SUMMARY
The EEG in Sturge-Weber syndrome (SWS) was theorized over 50 years ago as changing over time from normality to focal asymmetry to lastly epileptiform. We sought to validate these findings in a larger cohort today. Children with confirmed SWS and routine EEG at our center were evaluated retrospectively. An EEG score (0–3) was created and linked to patient current age, overall neurologic function, and seizure frequency. Eighty-one EEGs from 44 patients with SWS (mean age 2.0 years (range: 0.2 – 37.9 years) were evaluated and assigned an EEG score. The mean age for patients with an EEG score of 0–1 (normal or focal slowing) was 3.2 years (SEM 0.6), whereas those with an EEG score of 2–3 (focal sharp waves or frequent spike-wave bursts) was 8.7 years (SEM 1.7) (p=0.006). There was no correlation between the EEG score and either the SWS overall neuroscore or seizure subscore (measuring frequency). The EEG in patients with SWS does appear to evolve over time, becoming more abnormal with more frequent epileptiform activity, as suspected in smaller studies decades ago. This progressive change, however, did not correlate with the child’s neurologic function or seizure frequency.
Keywords: asymmetry, Sturge-Weber, EEG, children, epilepsy
1. INTRODUCTION
Sturge-Weber syndrome (SWS) is a condition with epilepsy, facial port-wine stain, glaucoma and typically unilateral leptomeningeal angioma (Sturge 1879; Weber 1929). Seizures have been reported in the large majority of those with SWS and occur in 75% before the age of 1 year, at a median of 6 months (Sujansky and Conradi, 1995).
Due to the high prevalence of epilepsy in children with SWS, the majority of patients will have an EEG obtained, even at a young age. Prior to the availability of MRI, EEG was also able to help demonstrate asymmetry, with the area of focal slowing corresponding to the leptomeningeal angioma. As a result, in the early literature reporting SWS, there were several publications regarding EEG findings. Most of the earliest publications were single case reports as early as 1941 (Cohen and Kay, 1941), typically describing asymmetry (Table 1). The largest by Peterman in 1958 reported 35 children with SWS, of which 26 had EEGs (Peterman et al, 1958). The vast majority (25/26) were reported as abnormal with findings including both slowing and spike-waves. Dora Chao in 1959 subsequently described 9 children with SWS and was the first to ascribe a chronological progression of EEG findings over time from normality during infancy to focal slowing to lastly sharp waves (Chao et al, 1959).
TABLE 1.
Selected historical studies to date focusing on qualitative EEG findings and SWS.
| Author (year) | Number of patients with EEGs | Findings | Evolution of EEG over time? |
|---|---|---|---|
| Cohen (1941) | 1 | Asymmetry | ND |
| Radermecker (1951) | 1 | Asymmetry | ND |
| Lichtenstein (1954) | 1 | Asymmetry | ND |
| Livingston (1956) | 3 | Diffusely slow (1), asymmetry (2) | ND |
| Peterman (1958) | 26 | Asymmetry and sharp waves | ND |
| Chao (1959) | 9 | Asymmetry and spike waves | Yes |
| Andriola (1972) | 1 | Asymmetry and spike waves | Yes |
| Brenner (1976) | 16 | Asymmetry and spike waves | No |
| Fukuyama (1979) | 5 | Asymmetry and spike waves | Yes |
ND=Not described.
As MRI was introduced, the interest in EEG appeared to diminish and to our knowledge there have been no similar studies in SWS in the past 30 years. Despite this, EEG remains a valuable tool in SWS, and may be helpful in non-invasive screening for brain involvement in pre-symptomatic infants with a facial port-wine birthmark (Ewen et al, 2009). We hypothesized that the EEG evolution over time first reported by Chao over 50 years ago was accurate and could be replicated in a larger series of children with SWS.
2. PATIENTS AND METHODS
This study was approved by the Johns Hopkins and Kennedy Krieger Institute Committees for Clinical Investigation. Parents and subjects consented to have their information included in a single database. Categorical data were analyzed by Fisher exact test, medians were compared by a Mann-Whitney test, and means with a two-tailed t test assuming unequal variance. Because the EEG and SWS scales are non-parametric scales, correlation coefficients to compare them were analyzed with Spearman’s rho, 2- tailed. The significance level for all tests was p=0.05.
We reviewed the records of all consecutive children and adults with confirmed SWS seen and evaluated at the Hunter Nelson Sturge Weber Center at the Kennedy Krieger Institute from December 2002 until October 2012. Of these children, we obtained at Kennedy Krieger Institute a total of 195 EEGs in 88 children for clinical reasons or as part of a research protocol; patient or parent consent was obtained to study the EEGs and approval obtained from the Johns Hopkins Institutional Review Board. Two children were excluded due to lack of consent for research analysis. As some children were evaluated for facial port-wine stain and were later found not to have SWS, and some were unknown in regards to imaging these children were also excluded; therefore 44 had confirmed SWS by MRI and in total 81 EEGs were then available for analysis.
SWS neuroscores were obtained in 27 children (Kelley et al., 2005; Ewen et al., 2009). This score was obtained at clinic visit and is a composite of seizure frequency, hemiparesis, cognition, and vision subscores. The seizure subscore focuses primarily on seizure frequency rather than severity and is ranked from 0–4, with 0 = no seizures ever, 1 = one or more seizures, currently controlled, 2 = breakthrough seizures, 3 = monthly seizures, and 4 = at least weekly seizures (Kelley et al., 2005). Information regarding patient demographics were obtained at each EEG and clinic visit and analyzed.
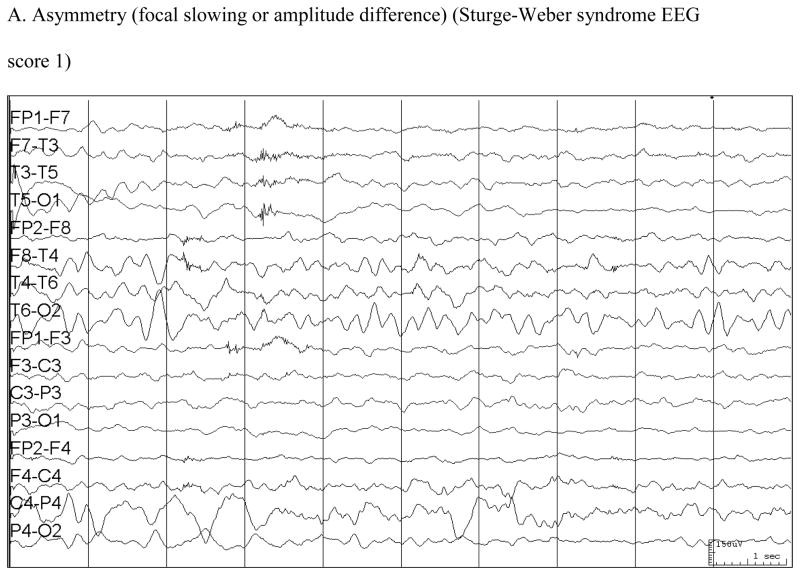
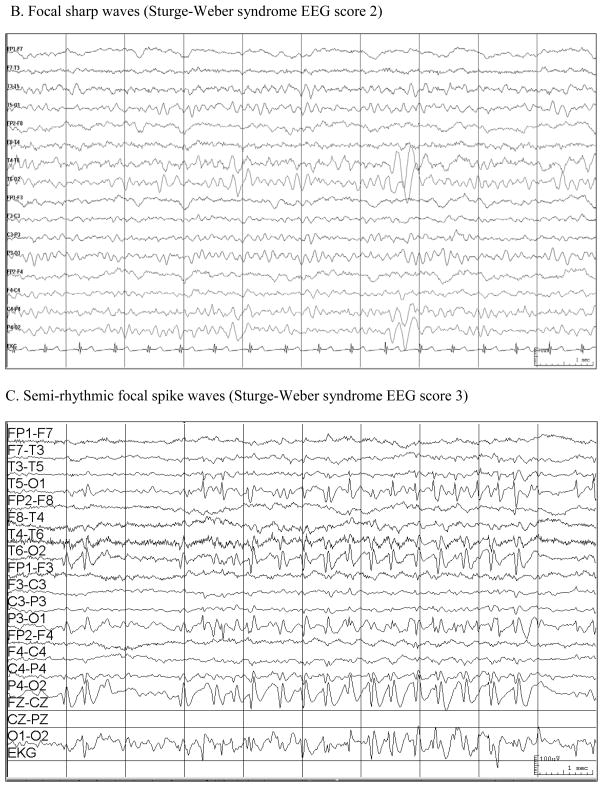
An EEG scale was created as a pattern of findings became evident (Table 2) (Figure). EEGs were read by the investigator (EK) at the time they were obtained over this 10-year period and a report was generated. No EEGs were re-reviewed for this analysis to avoid potential bias in review. EEG scores were assigned by an investigator blinded to age, neuroscores, and seizure activity of the subjects.
TABLE 2.
Sturge-Weber syndrome EEG score.
| 0 = Normal |
| 1 = Focal asymmetry (slowing or loss of normal background activity) |
| 2 = Sporadic, unilateral sharp waves (often occipital) |
| 3 = Frequent, at times semirhythmic, unilateral runs of spikes (often occipital). Occasionally with secondary generalization. |
Figure.
3. RESULTS
3.1. Correlation of EEG scale with age
Eighty-one EEGs were reviewed in detail from 44 children and adults with SWS. The mean age at EEG was 2.0 years (range: 0.2 – 37.9 years). Twenty-six patients had left hemispheric SWS by MRI, 13 right, and 5 bilateral. Sixteen (20%) records were normal (EEG score 0), 40 (50%) had focal asymmetry (EEG score 1), 15 (18%) had sporadic unilateral sharp waves (EEG score 2), and 10 (12%) had frequent spikes (EEG score 3).
An older age at EEG was positively associated with a more abnormal EEG score. The mean age for patients with an EEG score of 0–1 was 3.2 years (SEM 0.6), whereas those with an EEG score of 2–3 was 8.7 years (SEM 1.7) (p=0.006). No difference was seen between scores 0 (mean 2.0 years) and 1 (mean 3.7 years) p=0.12 as well as between scores 2 (mean 9.7 years) and 3 (mean 7.1 years), p=0.43, hence the decision to group these scores together for analysis. The relationship was less significant when duplicate subjects (those with multiple EEGs) only had their initial EEG analyzed, 4.7 years for EEG score 0–1 versus 9.4 years for EEG score 2–3 (p=0.06).
There did not appear to be a similar evolution over time for children who had multiple EEGs. Only 10 children with SWS had repeated EEGs at our center, however, which limits the ability to ascertain a trend. Of these 10 children, only 3 had an increasing EEG scale between their first and last EEG.
3.2. Correlation of EEG scale and SWS seizure subscore
Our next analysis was to determine if increasing EEG scores was related to increased disease burden. We therefore examined 55 cases where the SWS neuroscores was obtained at the same time as EEGs. There was no association between EEG scores (0–1 (n=39) and 2–3 (n=16)) and SWS seizure subscores (either 0–1 (n=31) and 2–4 grouped (n=24), p=0.54; or grouped 0–2 (n=49) and 3–4 (n=6), p=0.23). Without grouping scores, utilizing a correlation coefficient for all possible scores, there was also no statistically positive correlation, although a trend was noted for a very weak correlation 0.234 (p=0.09). Similarly, the correlation coefficient between EEG score and the larger overall SWS neuroscore (encompassing seizures as well as other clinical findings) was even weaker and less significant, 0.141 (p=0.31).
3.3. Association of age and SWS seizure subscore
Subject age and seizure subscore correlated weakly (Spearman’s rho =0.464, p=0.0001). However within the two age subgroups, an association between age and SWS seizure subscore did not reach significance, with a mean age of 6.3 years (SEM 1.8 years) for those with a SWS seizure subscore of 3–4 and 2.8 years (SEM 0.46) for those with subscores 0–2 (p=0.12).
4. DISCUSSION
This study represents the largest study of SWS and EEG findings to date to our knowledge. We validated theories from several decades ago that as patients with SWS age, their EEG appears to change. Many of the younger children had normal EEGs, with older children then developing an asymmetry of slowing as well as background activity, a mean of approximately 1 year later. Two to three years later the EEG appears to reveal focal sharp waves and more frequent spikes. Although we did not see an evolution in the 10 children with repeated EEGs, this number may be too small to replicate the findings from Chao (Chao et al, 1959). Alternatively modern anticonvulsants or treatment with low-dose aspirin may currently produce some modification in the previously noted EEG findings.
Surprisingly, the evolution of the EEG did not necessarily indicate that these children were having worsening neurologic function or seizure control. In some ways this is similar to what has been described as the severe, clustering seizure pattern noted in 39% of children with SWS (Kossoff et al, 2009). This often worrisome seizure pattern did not appear to correlate with the SWS neuroscore or seizure subscore either. This may represent the somewhat variable nature of this particular outcome measure. In addition, these were routine 30 minute EEGs and may have also fluctuated even within the same day in these patients. Lastly, there were relatively few patients with high SWS seizure subscores and EEG scores in this cohort. A larger series with more abnormal EEG and higher seizure burdens may be required to discern a correlation.
We therefore suggest using this EEG scale in SWS as a predominantly descriptive factor that mirrors advancing patient age. In some ways this is similar to what has been reported in the EEG of children with Rett syndrome over time (Hagne et al, 1989). PET, MR spectroscopy, and quantitative EEG measures have shown limited but potential ability to predict neurologic function (Lee et al, 2001; Lin et al, 2006; Hatfield et al., 2007). This EEG score, at least based on this study, should not at this time overall be used to guide medication management, predict prognosis, or suggest deterioration (without clinical correlation). Further, larger studies would be needed to correlate this EEG evolution with any clinical factors, especially including more children with repeated EEGs.
Acknowledgments
This work was supported in part by Hunter’s Dream for a Cure.
Abbreviations
- SWS
Sturge-Weber syndrome
Footnotes
None of the authors have any conflicts of interest to disclose.
Contributor Information
Eric H. Kossoff, The Johns Hopkins Hospital.
Cathy Bachur, Kennedy Krieger Institute.
Angela M. Quain, Eastern Virginia Medical School.
Joshua B. Ewen, Kennedy Krieger Institute.
Anne M. Comi, Kennedy Krieger Institute.
References
- Andriola M, Stolfi J. Sturge-Weber Syndrome: report of an atypical case. Amer J Dis Child. 1972;123:507–510. doi: 10.1001/archpedi.1972.02110110135019. [DOI] [PubMed] [Google Scholar]
- Brenner RP, Sharbrough FW. Electroencephalographic evaluation in Sturge- Weber syndrome. Neurology. 1976;26:629–632. doi: 10.1212/wnl.26.7.629. [DOI] [PubMed] [Google Scholar]
- Chao DH-C. Congenital neurocutaneous syndromes of childhood III. Sturge- Weber disease. J Pediatr. 1959;55:635–649. doi: 10.1016/s0022-3476(59)80247-x. [DOI] [PubMed] [Google Scholar]
- Cohen HJ, Kay MN. Associated facial hemangioma and intracranial lesion (Weber-Dimitri disease) Am J Dis Child. 1941;62:606–612. [Google Scholar]
- Ewen JE, Kossoff EH, Crone NE, et al. Use of Quantitative EEG in Infants with Port-Wine Birthmark to Assess for Sturge-Weber Brain Involvement. Clin Neurophys. 2009;120:1433–1440. doi: 10.1016/j.clinph.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyama Y, Tsuchiya S. A study on Sturge-Weber syndrome: report of a case associated with infantile spasms and electroencephalographic evolution in five cases. Eur Neurol. 1979;18:194–204. doi: 10.1159/000115076. [DOI] [PubMed] [Google Scholar]
- Hagne I, Witt-Engerstrom I, Hagberg B. EEG development in Rett syndrome: A study of 30 cases. Electroencephalogr Clinical Neurophysiol. 1989;72:1–6. doi: 10.1016/0013-4694(89)90025-4. [DOI] [PubMed] [Google Scholar]
- Hatfield LA, Crone NE, Kossoff EH, et al. Quantitative EEG asymmetry correlates with clinical severity in unilateral Sturge-Weber syndrome. Epilepsia. 2007;48:191–195. doi: 10.1111/j.1528-1167.2006.00630.x. [DOI] [PubMed] [Google Scholar]
- Kelley TM, Hatfield LA, Lin DD, Comi AM. Quantitative analysis of cerebral cortical atrophy and correlation with clinical severity in unilateral Sturge-Weber syndrome. J Child Neurol. 2005;20:867–870. doi: 10.1177/08830738050200110201. [DOI] [PubMed] [Google Scholar]
- Kossoff EH, Ferenc L, Comi AM. An infantile-onset, severe, yet sporadic seizure pattern is common in Sturge-Weber syndrome. Epilepsia. 2009;50:2154–2157. doi: 10.1111/j.1528-1167.2009.02072.x. [DOI] [PubMed] [Google Scholar]
- Lee JS, Asano E, Muzik O, et al. Sturge-Weber syndrome: correlation between clinical course and FDG PET findings. Neurology. 2001;57:189–195. doi: 10.1212/wnl.57.2.189. [DOI] [PubMed] [Google Scholar]
- Lichtenstein BW. Sturge-Weber-Dimitri Syndrome: Cephalic form of neurocutaneous hemangiomatosis. Arch Neurol. 1954;71:291–301. doi: 10.1001/archneurpsyc.1954.02320390021002. [DOI] [PubMed] [Google Scholar]
- Lin DD, Barker PB, Hatfield LA, Comi AM. Dynamic MR perfusion and proton MR spectroscopic imaging in Sturge-Weber syndrome: correlation with neurological symptoms. J Magn Reson Imaging. 2006;24:274–281. doi: 10.1002/jmri.20627. [DOI] [PubMed] [Google Scholar]
- Livingston S, Eisner V, Brown WH, Boks LL. The Sturge-Weber Syndrome. Postgrad Med. 1956;19:221–230. doi: 10.1080/00325481.1956.11708288. [DOI] [PubMed] [Google Scholar]
- Peterman AF, Hayles AB, Dockerty MB, Love JG. Encephalotrigeminal angiomatosis (Sturge-Weber disease): Clinical study of thirty-five cases. JAMA. 1958;167:2169–2176. doi: 10.1001/jama.1958.02990350007002. [DOI] [PubMed] [Google Scholar]
- Radermecker J. L’electroencephalographie dans l’angiomatose encephalotrigeminee de Sturge-Weber-Krabbe. Acta Neurol Belg. 1951;51:427–451. [PubMed] [Google Scholar]
- Sturge WA. A case of partial epilepsy, apparently due to a lesion of one of the vaso-motor centers of the brain. Trans Clin Soc London. 1879;12:162–167. [Google Scholar]
- Sujansky E, Conradi S. Sturge-Weber syndrome: age of onset of seizures and glaucoma and the prognosis for affected children. J Child Neurol. 1995;10:49–58. doi: 10.1177/088307389501000113. [DOI] [PubMed] [Google Scholar]
- Weber FP. A note on the association of extensive hemangiomatous naevus of the skin with cerebral (meningeal) hemangioma, especially cases of facial vascular naevus with contralateral hemiplegia. Proc Roy Soc Med. 1929;22:431. doi: 10.1177/003591572902200422. [DOI] [PMC free article] [PubMed] [Google Scholar]