Abstract
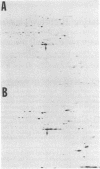
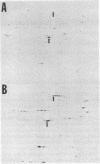
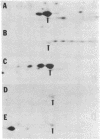
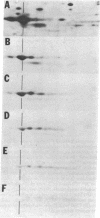
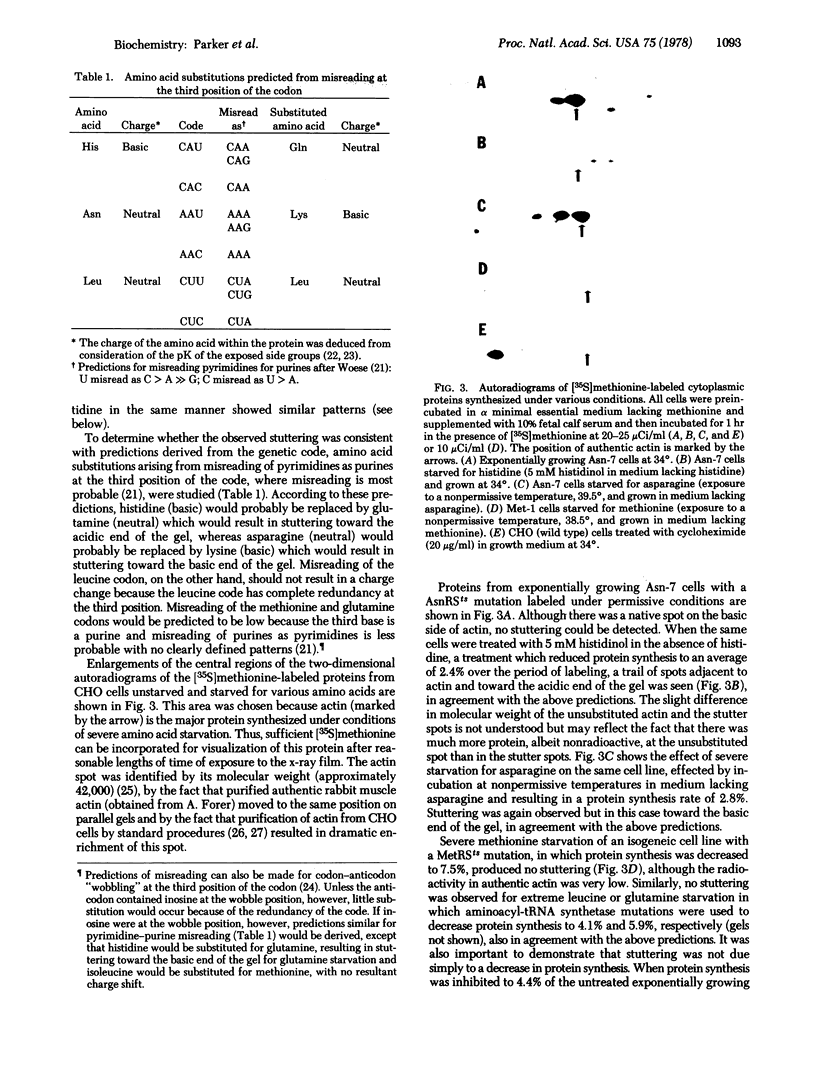
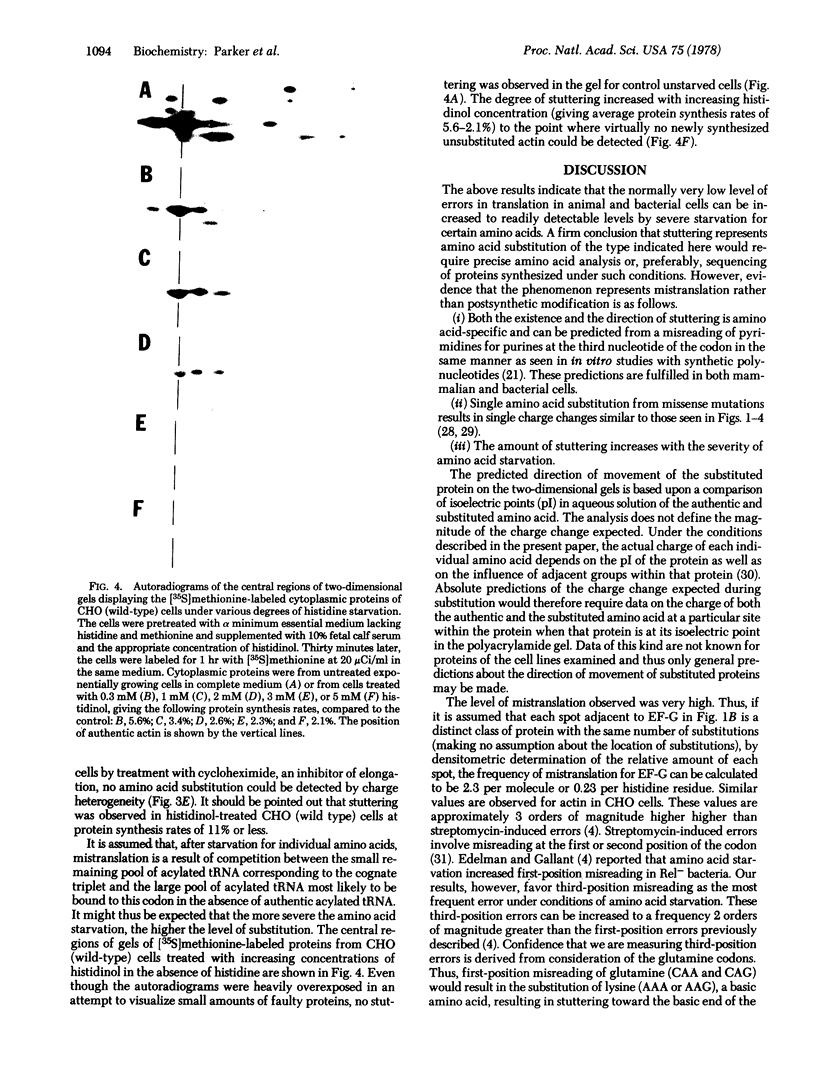
In both bacterial and mammalian cells, extreme starvation for certain amino acids resulted in translational errors that could be easily detected by two-dimensional polyacrylamide gel electrophoresis. On two-dimensional gels, the faulty proteins were shown as a trail of spots with molecular weights similar to those of the authentic proteins but separated in the isoelectric focusing dimension, a phenomenon we call "stuttering." The direction of charge shift depended on the amino acid substituted and could be predicted from misreading of pyrimidines for purines at the third position of the codon. It is expected that this phenomenon will provide a rapid means of measuring the fidelity of the translational machinery from cell type to cell type.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K., Kawakita M., Kaziro Y., Kondo T., Ui N. Studies on the polypeptide elongation factors from E. coli. 3. Molecular characteristics of EF-Tu-guanosine diphosphate, EP-Ts, and EF-Tu-Ts complex. J Biochem. 1973 May;73(5):1095–1105. doi: 10.1093/oxfordjournals.jbchem.a130164. [DOI] [PubMed] [Google Scholar]
- Bettex-Galland M., Lüscher E. F., Weibel E. R. Thrombosthenin--electron microscopical studies on its localization in human blood platelets and some properties of its subunits. Thromb Diath Haemorrh. 1969 Dec 31;22(3):431–449. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. S., Hagenmaier H., Pollard H., Schechter A. N. Proton magnetic resonance study of the histidine residues of sperm whale and horse myoglobins. J Mol Biol. 1972 Nov 14;71(2):513–519. doi: 10.1016/0022-2836(72)90367-1. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Elzinga M. The primary structure of actin from rabbit skeletal muscle. Completion and analysis of the amino acid sequence. J Biol Chem. 1975 Aug 10;250(15):5915–5920. [PubMed] [Google Scholar]
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- Edelmann P., Gallant J. Mistranslation in E. coli. Cell. 1977 Jan;10(1):131–137. doi: 10.1016/0092-8674(77)90147-7. [DOI] [PubMed] [Google Scholar]
- Gershon H., Gershon D. Inactive enzyme molecules in aging mice: liver aldolase. Proc Natl Acad Sci U S A. 1973 Mar;70(3):909–913. doi: 10.1073/pnas.70.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein S., Singal D. P. Alteration of fibroblast gene products in vitro from a subject with Werner's syndrome. Nature. 1974 Oct 25;251(5477):719–721. doi: 10.1038/251719b0. [DOI] [PubMed] [Google Scholar]
- Hansen B. S., Vaughan M. H., Wang L. Reversible inhibition by histidinol of protein synthesis in human cells at the activation of histidine. J Biol Chem. 1972 Jun 25;247(12):3854–3857. [PubMed] [Google Scholar]
- Holliday R., Tarrant G. M. Altered enzymes in ageing human fibroblasts. Nature. 1972 Jul 7;238(5358):26–30. doi: 10.1038/238026a0. [DOI] [PubMed] [Google Scholar]
- Kalousek F., Konigsberg W. H. Purification and characterization of histidyl transfer ribonucleic acid synthetase of Escherichia coli. Biochemistry. 1974 Feb 26;13(5):999–1006. doi: 10.1021/bi00702a026. [DOI] [PubMed] [Google Scholar]
- Kaziro Y., Inoue N., Kuriki Y., Mizumoto K., Tanaka M., Kawakita M. Purification and properties of factor G. Cold Spring Harb Symp Quant Biol. 1969;34:385–393. doi: 10.1101/sqb.1969.034.01.045. [DOI] [PubMed] [Google Scholar]
- LOFTFIELD R. B. THE FREQUENCY OF ERRORS IN PROTEIN BIOSYNTHESIS. Biochem J. 1963 Oct;89:82–92. doi: 10.1042/bj0890082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis C. M., Holliday R. Mistranslation and ageing in Neurospora. Nature. 1970 Nov 28;228(5274):877–880. doi: 10.1038/228877a0. [DOI] [PubMed] [Google Scholar]
- Lewis C. M., Tarrant G. M. Error theory and ageing in human diploid fibroblasts. Nature. 1972 Oct 6;239(5371):316–318. doi: 10.1038/239316a0. [DOI] [PubMed] [Google Scholar]
- Linn S., Kairis M., Holliday R. Decreased fidelity of DNA polymerase activity isolated from aging human fibroblasts. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2818–2822. doi: 10.1073/pnas.73.8.2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftfield R. B., Vanderjagt D. The frequency of errors in protein biosynthesis. Biochem J. 1972 Aug;128(5):1353–1356. doi: 10.1042/bj1281353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M. Resolution of simian virus 40 proteins in whole cell extracts by two-dimensional electrophoresis: heterogeneity of the major capsid protein. Cell. 1976 Oct;9(2):289–298. doi: 10.1016/0092-8674(76)90119-7. [DOI] [PubMed] [Google Scholar]
- ORGEL L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing. Proc Natl Acad Sci U S A. 1963 Apr;49:517–521. doi: 10.1073/pnas.49.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orgel L. E. Ageing of clones of mammalian cells. Nature. 1973 Jun 22;243(5408):441–445. doi: 10.1038/243441a0. [DOI] [PubMed] [Google Scholar]
- Orgel L. E. The maintenance of the accuracy of protein synthesis and its relevance to ageing: a correction. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1476–1476. doi: 10.1073/pnas.67.3.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen S., Reeh S. V. Analysis of the proteins synthesized in ultraviolet light-irradiated Escherichia coli following infection with the bacteriophages lambdadrifd 18 and lambdadfus-3. Mol Gen Genet. 1976 Mar 30;144(3):339–343. doi: 10.1007/BF00341733. [DOI] [PubMed] [Google Scholar]
- Salser W., Fluck M., Epstein R. The influence of the reading context upon the suppression of nonsense codons. 3. Cold Spring Harb Symp Quant Biol. 1969;34:513–520. doi: 10.1101/sqb.1969.034.01.058. [DOI] [PubMed] [Google Scholar]
- Shakespeare V., Buchanan J. H. Increased degradation rates of protein in aging human fibroblasts and in cells treated with an amino acid analog. Exp Cell Res. 1976 Jun;100(1):1–8. doi: 10.1016/0014-4827(76)90319-0. [DOI] [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Steinberg R. A., O'Farrell P. H., Friedrich U., Coffino P. Mutations causing charge alterations in regulatory subunits of the cAMP-dependent protein kinase of cultured S49 lymphoma cells. Cell. 1977 Mar;10(3):381–391. doi: 10.1016/0092-8674(77)90025-3. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Harkins J. L., Stanners C. P. A mammalian cell mutant with a temperature-sensitive leucyl-transfer RNA synthetase. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3094–3098. doi: 10.1073/pnas.70.11.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Lofgren D. J., Adair G. M. CHO cell mutants for arginyl-, asparagyl-, glutaminyl-, histidyl- and methionyl-transfer RNA synthetases: identification and initial characterization. Cell. 1977 May;11(1):157–168. doi: 10.1016/0092-8674(77)90326-9. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Stanners C. P., Siminovitch L. Selection by [3H] amino acids of CHO-cell mutants with altered leucyl- and asparagyl-transfer RNA synthetases. Somatic Cell Genet. 1975 Apr;1(2):187–208. doi: 10.1007/BF01538547. [DOI] [PubMed] [Google Scholar]