, while the remaining 17 were considered not FA–AD affected. In all, the 290 AD-affected patients were divided in FA–AD-affected (n = 88, i.e. 30.3%) and not FA–AD-affected patients (n = 202, i.e. 69.7%) (Figure 1).
Figure 1.
Flow diagram.
The diagnosis of atopy based on clinical history was confirmed by skin-prick test (SPT) as well as by measuring serum total IgE (from 25 to 60 months <81 kU/l, from 61 to 156 months <101 kU/l) and specific IgE concentration levels (>0.36 kUA/l (ImmunoCap 0–100)).
SPTs were performed using a standard battery of aeroallergens and food allergens: house dust mite (Dermatophagoides pteronyssinus, D. farinae), Parietaria officinalis, grasses (Dactylis glomerata, Lolium perenne, Phaleum pratense), mould (Alternaria, Aspergillus, Cladosporium), dog fur, cat fur, egg, cow milk, casein, wheat, codfish, peanut, soy, tree nuts, tomato, and potato (Bayer DHS Diagnostic Epernox Cedex-France). Allergens were applied into a stencil stamped on the forearm with ink and pricked with a lancet (Bayer DHS Diagnostic). Histamine chloride (10 mg/ml) was used as a positive control and the allergen diluent served as the negative control. The results were read after 15 min and 30 min. The test was considered positive if the wheal was at least 3 mm in diameter compared with negative control.
In the end, on the basis of the work-up of atopy, patients were further divided in two groups: IgE mediated and not IgE-mediated FA–AD affected.
Specific Hp IgG production was determined in serum using the Wampole® Helicobactor pylori IgG ELISA II (Wampole Laboratories, Cranbury, NJ). For each specimen, an immune status ratio (ISR) was calculated by dividing the optical density of the specimen by the mean optical density of the cut-off controls. Specimens were considered negative for Hp if the ISR was 0–0.90 and positive for Hp if the ISR was >0.90.12 In order to identify an existing Hp infection, the Hp-immunized patients were then screened using an enzyme immunoassay for detection of H. pylori stool antigens (Premier Platinum HpSa plus, Cincinnati OH). An optical density <0.140 was valuated as negative, >0.141 as positive.
All children had been off steroids or other immunosuppressive treatments for at least 3 months before investigation. The children suspected for Hp infection had not taken antibiotics, antisecretory drugs and bismuth subsalicylate for at least 4 weeks before performing the HpSa test. Consent was obtained from all enrolled subjects and their parents in accordance with the protocol approved from the Institutional Review Board of the University of Naples.
Skewness and Kurtosis tests were used to evaluate if the distribution of continuous variables was normal. According to categorical variables distribution, the values were expressed as mean ± standard deviation (SD), t-test was used to compare the difference between mean values and a χ2 was used to analyse the difference between the frequencies. A p-value <0.05 was considered significant.
The inverse odds ratio (1\OR) was calculated to evaluate a possible association of Hp immunization and both FA and atopy. The tests were considered significant when a 95% confidence interval excluded unity.
Results
Comparing mean age, frequencies of sex-specific prevalence of males and family history of atopic disease, no significant difference was found between the AD-affected patients divided in different groups (Tables 1 and 2). On the contrary, in Table 1, we found a significantly higher prevalence of atopy in FA–AD vs. not FA–AD-affected children (p = 0.003), but high atopy frequencies in both groups of AD-affected children. Of note, we also found a significantly higher prevalence of Hp serology positivity in not FA–AD vs. FA–AD patients (p = 0.032), and a strong statistically significant inverse association between FA and Hp immunization (1/OR 0.32, 95%CI 0.11–0.95). Hp infection was diagnosed in 2 out of 4 (50%) FA–AD and in 9 out of 26 (34.6%) not FA–AD Hp-immunized patients. No significant difference between the infection rates was observed.
Table 1.
Clinical and laboratory characteristics of 290 AD-affected children divided into two groups on the basis of the work-up for FA
| AD-affected children 290 | FA–AD-affected children 88 (30.3%) | Not FA–AD-affected children 202 (69.7%) | p |
|---|---|---|---|
| Age expressed in months (mean age +/−SD) | 79.4 ± 48 | 83.6 ± 50 | |
| Gender (male %) | 54.1% | 48.3% | |
| Family history of atopic disease (%) | 64.3 % | 59.2 % | |
| Atopy diagnosis (%) | 72% | 53% | 0.003 |
| Hp serology positivity (%) 30 (10.34%) | 4 (4.5%) | 26 (12.8%) | 0.032 1/OR 0.32, 95%C.I. 0.11–0.95 |
| Hp infection rate (%) 11 (36.6%) | 2 (50%) | 9 (34.6%) |
AD: atopic dermatitis; FA: food allergy.
p-values are expressed only for significant results (<0.05).
1/OR: inverse odds ratio; the test was considered significant when a 95% confidence interval excluded unity.
Table 2.
Clinical and laboratory characteristics of FA–AD-affected children divided into IgE and non-IgE mediated on the basis of the work-up for atopy
| FA–AD-affected children 88 | IgE-mediated FA–AD-affected patients 65 (73.8%) | Not IgE-mediated FA–AD-affected patients 23 (26.2%) | p |
|---|---|---|---|
| Age expressed in months (mean age +/−SD) | 75.3 ± 45 | 84.7 ± 51 | |
| Gender (male %) | 55.3% | 46.8% | |
| Family history of atopic disease (%) | 68.2% | 57% | |
| Hp serology positivity (%) 4 (4.54%) | 0/65 | 4/23 (17.4%) | 0.0006 |
| Hp infection rate (%) (50%) | 2 (50%) |
AD: atopic dermatitis; FA: food allergy.
p: expressed only for values <0.05 considered as significant.
Table 2 shows some clinical and laboratory differences between IgE-mediated and not IgE-mediated FA–AD-affected patients. We identified Hp serology positivity only in not-IgE mediated FA–AD patients (n = 4, p = 0.0006). Only two out of four not IgE-mediated FA–AD Hp-immunized patients were diagnosed as actually infected by Hp.
Discussion
Hp serology has been selected as a suitable test for detection of Hp immunization prevalence, as recommended by the evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children.18 Indeed, antibody detection could be used for serological-epidemiological surveys, but should not be used to perform the diagnosis of Hp infection or to monitor the success of therapy.
The overall prevalence of Hp immunization in our case-control study (10.34%) is much lower than that reported in literature to date for age-matched healthy children. In fact, Yucel et al. found a 30.9% prevalence rate in Turkish children (mean age 6.8 ± 3.0 years).19 Kori et al. observed a prevalence rate of 24.7% in daycare children from Israel, with higher rates in the 13–60-month-old group (32.5%).20
Nowadays is generally accepted that Hp immunization decreases in the epidemiological increase of allergic disorders, so that the low prevalence of Hp serology positivity observed in our patients could pertain to a bias due to a selected sample survey. In fact, the children referring to us were often atopics, as indicated by the high rate of atopy diagnosis valuated in both FA and not FA–AD-affected patients.
A positive association between Hp infection and FA has been described by Corrado et al.13 and Galadari et al.14 On the contrary, our results in FA–AD-affected children show a strong significant inverse association between FA and Hp IgG production rate. Hp infection rates, based only on Hp-immunized patients, do not show any significant difference between FA–AD and not FA–AD or between IgE-mediated and not IgE-mediated FA–AD patients. Thus it seems conceivable that Hp immunization, but not existing Hp infection, could interfere with both atopy and FA development in AD. On the basis of large epidemiological studies, a consistent negative association between Hp infection and the presence of allergic disorders has recently been proposed.21,22
This phenomenon can be explained by the inhibition of the allergic Th2 inflammation by Th1 responses elicited by Hp, able to induce the production of IFN-γ, IL-12, IL-18 and IL-23.23 The neutrophil-activating protein of Hp (HP-NAP) has the potential role to redirect the in vitro allergen-specific T-cell response from a predominant Th2 to a Th1 response.24 So, it is biologically plausible that Hp could be protective against atopy.
Overall, these results suggest that Hp immunization could protect against atopy in AD-affected children as in many allergic diseases. Further systematic studies are warranted to understand this topic.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Pellicano R, Franceschi F, Saracco G, et al. Helicobacters and extragastric diseases. Helicobacter 2009; 14: 58–68. [DOI] [PubMed] [Google Scholar]
- 2.Realdi G, Dore MP, Fastame L. Extradigestive manifestations of Helicobacter pylori infection: Fact and fiction. Dig Dis Sci 1999; 44: 229–236. [DOI] [PubMed] [Google Scholar]
- 3.Shiotani A, Okada K, Yanaoka K, et al. Beneficial effect of Helicobacter pylori eradication in dermatologic diseases. Helicobacter 2001; 6: 60–65. [DOI] [PubMed] [Google Scholar]
- 4.Magen E, Mishal J, Schlesinger M, et al. Eradication of Helicobacter pylori infection equally improves chronic urticaria with positive and negative autologous serum skin test. Helicobacter 2007; 12: 567–571. [DOI] [PubMed] [Google Scholar]
- 5.Michel M, Cooper N, Jean C, et al. Does Helicobater pylori initiate or perpetuate immune thrombocytopenic purpura? Blood 2004; 103: 890–896. [DOI] [PubMed] [Google Scholar]
- 6.Corrado G, Luzzi I, Pacchiarotti C, et al. Helicobacter pylori seropositivity in children with atopic dermatitis as sole manifestation of food allergy. Pediatr Allergy Immunol 2000; 11: 101–105. [DOI] [PubMed] [Google Scholar]
- 7.Rebora A, Drago F. Helicobacter pylori and rosacea. J Am Acad Dermatol 2000; 43: 884–884. [DOI] [PubMed] [Google Scholar]
- 8.Fritscher AM, Cherubini K, Chies J, et al. Association between Helicobacter pylori and recurrent aphthous stomatitis in children and adolescents. J Oral Pathol Med 2004; 33: 129–132. [DOI] [PubMed] [Google Scholar]
- 9.Rigopoulos D, Katsambas A, Karalexis A, et al. No increased prevalence of Helicobacter pylori in patients with alopecia areata. J Am Acad Dermatol 2002; 46: 141–141. [DOI] [PubMed] [Google Scholar]
- 10.Fu KI, Yagi S, Mashimo Y, et al. Regression of Helicobacter pylori-negative duodenal ulcers complicated by Schonlein–Henoch purpura with H. pylori eradication therapy: The first report. Dig Dis Sci 2005; 50: 381–384. [DOI] [PubMed] [Google Scholar]
- 11.El Miedany YM, Baddour M, Ahmed I, et al. Sjogren’s syndrome: Concomitant H. pylori infection and possible correlation with clinical parameters. Joint Bone Spine 2005; 72: 135–141. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Blaser MJ. Helicobacter pylori colonization is inversely associated with childhood asthma. J Infect Dis 2008; 198: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corrado G, Luzzi I, Lucarelli S, et al. Positive association between Helicobacter pylori infection and food allergy in children. Scand J Gastroenterol 1998; 33: 1135–1139. [DOI] [PubMed] [Google Scholar]
- 14.Galadari IH, Sheriff MO. The role of Helicobacter pylori in urticaria and atopic dermatitis. Skin Med 2006; 5: 172–176. [DOI] [PubMed] [Google Scholar]
- 15.Hanifin JM. Atopic dermatitis. In: Ellis EF, Adkinson NF, Jr, Yonginger JW, Buss WW. (eds). Allergy principles and practice, St. Louis: Mosby, 1993, pp. 1581–1581. [Google Scholar]
- 16.Moneret-Vautrin DA. Cow’s milk allergy. Allerg Immunol 1999; 31: 201–210. [PubMed] [Google Scholar]
- 17.RanceF, AbbalM, Lauwers-Cances V. Improved screening for peanut allergy by the combined use of skin prick tests and specific IgE assays. J Allergy Clin Immunol 2002; 109: 1027–1033. [DOI] [PubMed] [Google Scholar]
- 18.Koletzko S, Jones NL, Goodman KJ, et al. (H. pylori Working Groups of ESPGHAN and NASPGHAN). Evidence-based guidelines from ESPGHAN and NASPGHAN for Helicobacter pylori infection in children. J Pediatr Gastroenterol Nutr 2011; 53: 230–243. [DOI] [PubMed] [Google Scholar]
- 19.Yucel O, Sayan A, Yildiz M. The factors associated with asymptomatic carriage of Helicobacter pylori in children and their mothers living in three socio-economic settings. Jpn J Infect Dis 2009; 62: 120–124. [PubMed] [Google Scholar]
- 20.Kori M, Goldstein E, Granot E. Helicobacter pylori infection in young children detected by a monoclonal stool antigen immunoassay. Pediatr Infect Dis J 2009; 28: 157–159. [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Blaser MJ. Inverse associations of Helicobacter pylori with asthma and allergy. Arch Intern Med 2007; 167: 821–827. [DOI] [PubMed] [Google Scholar]
- 22.Reibman J, Marmor M, Filner J, et al. Asthma is inversely associated with Helicobacter pylori status in an urban population. PLoS One 2008; 3: e4060–e4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amedei A, Codolo G, Del Prete G, et al. The effect of Helicobacter pylori on asthma and allergy. J Asthma Allerg 2010; 3: 139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Amedei A, Cappon A, Codolo G, et al. The neutrophil-activating protein of Helicobacter pylori promotes Th1 immune responses. J Clin Invest 2006; 116: 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]