Abstract
The most common recurrent copy number variants associated with autism, developmental delay, and epilepsy are flanked by segmental duplications. Complete genetic characterization of these events is challenging because their breakpoints often occur within high-identity, copy number polymorphic paralogous sequences that cannot be specifically assayed using hybridization-based methods. Here, we provide a protocol for breakpoint resolution with sequence-level precision. Massively parallel sequencing is performed on libraries generated from haplotype-resolved chromosomes, genomic DNA, or molecular inversion probe–captured breakpoint-informative regions harboring paralog-distinguishing variants. Quantifying sequencing depth over informative sites enables breakpoint localization, typically within several kilobases to tens of kilobases. Depending on the approach employed, the sequencing platform, and the accuracy and completeness of the reference genome sequence, this protocol takes from a few days to several months to complete. Once established for a specific genomic disorder, it is possible to process thousands of DNA samples within as little as 3–4 weeks.
Keywords: breakpoint, segmental duplication, paralog, nonallelic homologous recombination, NAHR, whole-genome sequencing, WGS, molecular inversion probe, MIP, genomic disorder, sequencing, recurrent deletion, recurrent duplication, copy number variant, CNV
INTRODUCTION
Several regions of the human genome are predisposed to recurrent duplication and deletion1. Nonallelic homologous recombination (NAHR) between directly oriented segmental duplications, defined as contiguous sequences at least 1 kbp in length having at least 90% identity, results in recurrent gain and loss of the intervening sequence2–3. Collectively, such events affect hundreds of genes and have been associated with many diseases, including autism, schizophrenia, intellectual disability, epilepsy, macrocephaly, microcephaly, congenital defects, and severe obesity, among others4.
Although a variety of technologies reliably detect recurrent duplications and deletions, determining the breakpoints within duplicated sequences remains a significant challenge. Pulsed-field gel electrophoresis followed by genomic Southern blots was originally used to map breakpoints in patients with Charcot-Marie-Tooth disease type 1A having a duplication at 17p125. This method required the preparation of high-molecular-weight DNA, an often trial-and-error identification of informative restriction enzymes, and the design of probes adjacent to the breakpoints themselves. When array comparative genomic hybridization (array CGH) methods6 became the standard for copy number variant detection, they were often applied to initially refine breakpoints7,8; follow-up with long-range PCR, subcloning, and capillary sequencing in some cases then enabled the precise delineation of breakpoints8. This strategy worked well for breakpoints mapping in unique regions of the genome and would, in principle, prove effective in mapping breakpoints within small segmental duplications (<10 kbp). However, it cannot be successfully applied to most recurrent, NAHR-mediated microdeletions and duplications, whose breakpoints map to the largest and most highly identical segmental duplications. In these cases, refinement by array CGH is of limited utility because of probe cross-hybridization. As a result, the researcher can only narrow the breakpoints to within hundreds of kilobases of nearly identical duplicated sequence. Thus, subcloning and sequencing several long-range PCR products across these large duplicated regions would be required for breakpoint resolution—a particularly difficult proposition, given the generation of nonspecific PCR products.
More recent bioinformatics approaches involving the analysis of split-read or read-pair sequence signatures from massively parallel whole-genome sequencing (WGS) data9–10 are not reliable in these regions. These methods depend on the sequence read or the read-pair itself traversing the junction formed via NAHR. However, short read lengths, short library insert sizes, and the paucity of distinguishing variants within breakpoint-containing segmental duplications make detection of a junction-spanning sequence read or read-pair highly unlikely. Finally, breakpoint resolution is often confounded by copy number polymorphisms, gaps in the reference genome, and alternative structural haplotypes affecting breakpoint regions11–12. Incomplete knowledge of the sequence, structure, and genetic variation at these loci presents a substantial barrier to breakpoint localization regardless of the method employed.
Despite its difficulty, accurate breakpoint resolution is critical for understanding the origins and consequences of recurrent duplications and deletions. Obtaining breakpoint data from multiple independent events may elucidate factors influencing NAHR susceptibility and may help identify potential hotspots. It is becoming apparent, for example, that specific structural configurations within the genome increase susceptibility to some genomic disorders, whereas others are protective with respect to recurrent rearrangements12,13. Furthermore, precise breakpoint mapping will reveal the effects of recurrent rearrangements on genes within breakpoint regions. Most such genes have hardly been characterized, and their disruption may contribute to both disease phenotypes and phenotypic variability associated with some genomic disorders14. Here we detail a protocol for resolving breakpoints including a series of experimental approaches (Fig. 1) and provide general guidelines for its successful application to particular cases of interest. Although hybridization-based approaches are still the primary method by which copy number variants are discovered, we focus mainly on two massively parallel sequencing strategies—analysis of WGS data and targeted capture and sequencing of informative regions using molecular inversion probes (MIPs)—as they provide the greatest potential for breakpoint resolution within duplicated sequence. More detailed protocols for the other methodologies outlined here have been previously published15,16.
Figure 1. General workflow for breakpoint resolution.
The diagram outlines the typical stages involved in sequence-based breakpoint resolution and indicates some relevant associated experiments and computational analyses. Optional sub-sections of the procedure described briefly in this protocol are highlighted in yellow, while strategies for attaining sequence-level breakpoint resolution covered in more detail are highlighted in green. Targeted array CGH is displayed near the top of the figure because until recently, it was the method of choice for breakpoint resolution after CNV detection—the sequencing-based approaches were only developed within the last few years. Today, however, sequencing approaches are sensible starting points for breakpoint resolution. Note that depending on the particular region of interest, further genomic characterization of the region may be critical to successfully refine breakpoint locations (see Box 1 for discussion). Genomic characterization is often an iterative process (circular arrow), and because it facilitates data interpretation for all breakpoint resolution methods, it is included in the diagram near the center. CGH: comparative genomic hybridization. CNV: copy number variant. SUN: singly unique nucleotide. SUNK: singly unique nucleotide k-mer.
Concept and development
We originally leveraged massively parallel WGS to localize breakpoints for three individuals with the 17q21.31 microdeletion syndrome14. This recurrent microdeletion usually occurs via NAHR (Fig. 2a) between directly oriented segmental duplications that are ~145 kbp in length and have >99% sequence identity. To refine breakpoints within the segmental duplications, singly unique nucleotides (SUNs) were identified from a sequence alignment between paralogs (Fig. 2b). The idea of using paralogous sequence variants to characterize duplicated regions is not new17–21. SUNs, however, represent a specific type of paralogous sequence variant because they uniquely distinguish one paralog from all other sequences in the genome, thereby enabling accurate sequence and copy number genotyping for specific paralogs genome-wide22. Conceptually, any sequencing read carrying a SUN can be unambiguously assigned to a specific paralog even though it maps within segments of nearly identical sequence. Furthermore, quantifying read-depth from sequencing data over SUNs helps to refine recurrent deletion and duplication breakpoints (Fig. 2c). Specifically, in the case of patients with the 17q21.31 microdeletion syndrome, we used WGS together with SUN read-depth analysis to narrow breakpoints to intervals of less than 25 kbp (Fig. 3)14. More recently, we developed a conceptually similar method based on targeted capture of SUN-containing regions using MIPs and applied it to resolve breakpoints for NAHR-mediated deletions and duplications of RHD to ~6 kbp (Fig. 4)23. We have found that these sequencing-based approaches ultimately refine breakpoints within segmental duplications to the highest attainable sequence-level resolution and compare favorably in time and expense to more conventional approaches.
Figure 2. Sequencing-based breakpoint resolution strategy.
a) NAHR between segmental duplications (red and blue arrows) results in the deletion and duplication of intervening sequence as well as the proximal end of one of these paralogs and the distal part of the other. b) Alignment of segmental duplication sequences enables the identification of SUNs. c) Quantifying WGS read-depth at each SUN reveals a reciprocal copy number transition, a signature of NAHR, corresponding to the breakpoint region.
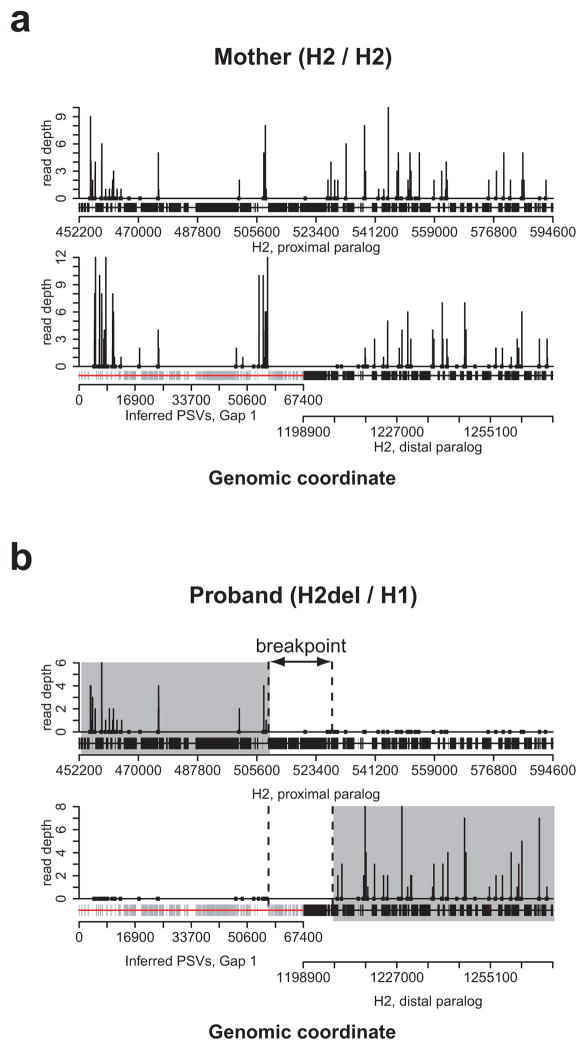
Figure 3. Resolution of 17q21.31 microdeletion breakpoints using WGS data.
Read-depth (vertical lines) at breakpoint-informative variants (dots) is shown over an alignment of the paralogous segmental duplications mediating the microdeletion for the patient’s mother (a), who lacks the microdeletion, and for the patient (b). Structural haplotypes for both chromosomes for each individual are given in parentheses. Variation in read-depth between informative variants occurs even in the absence of any copy number variation at these loci. However, because the patient is heterozygous for the deletion-bearing haplotype, a read-depth of zero at informative variants is observed over deleted regions, allowing the breakpoint to be unambiguously refined to an ~22 kbp window (between dashed lines). Note that a region of sequence (red line) nearly 70 kbp in size was missing from the reference genome and had to be resolved before sequencing data could be accurately interpreted (Box 1). Adapted from reference 14.
Figure 4. Resolution of NAHR-associated RHD duplication and deletion breakpoints by MIP capture and sequencing.
a) NAHR between ~9-kbp segmental duplications (purple and gray triangles) flanking RHD results in its deletion and duplication. b) 39 MIPs were used for genotyping the copy number of RHD and flanking segmental duplications in diploid DNA from HapMap individuals NA20814 and NA19204. Each point indicates a paralog-specific copy number estimate, calculated at each locus as the product of the paralog-specific relative read-depth and the aggregate estimated copy number. Data from the four MIPs targeting SUN-containing regions in the flanking segmental duplications refine the breakpoints to ~6 kbp intervals (yellow highlights). Modified from reference 23.
Applications and limitations
This protocol focuses on breakpoint resolution; however, read-depth analysis utilizing SUNs has more broadly enabled genetic characterization of duplicated genes22–24. Specifically, this approach has been used to genotype paralog-specific copy number, discover structural variation, detect interlocus gene conversion, and assay paralog-specific gene expression. Although these studies were performed on human DNA, the method should prove similarly useful, in principle, for large duplicated regions in the genome of any organism. Whether or not sequencing data can be used for any of the mentioned purposes, however, depends on the identification of SUNs. Because the level of attainable breakpoint resolution is determined by these markers, careful analysis of their density and spatial distribution should always serve as an initial step to assess whether using a sequencing approach described here makes sense for a particular case. To that end, we provide tables including the number of 30 bp SUN K-mers (SUNKs)22 in different-sized windows (1 kbp, 5 kbp, 10 kbp, 25 kbp, and 50 kbp) across the GRCh37 reference genome (http://eichlerlab.gs.washington.edu/breakpoint_protocol_supplementary_data).
Having an accurate genome sequence and understanding haplotypic and copy number variation are critical foundations upon which all subsequent steps depend (Box 1). Sequences corresponding to breakpoint regions are frequently misassembled, even in the finished human reference genome, and often contain gaps due to the high sequence identity and highly duplicated nature of sequences therein24–26. Furthermore, such sequences are enriched for copy number polymorphisms and structural variation. All these factors complicate singly unique nucleotide identification and warrant careful consideration before proceeding to study a particular microdeletion or microduplication using this protocol.
Box 1. Genome sequence and haplotype characterization.
In order to apply sequencing-based components of this protocol, it is crucial to accurately identify SUNs that distinguish breakpoint-associated segmental duplications from one another. How can one tell whether breakpoint-associated sequences are accurate and complete in a reference genome? Unless sequence gaps exist in these regions, evaluating the reference genome with regards to these criteria is challenging. We have found a variety of strategies particularly useful for this purpose: (1) mapping end sequences from clones from large-insert genomic libraries to the reference genome and searching for discordancies between mapping locations and the known insert size range33–34, (2) performing fluorescent in situ hybridization experiments using probes targeting breakpoint regions and surrounding loci, and (3) generating and comparing copy number profiles across breakpoint regions for hundreds of individuals from populations around the globe using massively parallel WGS data22. Collectively, these analyses and experiments provide some insight into the variability and complexity of the particular breakpoint regions in question and inform the researcher on whether their further genomic characterization and high-quality sequencing are necessary to develop an alternate reference genome.
In cases where the reference genome is inaccurate or incomplete, we recommend sequencing bacterial artificial chromosomes (BACs) from genomic libraries using Sanger24 or Pacific Biosciences technology35 and assembling a contig for each distinct structural haplotype. BAC libraries from hydatidiform mole source material, containing a single haplotype and lacking allelic variation, are especially useful for high-identity duplications in the human genome24. High-quality sequence characterization of a particular set of breakpoint-associated regions using this approach takes from one to several months to complete. Nevertheless, this process is fundamental for accurate SUN identification and the eventual interpretation of sequencing data. Thus, genomic characterization is the starting point for any breakpoint refinement effort whenever the reference genome fails to provide accurate sequence data capturing the full diversity and complexity of breakpoint-associated loci.
In general, the more highly identical and the higher the total copy of breakpoint-associated segmental duplications, the more difficult breakpoint resolution becomes. The number of SUNs decreases as sequence identity increases, and accurate copy number prediction grows more challenging as the number of homologous sequences increases. Biological factors can sometimes simplify the procedure. For example, the patients with the 17q21.31 microdeletion syndrome included in our study were all heterozygous for the haplotype on which the microdeletions occurred. As a result, breakpoint resolution amounted to assessing the presence or absence of haplotype-specific SUNs in WGS data14, a straightforward task compared to inferring deleted regions from a decrease in read-depth over SUNs, which would be necessary if an individual were homozygous for a single haplotype. As a second example, RHD deletion and duplication breakpoints were readily refined23 because the breakpoint-containing paralogs are not present at high total copy number at many locations throughout the genome. Other biological factors, particularly frequent interlocus gene conversion between associated segmental duplications, sometimes complicate accurate breakpoint localization.
As noted above, the precision of breakpoint resolution for recurrent duplications and deletions is inherently limited by the number and spatial distribution of distinguishing variants within associated segmental duplications and depends upon where the breaks occur relative to these variants. Breakpoints occurring at different locations within long stretches of identical sequence between associated segmental duplications will be indistinguishable, as deletion or duplication products of NAHR anywhere in such regions all have identical sequences. Thus, although sequencing-based analysis enables pinpointing breakpoints to one of these identical stretches—providing the highest attainable breakpoint resolution for any recurrent event—in some cases, inferred breakpoint intervals will remain large because there are no additional informative markers to refine the region further.
Experimental design
Breakpoint mapping generally involves discovery and confirmation of a duplication or deletion, refinement of breakpoint locations, and validation. Multiple approaches are available for completing each of these stages, and we consider several refinement strategies here. Although massively parallel sequencing is not the only technology applicable to breakpoint refinement for recurrent events, sequencing is ultimately required to obtain sequence-level resolution. Here we overview several breakpoint localization methods, offer suggestions regarding their application in different situations, and diagram their relationships to one another in the context of discovery, refinement, and validation (Fig. 1).
Array CGH (steps 1–3 and 9–10)
A conventional first step in resolving breakpoints is performing array CGH using a custom-designed, high-density oligonucleotide microarray with probes spanning the region of interest and extending beyond the hypothesized breakpoint locations. This analysis provides useful qualitative information about events of interest, confirming them, defining minimal affected regions, and suggesting particular segmental duplications likely to harbor breakpoints. In a best case scenario, initial array CGH will refine breakpoint locations to specific segmental duplications—probe cross-hybridization generally prevents further narrowing breakpoint locations within the duplications. Given the higher resolution of the sequencing-based approaches outlined below, their ability to assay several pairs of candidate breakpoint-associated segmental duplications in a single experiment, and the high cost of custom array design, array CGH is not necessary for breakpoint mapping. However, array CGH provides orthogonal data that is often useful to validate results and in cases in which analysis of sequencing data fails to define clear breakpoint intervals.
Haplotype isolation (steps 4–8)
One strategy to achieve breakpoint resolution begins with the generation of human-mouse somatic cell hybrids to physically isolate the chromosome harboring the duplication or deletion and, separately, its parental progenitor14–15, 27. Isolation of these chromosomes in somatic cell hybrids simplifies breakpoint localization for any method because afterwards, confounding effects of unaffected homologs need not be considered. This strategy can be leveraged to perform an array CGH experiment comparing the affected and parental chromosomes or to simplify analysis of sequencing data. For example, we determined that two distinct types of NAHR accounted for the three deletions we observed in patients with the 17q21.31 microdeletion syndrome. We reached that conclusion based on observing two distinct patterns of haplotype-specific array CGH data14. Regarding sequencing data analysis, SUNs can be assessed for presence or absence rather than relative read-depth when the input library is derived from any chromosome in isolation from its homolog. Isolation of at least the affected chromosome is, therefore, in theory an optimal early step in any breakpoint mapping effort. However, we recognize that generating somatic cell hybrids is a time-consuming, expensive, and highly specialized process, demanding specific expertise and experience and not amenable to large-scale application. Therefore, haplotype isolation–based breakpoint resolution has been applied only for small numbers of cases where the benefits described above promise considerable improvement in the quality of the results obtained over other options not requiring haplotype isolation. An emerging alternative to creating somatic cell hybrids for achieving haplotype isolation with greater efficiency is sub-haploid complexity reduction and sequencing, for instance with fosmids or in vitro dilution28–29.
Any current haplotype isolation method remains very expensive and time-consuming relative to sequencing, and thus we recommend pursuing such a strategy only if WGS or targeted sequencing following one of the approaches described below fails to define breakpoint intervals. In such cases, haplotype isolation may prove worthwhile, particularly if the individual is homozygous for the structural haplotype on which the event occurred and the breakpoint-associated segmental duplications have many paralogs throughout the genome—situations that are more challenging than that we faced in the 17q21.31 study and our RH example. Note that a failure of the sequencing-based approaches could indicate inaccuracy of the genomic sequences used for SUN identification or their irrelevance in that particular case due to NAHR having occurred on a structural haplotype distinct from those sequences, with accordingly different SUNs (see Box 1). As noted above, performing array CGH or haplotype-specific array CGH would probably yield some biological insight in cases where sequencing strategies fail, particularly if array data can be generated for both unresolved and resolved cases involving the same genomic region and compared in a manner akin to our 17q21.31 analysis14.
WGS of a trio (steps 11–20)
An alternative to haplotype isolation is high-coverage sequencing of diploid genomic DNA from the affected individual and both parents (a trio) and directly assaying sequence read-depth across the breakpoint-informative regions to define the unequal crossover event14. Implementation of this approach enables the researcher to achieve sequence-level breakpoint resolution using bioinformatics analyses. Performing WGS provides a comprehensive view of genetic variation across the genome in addition to refined breakpoints, a feature particularly valuable in studies exploring genetic bases for variable expressivity of a recurrent event. One limitation of this strategy is its relatively high cost, which currently precludes its application to patient cohorts of even moderate size.
MIP capture and sequencing (steps 21–103)
The majority of reads from sequencing libraries prepared from genomic DNA or even from an isolated affected chromosome are not useful for breakpoint refinement, because they do not map to breakpoint regions or do not contain relevant SUNs. The approach described in this paragraph circumvents this problem by first enriching the sequencing library to obtain high coverage precisely over the regions that are most informative for breakpoint delineation23. Targeted capture using MIPs is particularly well suited for this purpose, as ~3,000 MIPs can be combined in a single reaction to simultaneously assay many loci30–32. MIPs are short oligonucleotides (70–80 bp) used to capture specific genomic targets <200 bp in length. They have been successfully applied to routinely genotyping thousands of samples and are generally cost-effective compared to WGS when used to assay moderate to large numbers of samples.
Despite its advantages, MIP capture is not always preferable to WGS for breakpoint resolution. Not all SUNs are targetable using MIPs. Regions of particularly low or high GC content (<30% or >60%) are often refractory to successful capture30–31, and high-copy repeats cannot be specifically targeted. As currently implemented, analysis of WGS data focuses on absolute read-depth over SUNs, whereas MIP sequence data analysis considers read-depth over SUNs relative to that over captured paralogous targets. This relative analytical framework makes regions of high aggregate copy number difficult to accurately analyze using the MIP method23. Furthermore, because such regions often contain very few SUNs, successfully interrogating as many individual SUNs as possible is critical for breakpoint resolution within these regions. WGS, in principle, yields data for all SUNs, including those that MIPs would not capture. In general, however, we believe that MIP-based breakpoint resolution will prove more broadly useful than its counterpart based on WGS, at least until the cost of the latter approach becomes less prohibitive.
MATERIALS
REAGENTS
Custom oligonucleotide microarrays (Agilent Technologies)
Array CGH reagents16
Microsatellite genotyping reagents36
Somatic cell hybrid reagents15
Molecular inversion probes (MIPs, Integrated DNA Technologies)
T4 DNA Ligase Buffer with 10 mM ATP (New England BioLabs cat. no. B0202S)
T4 polynucleotide kinase (New England BioLabs, cat. no. M0201L)
Ampligase buffer (Epicentre, cat. no A1905B)
10 mM dNTP mix (Roche NimbleGen, cat. no. 11581295001)
Hemo Klentaq (New England BioLabs, cat. no. M0332L)
Ampligase (Epicentre, cat. no. A0110K)
Nuclease-free water (Ambion, cat. no. AM9906)
Genomic DNA from individuals to be analyzed CAUTION: All human genetic studies must be approved by an institutional review board, and all participating subjects must provide informed consent.
Exonuclease I (New England BioLabs, cat. no. M0293L)
Exonuclease III (New England BioLabs, cat. no. M0206L)
2X iProof PCR master mix (Bio Rad, cat. no. 172-5311)
Primer SLXA_PE_MIPBC_FOR (100 μM, Supplementary Table 1, Operon)
SYBR green (Life Technologies cat. no. S-7563, dilute from 10,000X to 100X in DMSO)
Reverse barcode primers (10 μM, Supplementary Table 1, Integrated DNA Technologies)
Magnetic beads (Agencourt, Beckman Coulter, cat. no. A63881)
Ethanol 200 proof (Decon Laboratories, Inc., cat. no. 2716)
Deionized water (MilliQ)
Elution buffer (Qiagen, cat. no. 19086)
Sequencing primers (100 μM, Supplementary Table 1, Operon)
HiSeq or MiSeq kit (Illumina, v2 PE 300 cycles)
EQUIPMENT
1.7 mL Eppendorf tube incubators (VWR)
Hybridization oven (Shel Lab)
Microarray scanner (Agilent technologies)
Agilent feature extraction software
Microsatellite genotyping equipment36
Somatic cell hybrid equipment15
SPARC computer
Microsoft Excel
Microcentrifuge (Eppendorf)
1.7 mL PCR tube mini centrifuge (Fisher Scientific)
96-well cold blocks (Eppendorf, cat. no. Z606634)
Lightcycler (Bio Rad)
Optical qPCR tubes, 8-strip (Bio Rad, cat. no. TLS-0851)
Optical qPCR caps, 8-strip (Bio Rad, cat. no. TCS-0803)
200 μL 8-strip PCR tube mini centrifuge (Fisher Scientific)
Magnet tube rack (Life Technologies MagnaRack, cat. no. CS15000)
eGel system (Life Technologies, cat. no. G6512ST)
Qubit DNA quantification system (cat. no. Q32871)
Illumina MiSeq, HiSeq2000, or HiSeq 2500 sequencer
Hardware (64-bit computer running Linux with at least 5 GB RAM—a high-memory, multicore computer is best)
Software (see Box 2)
Box 2. Software setup.
Custom analysis programs.
Download all custom analysis programs from GitHub
(http://github.com/xnuttle/breakpoint_resolution_wgs_mips) and save them in a single directory, for example, ‘/software/brkpt/’. Define the environmental variable BRKPT_SOFTWARE by using the command below or including the command below as a line in your bash profile, replacing the example path shown here with the actual path to the directory where the custom analysis programs have been saved:
$ export BRKPT_SOFTWARE=/software/brkpt
mrFAST
Download mrFAST37 version 2.6.0.0 from SourceForge (http://mrfast.sourceforge.net/). Refer to the mrFAST user manual (http://mrfast.sourceforge.net/manual.html) for detailed instructions on setup and usage.
R
Download R version 2.15 from the R Project website (http://www.r-project.org). Install the R package ‘ggplot2’ by running the following command in the R console:
> install.packages(“ggplot2”)
REAGENT SETUP
Molecular inversion probes. Order MIPs with the following specifications: a scale of 25 nmole, with standard desalt purification, in deep well plates, shipped wet-frozen, with a full yield per well, at a concentration of 100 μM in buffer/IDTE (1X TE buffer) at pH 8. Upon arrival, MIPs should be stored at 4°C (for up to ~1 year) or -80°C (for long-term storage).
Reverse barcode primers. Order reverse barcode primers (Supplementary Table 1) with the following specifications: a scale of 25 nmole, with standard desalt purification, in deep well plates, shipped wet-frozen, with a full yield per well, at a concentration of 100 μM in buffer/IDTE (1X TE buffer) at pH 8. Prepare each working reverse barcode primer plate by adding 20 μl of each reverse barcode primer and 180 μl of elution buffer to each well of a new 96-well plate and mixing thoroughly by pipetting up and down.
EQUIPMENT SETUP
WGS
Depending on the desired turnaround time, either the Illumina HiSeq 2000 or the HiSeq 2500 can be used for sequencing. (See http://www.illumina.com/systems/sequencing.ilmn and http://res.illumina.com/documents/systems/hiseq/datasheet_hiseq_systems.pdf for comparisons of various sequencing platforms.) The final sequence coverage for each individual should be >15X (follow standard protocols with 101 bp paired-end reads and 300–500 bp insert libraries). This level of coverage ensures that several breakpoint-informative sequence reads will be obtained. Sequencing runs should include forward and reverse reads of at least 100 bp each as well as an 8 bp index read. Sequences of the index reads will be the reverse complements of sample barcode sequences incorporated into the sequencing library during library preparation.
MIP sequencing
Depending on the desired coverage per MIP target and the desired turnaround time, any of the Illumina MiSeq, HiSeq 2000, or HiSeq 2500 can be used for sequencing. (See http://www.illumina.com/systems/sequencing.ilmn and http://res.illumina.com/documents/systems/hiseq/datasheet_hiseq_systems.pdf for comparisons of various sequencing platforms.) For a pool of ~2,000 MIPs, up to 192 samples can be pooled and sequenced to good coverage on a single lane of HiSeq 2000 with 101 bp paired end reads and an 8 bp index read. With the reverse barcode primers provided here (Supplementary Table 1), up to 384 samples can be pooled and analyzed in a single run. Sequencing runs should include forward and reverse reads of at least 100 bp each as well as an 8 bp index read. Sequences of the index reads will be the reverse complements of sample barcode sequences incorporated into the sequencing library during library preparation. Sequencing primers are reported in Supplementary Table 1.
Centrifugation
Many steps in this protocol specify using a centrifuge or microcentrifuge but do not provide information regarding the centrifugation speed, duration, and temperature. For these steps, the exact centrifugation speed, duration, and temperature do not matter, so long as the centrifugation effectively ensures all liquids in plates or tubes collect at the bottom of the wells or tubes.
Hardware
Many programs benefit from parallelization in a parallel computing environment, such as a high-performance Linux-based cluster integrated with network-available storage. Specifically, we use a Linux-based high-performance cluster with 110 nodes with an aggregate 1048 CPU cores. We have 491 terabytes (TB) of usable network available storage, a mix of EMC SAN based storage (22 TB), a CORAID storage server (48 TB), three large Sun Microsystems storage servers (131 TB), and three Dell SAS servers (290 TB). To facilitate rapid analysis of data across systems, all storage can be made available to all cluster nodes, application servers, and desktop systems. The cluster queuing system is Sun Grid engine 6.1.
PROCEDURE
Targeted array CGH TIMING 2–3 days (plus the time it takes to receive oligonucleotide microarrays from supplier)
CRITICAL The implementation of this sub-section of the Procedure (steps 1–3) is optional (see Experimental design for a relevant discussion on its suggested implementation)
-
1
Design a custom oligonucleotide microarray covering the region of interest using the Agilent eArray design suite (http://www.genomics.agilent.com/en/product.jsp?cid=AG-PT-122&tabId=AG-PR-1047&_requestid=1207000). Probes should be 60 bp in length and designed at a high-density over the target region (approximately one probe every 900 bp). To ensure adequate coverage of the region of interest, probe design should cover a larger region including at least 25 kbp of flanking sequence on each side of the breakpoint-associated segmental duplications. The final probe set should include a substantial fraction of probes targeting unique regions outside of the region of interest (e.g. a genomic backbone), as such regions facilitate calibration of diploid state (see step 3 below). Order the oligonucleotide arrays.
PAUSE POINT: Ordered custom oligonucleotide arrays take several weeks to months to arrive.
-
2
Perform array CGH experiments using a sample from the individual whose DNA has the duplication or deletion under investigation and a sample from an individual whose DNA does not to approximate regions where breakpoints occur. Provided that DNA from the affected individual and both parents is available in sufficient quantity for these experiments (250 ng per individual per hybridization) and desired follow-up experiments, we recommend performing three separate array CGH experiments (comparing the affected individual to the mother, the affected individual to the father, and the mother to the father). A protocol detailing the array CGH procedure has been previously published16.
-
3
Analyze the array CGH data using Agilent feature extraction software (http://www.genomics.agilent.com/en/Microarray-Scanner-Processing-Hardware/Feature-Extraction-Software/?cid=AG-PT-144&tabId=AG-PR-1050) and custom analysis programs to generate plots of log2 fluorescence intensity ratios across the spatial extent of the targeted region. For quality control, we recommend following the manufacturer’s instructions, ensuring the derivative log ratio spread is <0.23 per sample. To highlight probes signaling a deletion or a duplication, visualize points with log2 ratios >1.5 standard deviations from the mean log2 ratio in the experiment using different colors. If probes in the region of interest constitute a substantial fraction of the total probe set (>5%), exclude these probes when calculating the mean and standard deviation of log2 ratios. Note that the signals from deletions and duplications affecting duplicated sequences will not be as strong as those observed for such events affecting unique sequences, because the relative loss or gain of DNA is smaller for sequences originally present at higher copy numbers.
Isolation of the affected chromosome and its parental progenitor(s) TIMING 2–3 months
CRITICAL The implementation of this sub-section of the Procedure (steps 4–8) is optional (see Experimental design for a relevant discussion on the merits of implementing it)
-
4
Genotype DNA from the affected individual as well as from both parents using microsatellite markers (Marshfield Map38) along the chromosome of interest, including multiple markers in proximity to the deletion or duplication locus (within ~5 Mbp). The Marshfield map is a collection of short-tandem repeat markers developed in the 1990s to map human genetic traits, integrate physical mapping data, and assess patterns of recombination38. Obtaining genotypes at these markers will enable the experimenter to infer the haplotypes of the affected individual and of both parents. A protocol detailing microsatellite genotyping has been published36.
-
5
Generate human-mouse somatic cell hybrids by electrofusion of human Epstein-Barr-Virus-transformed lymphoblast cells with mouse E2 cells and expand transformants for 18 days, using a standard protocol for the construction of somatic cell hybrids15.
-
6
Genotype somatic cell hybrid colonies (50–100) for the same microsatellite markers as above, following published protocols15,36. Generating somatic cell hybrids yields several colonies, most of which do not contain the affected chromosome or a parental homolog in isolation. Genotyping microsatellites from several colonies enables the experimenter to identify these colonies of interest and provides insight into the integrity of the affected and parental homologous chromosomes they contain.
-
7
Genotype a denser panel of microsatellite markers, including several in close proximity (~1 Mb) to the deletion or duplication event, using DNA from a single colony that has the affected chromosome intact and in isolation and from four single colonies each having one parental homolog intact and in isolation. Follow the same published protocols as above15,36. The genotyping results will provide insight into the specific parental chromosome or chromosomes involved in the NAHR event, recombination patterns, and the timing of the NAHR event in meiosis (i.e., meiosis I or II).
-
8
Propagate a single colony harboring the intact, isolated affected chromosome and a single colony harboring the intact, isolated parental homolog involved in NAHR (determined in the previous step). If two parental homologs were involved in the deletion or duplication event (i.e., NAHR was interchromosomal rather than interchromatidal), propagate at least three single colonies, one harboring each intact, isolated parental homolog involved in NAHR and one harboring the intact, isolated affected chromosome. Follow the published protocol15 to propagate relevant colonies and isolate DNA from each that can be used for haplotype-specific array CGH or sequencing experiments.
Haplotype-specific array CGH TIMING 2–3 days
CRITICAL: The implementation of this sub-section of the Procedure (steps 9–10) is optional and can only be performed after the completion of steps 4–8 (see Experimental design for details and discussion)
-
9
Implement steps 1–3 described above using DNA from one expanded somatic cell hybrid colony harboring the affected chromosome as test and another harboring its progenitor as reference to obtain log2 fluorescence intensity ratios. Provided that NAHR was interchromatidal in origin (the most typical case), this experiment allows for the direct comparison of the affected chromosome with an effectively isogenic background over the region of interest. This comparison is possible because the rearranged chromosome differs from the parental donor chromosome primarily as a result of the duplication or deletion event.
-
10
Assuming a model of NAHR, identify all directly orientated segmental duplications using the Segmental Duplications track on the UCSC Genome Browser (http://www.genome.ucsc.edu/) or the whole-genome assembly comparison pipeline3, if sequences of interest are not accurate or complete in the reference genome (see Box 1). This pipeline defines all segmental duplications over 1 kbp in length and having >90% sequence identity in a genome. For all duplication pairs, analyze log2 ratio patterns over the spatial extent of the targeted region14. Comparing the observed log2 ratios with expected log2 ratios under different hypothesized NAHR scenarios enables the experimenter to define candidate breakpoint-harboring duplication pairs and eliminate others from consideration.
Massively parallel WGS and SUNK analysis TIMING variable from ~1–3 weeks, depending on sequencing platform
CRITICAL: Please note that implementation of this sub-section of the Procedure (steps 11–20) is an alternative to the the MIP-based approach (steps 21–103, see Experimental design for a relevant discussion)
-
11
Sequence libraries prepared from genomic DNA or haplotype-resolved chromosomes from the affected individual and both parents on an Illumina HiSeq according to the manufacturer’s instructions and specifications detailed above (see WGS under EQUIPMENT SETUP). Please note that sequencing will take anywhere from ~1 day to multiple weeks to complete, depending on the platform used. Once the sequencing run is complete, data should be stored and backed up before beginning the analysis. Please note as well that the first few steps of the analysis (steps 12–15 below) do not require the sequencing data and can be completed while waiting for the sequencing run to finish.
-
12
Obtain paralogous breakpoint-associated sequences and align them using an alignment program such as ClustalW2 (http://www.clustal.org/clustal2/). Make two fasta files from the alignment output, one containing each aligned sequence. These fasta files should include ‘−’ characters within the nucleotide sequences at positions corresponding to alignment gaps. Both unaligned and aligned breakpoint-associated sequences must start with the first base in the alignment and end with the last base in the alignment. Name the unaligned sequences ‘prox.fasta’ and ‘dist.fasta’ (corresponding to the proximal and distal breakpoint-associated segmental duplications mediating the rearrangement) and the aligned sequences ‘prox_aligned.fasta’ and ‘dist_aligned.fasta’. Make sure the names of the sequences correspond to the file names (e.g., the file ‘prox.fasta’ should have ‘>prox’ as its first line). Save all sequences in the same directory and name this directory ‘brkpt_WGS’, referred to henceforth as the project directory.
-
13
Determine the reference sequence coordinates corresponding to the contig sequences—specifically, the reference coordinates of the first and last bases in the ‘prox.fasta’ and ‘dist.fasta’ files. Create a tab-delimited text file in the project directory detailing this information, with the chromosome name (i.e. ‘chr1’) in the first column, the base-1 start coordinate in the second column, and the base-1 end coordinate in the third column. Name this file ‘seqs.refcoords’. These regions must be listed in order of their reference genomic coordinates.
-
14Identify breakpoint-informative SUNKs (36 bp) and SUNs by running the script ‘wgs_analysis_pt1.sh’ from the project directory on a high-memory machine. This program will generate the text files ‘brkpt.suns’, ‘brkpt.sunks’, and ‘brkpt.sunsunks’. It requires high memory (for an example test run, the ‘top’ command showed that VIRT was 52.0g and RES was 27g) to run and takes several hours to finish; we thus recommend running it overnight. Please note that the following step 15 can be completed before this step finishes and before the sequencing run is complete.
$ bash $BRKPT_SOFTWARE/wgs_analysis_pt1.sh
?TROUBLESHOOTING
-
15
Create a tab-delimited file listing the names of all samples pooled in the sequencing run in the first column and their corresponding barcode reverse complement sequences in the second column. Name this file ‘brkpt.barcodekey’ and save it in the project directory.
-
16
After the sequencing run has completed, follow the manufacturer’s instructions regarding bcl conversion to convert raw sequencing base call data to qseq text files. Make a new directory in the project directory called ‘raw_qseq_files’ and store the qseq text files in this new directory. Do not compress these files—running the script in the next step will do that.
-
17Change into the ‘raw_qseq_files’ directory and run the script ‘wgs_analysis_pt2.sh’ to generate gzipped fastq files that will be searched for breakpoint-informative SUNKs. These files will be generated in the ‘raw_qseq_files’ directory and moved to a directory within the parent directory called ‘fastqs’.
$ cd raw_qseq_files $ bash $BRKPT_SOFTWARE/wgs_analysis_pt2.sh
-
18Quantify read-depth over breakpoint-informative SUNs by running the script ‘wgs_analysis_pt3.sh’ from the ‘raw_qseq_files’ directory. Directories for each sample in the ‘brkpt.barcodekey’ file will be created within the ‘fastqs’ directory. In each of these sample directories, final output is written to the file ‘brkpt.suns.depth’, with the last column of this file showing the observed read depth over each breakpoint-informative SUN.
$ bash $BRKPT_SOFTWARE/wgs_analysis_pt3.sh
-
19Analyze and visualize the data in R. To view data for a single individual, copy that individual’s ‘brkpt.suns.depth’ file and the ‘$BRKPT_SOFTWARE/pdf_brkpt_WGS.r’ file to a directory R can access, open R, and set the R working directory to that directory. Then run the following commands in the R console to generate a file with a name like ‘sample_brkpt.pdf’, written to that directory. Replace ‘sample’ in the first command below with the name of the individual:
>indiv<-“sample” >source(“pdf_brkpt_WGS.r”)
-
20
Manually inspect the pdf file with a name like ‘sample_brkpt.pdf’ showing read depth data over breakpoint-informative SUNs for the individual of interest. Breakpoint signatures should be apparent as a decrease or increase of paralog-specific read-depth over the extent of the deletion or duplication (e.g. Fig. 2c, see Anticipated results for further discussion).
MIP design TIMING 1 day, plus the time it takes to receive MIP oligonucleotides from the supplier (~1–2 weeks)
CRITICAL: As mentioned above, steps 21–103 below should be performed as an alternative to the WGS approach (steps 11–20, see Experimental design for relevant discussion)
-
21
Obtain paralogous breakpoint-associated sequences and align them using an alignment program such as ClustalW2 (http://www.clustal.org/clustal2/). Make two fasta files from the alignment output, one containing each aligned sequence. These fasta files should include ‘−’ characters within the nucleotide sequences at positions corresponding to alignment gaps. Both unaligned and aligned breakpoint-associated sequences must start with the first base in the alignment and end with the last base in the alignment. Name the unaligned sequences ‘prox.fasta’ and ‘dist.fasta’ (corresponding to the proximal and distal breakpoint-associated segmental duplications mediating the rearrangement) and the aligned sequences ‘prox_aligned.fasta’ and ‘dist_aligned.fasta’. Make sure the names of the sequences correspond to the file names (e.g., the file ‘prox.fasta’ should have ‘>prox’ as its first line). Save all sequences in the same directory and name this directory ‘brkpt_MIPs’, referred to henceforth as the project directory.
-
22Generate initial MIP designs. The script ‘mip_design_pt1.sh’ calls several programs to design an initial set of MIPs targeting breakpoint-informative SUNs, detailed in the output file ‘brkpt.mipdesign’. It typically takes from 30 min to 2 h to run. Run this script from the project directory:
$ bash $BRKPT_SOFTWARE/mip_design_pt1.sh
-
23
Import data from the tab-delimited text file ‘brkpt.mipdesign’ into Microsoft Excel, so that each column in the file is imported into a separate column in the spreadsheet and the data begin in position A1. The first line of the file ‘prox.mipdesign’ details the meaning of the data in each of the columns except for the last column, which contains the oligo sequences for all MIPs initially designed.
-
24
Sort the Excel spreadsheet by column S and delete all rows having a value in column S that includes ‘snp’. This action will ensure that all remaining MIP designs have hybridization arms targeting sequences that are identical between both breakpoint-associated sequences.
-
25
Sort the Excel spreadsheet by column G, which contains the total number of copies of the extension hybridization arm sequence found in the human genome. Delete all rows having values in this column greater than a threshold cutoff. The exact value of this cutoff will differ on a case-by-case basis, but a good general rule to follow is that the cutoff should be approximately two times the number of copies of breakpoint-associated duplicated sequences in the haploid genome of interest. For example, if the breakpoint-associated sequences have no paralogous sequences elsewhere in the genome, this cutoff should be set around 4. Increasing this cutoff will increase the number of MIPs designed and potentially the spatial resolution of refined breakpoints but may result in more off-target MIP capture events. Columns L–M contain the alignment coordinates of regions targeted by remaining MIPs, so sorting by column L and examining the values in columns L–M in comparison to the length of the aligned sequence ‘prox_aligned.fasta’ provides some sense of the spatial resolution afforded by remaining MIPs.
-
26
Sort the Excel spreadsheet by column K, which contains the total number of copies of the ligation hybridization arm sequence found in the human genome, and delete all rows having values in the column greater than the same threshold cutoff imposed in step 25.
-
27Sort the Excel spreadsheet by column L to order remaining MIPs by the alignment coordinates of the regions they target. Copy all sequences in column N, paste them into a new text file named ‘target.seqs’ created in the project directory using a command line text editor such as vim (http://www.vim.org/), and save the text file. Then run the following command from the project directory to calculate GC content for all remaining MIP target regions. This information will be taken into account by a later program that selects a set of MIPs having good potential for successfully capturing targets harboring breakpoint-informative SUNs:
$ $BRKPT_SOFTWARE/calculate_target_GC target.seqs > target.gc
-
28Import data from the text file ‘target.gc’ into column X of the Excel worksheet such that the data begins in position X1. Cut the data from column X and paste it to the same rows in column S. Copy all data in the spreadsheet, paste it into a new text file named ‘brkpt.filtered.mipdesign’ created in the project directory using a command line text editor such as vim (http://www.vim.org/), and save the text file. Then run the script ‘mip_design_pt2.sh’ from the project directory to generate a file containing the final MIP oligos to order:
$ bash $BRKPT_SOFTWARE/mip_design_pt2.sh
-
29
Order MIPs (see REAGENT SETUP for order specifications). The final set of MIP oligonucleotides to order is specified in the last column of the file ‘brkpt.filtered.mippicks’ in the project directory.
PAUSE POINT: Ordered MIPs typically take 1 to 2 weeks to arrive.
MIP pooling and 5′ phosphorylation TIMING 2–3 h
-
30
On the same day the ordered MIPs are received in plates, allow these plates to thaw in a 4-°C refrigerator.
-
31
Remove thawed MIP plates from the 4-°C refrigerator and spin them down using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
32
For each MIP plate, pool 5 μl of each MIP into a 1.7-ml Eppendorf tube. This pooling can be easily accomplished with an 8-channel 0.5–10 μl mechanical pipette to first pool MIPs from each row of a plate into an 8-strip of 200-μl PCR tubes and then pooling the contents of each PCR tube into an Eppendorf tube.
-
33
Pool together the MIP pools for each plate by combining P μl of each plate pool into a new 1.7-ml Eppendorf tube, where P = 0.1 times the number of MIPs in each plate pool, calculated separately for each plate pool.
-
34
Add B μl of 10X T4 DNA Ligase Buffer with 10 mM ATP and K μl of T4 polynucleotide kinase to the same 1.7-ml tube containing the final MIP pool from step 33. Here B = (5/26)*(volume of MIPs in that pool) and K = (7/26)*(volume of MIPs in that pool).
-
35
If the total volume in the 1.7-ml tube after step 34 is less than 50 μl, add enough nuclease-free water to bring the final volume to 50 μl, then vortex the tube, spin it down using a microcentrifuge (see Centrifugation under EQUIPMENT SETUP), and transfer the tube content to a 200-μl PCR tube. Otherwise, use enough nuclease-free water to bring the final volume up to V μl, where V is the smallest multiple of 50 above the current total volume, vortex, centrifuge as detailed above, and then split up the final volume into multiple 200-μl PCR tubes each containing 50 μl of solution.
-
36
Phosphorylate the MIPs by incubating the PCR tubes from step 35 in a thermocycler. Set the thermocycler to run at 37 °C for 45 min, followed by 65 °C for 20 min and then 4 °C indefinitely, with a heated lid at 105 °C. Do not place the tubes in the thermocycler until the block temperature reaches 37 °C.
MIP capture TIMING ~1 day
-
37
After the reaction(s) from step 36 have completed (reached the 4-°C stage in the thermocycler), combine all reaction products from step 36 into a single 1.7-ml Eppendorf tube to form a stock of phosphorylated MIPs.
PAUSE POINT: Phosphorylated MIPs can be stored at 4 °C indefinitely.
-
38
Calculate the concentration C of each phosphorylated MIP in the stock, where C = 10/V μM, where V = the final volume of the stock determined in step 35.
-
39
Calculate the volume of MIP stock to add per MIP capture reaction. 1 ng of genomic DNA contains ~330 haploid genome copies. 200 ng of genomic DNA will be used per reaction; therefore, ~66,000 haploid genome copies will be present per reaction. Each MIP should be present at a ratio of 800 copies per haploid genome copy, so ~52,800,000 copies of each MIP are needed per reaction, or 8.8*10−5 picomoles. Thus, you will need to add M μl of MIP stock per reaction, where M = 8.8*10−5/C, where C = the concentration in μM calculated in step 38. Because the value of M is typically very low, it will likely be necessary to dilute the phosphorylated MIP stock using elution buffer to form a working stock solution and recalculate M based on the concentration of the working stock. M should ideally be between 0.1 and 1 μl.
-
40
Dilute genomic DNA samples to be analyzed to the same concentration S: 10–25 ng/μl.
-
41
Label an Eppendorf plate with information about the experiment and add U μl of samples from step 40 to it, where U = 200/S, where S is the concentration of each sample from step 40. Make sure to record the contents of each well for future reference.
-
42
Remove two 96-well cold blocks from the −20-°C freezer.
-
43
Make a working stock of 0.25 mM dNTPs by combining 1 μl of 10 mM dNTP mix with 39 μl of nuclease-free water.
-
44
Set up a master mix for the MIP capture reactions in a 2-ml Eppendorf tube, keeping the tube and its contents on ice when not adding components. In addition to the DNA to be analyzed, each reaction mixture contains 2.5 μl of ampligase buffer, M μl of working MIP stock (as calculated in step 39), 0.032 μl of 0.25 mM dNTPs, 0.32 μl of Hemo Klentaq, 0.01 μl of ampligase, and W μl of nuclease-free water, where W = 25 – (U+2.5+M+0.032+0.25+0.32+0.01), where U is the volume of sample added per well determined in step 41. Make enough master mix to prepare more reaction mixtures than the capture reactions to be performed, so as to allow for pipetting error. Preparation of this excess volume is recommended particularly if the experimenter is planning, as we recommend (see step 46), to transfer the master mix first to 8-well 200-μl PCR tubes so that the 8-channel 5–100 μl pipette can be used to add the master mix to the plate with samples.
-
45
Place the labeled plate with samples into a 96-well cold block.
-
46
Vortex the master mix, spin it down using a microcentrifuge (see Centrifugation under EQUIPMENT SETUP), and add MM μl of the master mix to each well of the plate with samples, where MM = 25 – U, where U is the volume of sample added per well determined in step 41. We recommend first transferring the master mix to 8-well 200-μl PCR tubes (placed in the remaining 96-well cold block) so that the 8-channel 5–100 μl pipette can be used to add the master mix to the plate. Mix the master mix with the samples by pipetting up and down a few times with the tips in the wells.
-
47
Seal the plate with a PCR plate seal.
CRITICAL STEP: The plate must be sealed very well, particularly along the edges, or some samples will likely evaporate during the capture reaction.
-
48
Spin down the plate using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
49
Incubate the sealed plate in a thermocycler for ~ 23 h. Set the thermocycler to run at 95 °C for 10 min, followed by 60 °C forever, with a heated lid at 105 °C. Do not place the tubes in the thermocycler until the block temperature reaches 95 °C.
Exonuclease treatment TIMING ~1 h
-
50
When the capture reactions from step 49 have nearly completed (~22.5 h after they were placed in the thermocycler), remove the ampligase buffer from the −20-°C freezer and allow it to thaw completely.
-
51
After the capture reactions from step 49 have completed (~23 h after they were placed in the thermocycler), remove two 96-well cold blocks from the −20-°C freezer and allow them to thaw for ~5 min.
-
52
Remove the plate from the thermocycler, place it into a 96-well cold block, and spin it down using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
53
Set up a master mix for the exonuclease reactions in a 1.7-ml Eppendorf tube, keeping the tube and its contents on ice when not adding components. In addition to the capture reaction products, each reaction contains 0.5 μl of exonuclease I, 0.5 μl of exonuclease III, 0.2 μl of ampligase buffer, and 0.8 μl of nuclease-free water. Make enough master mix for more reactions than capture reactions to allow for pipetting error, particularly if planning to transfer the master mix first to 8-well 200-μl PCR tubes, so that the 8-channel 0.5–10 μl pipette can be used to add the master mix to the plate with samples (recommended).
-
54
Vortex the master mix, spin it down using the microcentrifuge (see Centrifugation under EQUIPMENT SETUP), and add 2 μl of the master mix to each well of the plate with capture reactions. We recommend first transferring the master mix to 8-well 200-μl PCR tubes (placed in the remaining 96-well cold block) so that the 8-channel 0.5–10 μL pipette can be used to add the master mix to the plate. Mix the master mix with the capture reactions by pipetting up and down a few times with the tips in the wells.
-
55
Seal the plate with a PCR plate seal.
-
56
Spin down the plate using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
57
Incubate the sealed plate in a thermocycler. Set the thermocycler to run at 37 °C for 45 min, followed by 95 °C for 2 min and then 4 °C indefinitely, with a heated lid at 105 °C. Do not place the tubes in the thermocycler until the block temperature reaches 37 °C.
PAUSE POINT: Exonuclease-treated capture reactions can be stored at 4 °C for several days. Nevertheless, we recommend proceeding to quantitative PCR within a day of finishing the exonuclease treatment.
Quantitative PCR TIMING ~1 h
-
58
After the reactions from step 57 have completed (the 4-°C stage has been reached in the thermocycler), remove the plate from the thermocycler and spin it down using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
59
Remove a 96-well cold block from the −20-°C freezer.
-
60
Remove the reverse barcode primer plate (see REAGENT SETUP) from the 4-°C refrigerator and spin it down using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
61
Set up a master mix for 8 (or as many samples as being analyzed plus one if fewer than 7 samples are being analyzed) quantitative PCR reactions in a 1.7-ml Eppendorf tube, keeping the tube and its contents on ice when not adding components. In addition to exonuclease reaction products and reverse primers to be added separately, each reaction contains 12.5 μl of 2X iProof PCR master mix, 0.125 μl of forward primer solution (SLXA_PE_MIPBC_FOR, 100 μM, Supplementary Table 1), 0.125 μl of SYBR green 100X, and 6 μl of nuclease-free water. Prepare enough master mix for more reactions than capture reactions to allow for pipetting error.
-
62
Put one strip of 8-well optical PCR tubes in the cold block, vortex the master mix, spin it down using a microcentrifuge (see Centrifugation under EQUIPMENT SETUP), and add 18.75 μl of master mix to each optical PCR tube.
-
63
Add 5 μl of exonuclease reaction products from the first column of the exonuclease reaction plate, except for the last row, to the 8-well optical PCR tubes using the 8-channel 0.5–10 μl pipette. Mix the exonuclease reaction products with the master mix by pipetting up and down a few times with the tips in the wells. Add 5 μl of nuclease-free water to the optical PCR tube without added exonuclease reaction product to serve as a negative control and mix by pipetting up and down a few times with the tip in the well.
-
64
Add 1.25 μl of reverse barcode primers (Supplementary Table 1) from the first column of the reverse barcode primer plate to the 8-well optical PCR tubes using the 8-channel 0.5–10 μl pipette. Mix by pipetting up and down a few times with the tips in the wells.
CRITICAL STEP: Take extreme care not to contaminate the different reverse barcode primer solutions with each other—always use new pipette tips when working with this plate.
-
65
Seal the 8-well optical PCR tubes using an 8-strip of optical qPCR caps and spin down using a microcentrifuge (see Centrifugation under EQUIPMENT SETUP).
-
66
Incubate the sealed plate in a light thermocycler. Set the light thermocycler to run at 98 °C for 30 s, followed by 30 cycles at 98 °C for 10 s, 60 °C for 30 s, 72 °C for 20 s, a plate read, and 72 °C for 10 s, followed by 72 °C for 2 min and then 4 °C forever, with a heated lid at 105 °C. Do not place the tubes in the thermocycler until the block temperature reaches 98 °C.
-
67
Determine approximately how many cycles Y the quantitative PCR reactions took until the fluorescence curves reached their plateaus by manually inspecting the fluorescence curves. Also ensure that this value was tightly distributed between individual curves (the highest value should be within 2 cycles of the lowest value, excluding the value corresponding to the negative control curve, which should plateau far later than all other curves or not plateau at all). Typical values for Y are in the 17–24 cycle range, depending on the MIP pool used and the initial amount of DNA added to each MIP capture reaction.
?TROUBLESHOOTING
PCR TIMING ~1 h
-
68
Remove two 96-well cold blocks from the −20-°C freezer.
-
69
Remove the reverse barcode primer plate from the 4-°C refrigerator and spin it down using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
70
Set up a master mix for the PCR reactions in a 15-ml Falcon tube, keeping the tube and its contents on ice when not adding components. In addition to exonuclease reaction products and reverse primers to be added separately, each reaction contains 12.5 μl of 2X iProof PCR master mix, 0.125 μl of forward primer (SLXA_PE_MIPBC_FOR, 100 μM, Supplementary Table 1), and 6.125 μl of nuclease-free water. Make enough master mix for more reactions than capture reactions to allow for pipetting error, particularly if planning to transfer the master mix first to 8-well 200 μl-PCR tubes so that the 8-channel 5–100 μL pipette can be used to add the master mix to the plate with samples (recommended).
-
71
Label a 96-well PCR plate with information about the experiment, and place it into a cold block.
-
72
Vortex the master mix, spin it down using a microcentrifuge (see Centrifugation under EQUIPMENT SETUP), and add 18.75 μl of the master mix to each well of the plate with capture reactions. It is recommended to first transfer the master mix to 8-well 200-μl PCR tubes (placed in the remaining 96-well cold block) so that the 8-channel 5–100 μl pipette can be used to add the master mix to the plate.
-
73
Add 5 μl of exonuclease reaction products from each column of the exonuclease reaction plate to the PCR plate using the 8-channel 0.5–10 μl pipette. Mix the exonuclease reaction products with the master mix by pipetting up and down a few times with the tips in the wells.
-
74
Add 1.25 μl of reverse barcode primers (Supplementary Table 1) from each column of the reverse barcode primer plate to the PCR plate using the 8-channel 0.5–10 μl pipette. Mix by pipetting up and down a few times with the tips in the wells.
CRITICAL STEP: Take extreme care not to contaminate the different reverse barcode primer solutions with each other—always use new pipette tips when working with this plate.
-
75
Seal the PCR plate with a PCR plate seal.
-
76
Spin down the PCR plate using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
77
Incubate the sealed PCR plate in a thermocycler. Set the thermocycler to run at 98 °C for 30 s, followed by Y cycles (where Y is the value determined in step 67) at 98 °C for 10 s, 60 °C for 30 s, and 72 °C for 30 s, followed by 72 °C for 2 min and then 4 °C forever, with a heated lid at 105 °C. Do not place the tubes in the thermocycler until the block temperature reaches 98 °C.
PAUSE POINT: PCR reaction products can be stored at 4 °C for multiple days, though we recommend proceeding directly to cleanup (see below).
Cleanup TIMING 30 min
-
78
About 30 min before the reactions from step 77 have completed (the 4-°C stage has been reached in the thermocycler), remove the magnetic beads from the 4-°C refrigerator, and incubate them at room temperature (~21°C).
-
79
After the reactions from step 77 have completed (the 4-°C stage has been reached in the thermocycler), remove the PCR plate from the thermocycler and spin it down using a centrifuge (see Centrifugation under EQUIPMENT SETUP).
-
80
Pool 5 μl of each PCR reaction into a 1.7-ml Eppendorf tube. Pooling can be easily achieved with an 8-channel 0.5–10 μl mechanical pipette to first pool PCR reaction products from each row into an 8-strip of 200-μl PCR tubes and then pooling the contents of each PCR tube into an Eppendorf tube.
-
81
Add 1.8 μl of magnetic beads per microliter of pooled PCR reaction product to the PCR reaction pool. Mix well by pipetting up and down several times.
-
82
Incubate the PCR reaction product pool with added beads at room temperature for 10 min.
-
83
Place the tube containing the PCR reaction product pool and magnetic beads into a magnet tube rack and wait 5 min.
-
84
Prepare 20 ml of fresh 70% (vol/vol) ethanol from 200 proof ethanol and deionized water in a 50-ml Falcon tube.
-
85
Taking care not to disturb the beads, while keeping the Eppendorf tube in the magnet tube rack, pipette out the clear solution and discard into an empty 100-ml beaker.
-
86
Add fresh 70% (vol/vol) ethanol to the tube in the magnet tube rack such that the beads are fully covered (1 ml usually works well), and incubate for 30 s.
-
87
Taking care not to disturb the beads, while keeping the Eppendorf tube in the magnet tube rack, pipette out the 70% (vol/vol) ethanol and discard it into an empty 100-ml beaker.
-
88
Add fresh 70% (vol/vol) ethanol to the tube in the magnet tube rack such that the beads are fully covered (1 ml usually works well), and incubate for 30 s.
-
89
Taking care not to disturb the beads, while keeping the Eppendorf tube in the magnet tube rack, pipette out the 70% (vol/vol) ethanol and discard into an empty 100-ml beaker.
-
90
Allow the beads to air-dry for 5 min.
-
91
Remove the tube with beads from the magnet tube rack and add to it 100 μl of elution buffer, making sure to bring the beads into solution.
-
92
Mix well by pipetting up and down at least 10 times.
-
93
Place the tube with the beads in elution buffer back into the magnet tube rack and wait 1 min.
-
94
Taking care not to disturb the beads, while keeping the Eppendorf tube in the magnet tube rack, pipette out the elution buffer (which now contains cleaned up DNA from the PCR reactions) and transfer it to a new 1.7-mL Eppendorf tube. Label the tube with details regarding its contents. This tube contains the final library for sequencing (to be performed in step 96).
PAUSE POINT: Cleaned, pooled PCR reaction products can be stored at 4 °C for months or at −20 °C indefinitely.
Size confirmation by gel electrophoresis TIMING 15 min to 2 h
-
95
Run 4 μl of the final library for sequencing on a gel using the eGel system or by manually setting up an electrophoresis experiment with an agarose gel (1–2% (wt/vol)). Ensure that only a single major band of size ~288 bp corresponding to the desired MIP PCR products is present.
?TROUBLESHOOTING
Massively parallel sequencing TIMING variable from ~1 day–3 weeks, depending on sequencing platform
-
96
Set up a sequencing run on an Illumina MiSeq or HiSeq according to the manufacturer’s instructions and specifications detailed above (see MIP sequencing under EQUIPMENT SETUP), using the final library prepared in step 94 and the sequencing primers. We recommend measuring concentration using the Qubit dsDNA HS assay (included with the Qubit DNA quantification system). Please note that sequencing will take from ~1 day to multiple weeks to complete, depending on the platform used. Once the sequencing run is complete, store and back up data before beginning the analysis. The first step of the analysis (step 97) does not require the sequencing data and can be completed while waiting for the sequencing run to finish.
Data analysis TIMING ~3 h
-
97
Create a tab-delimited file listing the names of all samples pooled in the sequencing run in the first column and their corresponding reverse barcode primer reverse complement sequences in the second column. Name this file ‘brkpt.barcodekey’ and save it in the project directory.
-
98
After the sequencing run has completed, follow the manufacturer’s instructions regarding bcl conversion to convert raw sequencing base call data to qseq text files. Make a new directory in the project directory called ‘raw_qseq_files’ and store the qseq text files in this new directory. Do not compress these files—running the script in the next step will do that.
-
99Change into the new directory and run the script ‘mip_analysis_pt1.sh’ to generate gzipped fastq files that will be used for mapping. These files will be generated in the ‘raw_qseq_files’ directory and moved to a directory within the parent directory called ‘mrfast_mapping_input’.
$ cd raw_qseq_files $ bash $BRKPT_SOFTWARE/mip_analysis_pt1.sh
-
100
Map reads with mrFAST37 to a custom genome, consisting of chromosomes ‘prox’ and ‘dist’ containing the sequences in ‘prox.fasta’ and ‘dist.fasta’, respectively. Refer to the mrFAST user manual for detailed mapping instructions. Use the parameters ‘--pe --max 160 --min 144 -e 4 --discordant-vh --seqcomp --outcomp --maxoea 500’. Gzipped input fastq files can be found in the directory ‘mrfast_mapping_input’ in the project directory, and mapping output files should be written to the directory ‘mrfast_mapping_output’ directory in the project directory.
-
101Parse the mapping output gzipped sam files to generate a file containing paralog-specific read counts for each individual-MIP combination (‘brkpt.mipcounts’) by running the script ‘mip_analysis_pt2.sh’ from the ‘mrfast_mapping_output’ directory:
$ bash $BRKPT_SOFTWARE/mip_analysis_pt2.sh
-
102Analyze and visualize the data in R. Copy the ‘brkpt.mipcounts’ file, the ‘brkpt.barcodekey’ file, the ‘$BRKPT_SOFTWARE/pdf_brkpt_MIP.r’ file, and the ‘$BRKPT_SOFTWARE/mipplot_brkpt.r’ file to a directory R can access, open R, and set the R working directory to that directory. Then run the following commands in the R console to generate the file ‘brkpt.pdf’, written to that directory:
> base_name<-”brkpt” > source(“pdf_brkpt_MIP.r”)
-
103
Manually inspect each page of the pdf file ‘brkpt.pdf’ corresponding to data for each individual. The breakpoint location can be narrowed to the interval between two MIPs indicating a reciprocal paralog-specific copy number transition (see Fig. 4 and Anticipated results).
TIMING
Targeted array CGH (steps 1–3, optional) 2–3 days
Isolation of the affected chromosome and its parental progenitor(s) (steps 4–8, optional) 2–3 months
Haplotype-specific array CGH (steps 9–10, optional) 2–3 days
Massively parallel WGS and SUNK analysis (steps 11–20, alternative to steps 21–103) variable from 1–3 weeks, depending on sequencing platform
MIP design (steps 21–29, note that steps 21–103 are alternative to steps 11–20) 1 day, plus the time it takes to receive MIP oligonucleotides from the supplier (~1–2 weeks)
MIP pooling and 5′ phosphorylation (steps 20–36) 2–3 h
MIP capture (steps 37–49) ~1 day
Exonuclease treatment (steps 50–57) ~1 h
Quantitative PCR (steps 58–67) ~1 h
PCR (steps 68–77) ~1 h
Cleanup (steps 78–94) 30 min
Size confirmation by gel electrophoresis (step 95) 15 min to 2 h
Massively parallel sequencing (step 96) variable from ~1 day–3 weeks, depending on sequencing platform
Data analysis (steps 97–103), ~3 h
Anticipated results
The end product of WGS or MIP-based analysis should be a copy-number profile corresponding to paralog-specific read-depth over informative SUNs that distinguish the two segmental duplications that were involved in an unequal crossover event (NAHR). WGS analysis will yield plots resembling those in Fig. 3. The plot for an individual where an unequal crossover event has occurred should reveal a transition in paralog-specific copy-number over the spatial extent of aligned breakpoint-associated sequences (e.g. Fig. 3b). The region lacking breakpoint-informative SUNs where the transition in copy number occurs defines the boundary or the breakpoint interval. Perfect sequence identity between breakpoint-associated segmental duplications over this region prohibits the breakpoint interval from being refined any further. We note that paralog-specific read-depths of zero will only be observed in a few specific cases: when individual chromosomal haplotypes have been isolated, or when the proband has a deletion and is heterozygous for the structural haplotype on which the deletion occurred. MIP analysis yields similar results, including a plot showing paralog-specific read count frequencies on the y-axis and the alignment coordinate on the x-axis. Like the WGS analysis, the plots will exhibit two patterns of paralog-specific relative read-depth over the spatial extent of aligned breakpoint-associated sequences, separated by a region lacking breakpoint-informative SUNs that defines the breakpoint interval. This plot will differ slightly from the example in Fig. 4 by showing relative rather than absolute paralog-specific copy number estimates and using alignment coordinates rather than genomic coordinates.
Troubleshooting advice can be found in Table 1.
Table 1.
Troubleshooting table.
| Step | Problem | Possible reason | Solution |
|---|---|---|---|
| 14 | Error message: “Memory allocation failed!” | Insufficient computing memory | Rerun the script on a high-memory machine. Alternatively, use a less memory-intensive, successfully implemented strategy for identifying SUNKs detailed in our study of patients having the 17q21.31 microdeletion syndrome14. |
| 67 | Fluorescence curves for non-water reactions do not all plateau around the same value | DNA concentrations of samples used were not all within a narrow range | Measure the concentrations of samples used. If they are not all within a narrow range (within a few ng/μl), dilute the samples so that they come to be all within a few ng/μl of each other and perform the MIP capture and exonuclease treatment again with these samples. |
| 95 | Smaller band present in addition to band ~288 bp in size | Significant amount of smaller amplicons in PCR reaction products | Perform the cleanup steps again using a smaller ratio than 1.8 (as low as 0.6) for the volume of magnetic beads added per microliter of pooled PCR reaction product. Perform size confirmation by gel electrophoresis to ensure that having followed the modified cleanup protocol effectively removed the smaller band from the final library for sequencing. |
Supplementary Material
Acknowledgments
We thank J. Huddleston for assistance in testing analysis software and preparing it for public access; P. Sudmant for analyzing the number of SUNKs in windows across the reference genome; and T. Brown for assistance with manuscript preparation. X.N. is supported by a National Science Foundation Graduate Research Fellowship under grant no. DGE-1256082. This work was supported by US National Institutes of Health grants HG004120 and HG002385 to E.E.E. E.E.E. is an investigator of the Howard Hughes Medical Institute.
Footnotes
Author Contributions
X.N., A.I., J.S., and E.E.E. developed the protocol. X.N. and E.E.E. wrote the paper, with input and approval from all coauthors.
Competing Financial Interests
E.E.E. is on the scientific advisory boards (SABs) of DNAnexus, Inc. and was an SAB member of Pacific Biosciences, Inc. (2009–2013) and SynapDx Corp. (2011–2013).
References
- 1.Inoue K, Lupski JR. Molecular mechanisms for genomic disorders. Annu Rev Genomics Hum Genet. 2002;3:199–242. doi: 10.1146/annurev.genom.3.032802.120023. [DOI] [PubMed] [Google Scholar]
- 2.Stankiewicz P, Lupski JR. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002;18:74–82. doi: 10.1016/s0168-9525(02)02592-1. [DOI] [PubMed] [Google Scholar]
- 3.Bailey JA, Yavor AM, Massa HF, Trask BJ, Eichler EE. Segmental duplications: organization and impact within the current human genome project assembly. Genome Res. 2001;11:1005–1017. doi: 10.1101/gr.187101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stankiewicz P, Lupski JR. Structural variation in the human genome and its role in disease. Annu Rev Med. 2010;61:437–455. doi: 10.1146/annurev-med-100708-204735. [DOI] [PubMed] [Google Scholar]
- 5.Lupski JR, et al. DNA duplication associated with Charcot-Marie-Tooth disease type 1A. Cell. 1991;66:219–232. doi: 10.1016/0092-8674(91)90613-4. [DOI] [PubMed] [Google Scholar]
- 6.Pinkel D, et al. CGH (CGH) 5,976,790. US Patent. filed 27 Nov. 1995 and issued 2 Nov. 1999.
- 7.Wang NJ, et al. High-resolution molecular characterization of 15q11-q13 rearrangements by array CGH (array CGH) with detection of gene dosage. Am J Hum Genet. 2004;75:267–281. doi: 10.1086/422854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahoo T, et al. Prader-Willi phenotype caused by paternal deficiency for the HBII-85 C/D box small nucleolar RNA cluster. Nat Genet. 2008;40:719–721. doi: 10.1038/ng.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korbel JO, et al. Paired-end mapping reveals extensive structural variation in the human genome. Science. 2007;318:420–426. doi: 10.1126/science.1149504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mills RE, et al. Mapping copy number variation by population-scale genome sequencing. Nature. 2011;470:59–65. doi: 10.1038/nature09708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharp AJ, et al. Segmental duplications and copy number variation in the human genome. Am J Hum Genet. 2005;77:78–88. doi: 10.1086/431652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zody MC, et al. Evolutionary toggling of the MAPT 17q21.31 inversion region. Nat Genet. 2008;40:1076–1083. doi: 10.1038/ng.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Girirajan S, Eichler EE. Phenotypic variability and genetic susceptibility to genomic disorders. Hum Mol Genet. 2010;19:R176–R187. doi: 10.1093/hmg/ddq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Itsara A, et al. Resolving the breakpoints of the 17q21.31 microdeletion syndrome with next-generation sequencing. Am J Hum Genet. 2012;90:599–613. doi: 10.1016/j.ajhg.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Highsmith WE, Meyer KJ, Marley VM, Jenkins RB. Conversion technology for the separation of maternal and paternal copies of any autosomal chromosome in somatic cell hybrids. Curr Prot Hum Genet. 2007;3:3.6. doi: 10.1002/0471142905.hg0306s55. [DOI] [PubMed] [Google Scholar]
- 16.van den Ijssel P, Ylstra B. Oligonucleotide array CGH. In: Bergman NH, editor. Methods in Molecular Biology, vol. 396: Comparative Genomics. Vol. 2. Humana Press; Totowa, NJ, USA: 2007. pp. 207–221. [DOI] [PubMed] [Google Scholar]
- 17.Horvath JE, Schwartz S, Eichler EE. The mosaic structure of human pericentromeric DNA: a strategy for characterizing complex regions of the human genome. Genome Res. 2000;10:839–52. doi: 10.1101/gr.10.6.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saxena R, et al. Four DAZ genes in two clusters found in the AZFc region of the human Y chromosome. Genomics. 2000;67:256–267. doi: 10.1006/geno.2000.6260. [DOI] [PubMed] [Google Scholar]
- 19.Tilford CA, et al. A physical map of the human Y chromosome. Nature. 2001;409:943–945. doi: 10.1038/35057170. [DOI] [PubMed] [Google Scholar]
- 20.Estivill X, et al. Chromosomal regions containing high-density and ambiguously mapped putative single nucleotide polymorphisms (SNPs) correlate with segmental duplications in the human genome. Hum Mol Genet. 2002;11:1987–1995. doi: 10.1093/hmg/11.17.1987. [DOI] [PubMed] [Google Scholar]
- 21.Fredman D, et al. Complex SNP-related sequence variation in segmental genome duplications. Nat Genet. 2004;36:861–866. doi: 10.1038/ng1401. [DOI] [PubMed] [Google Scholar]
- 22.Sudmant PH, et al. Diversity of human copy number variation and multicopy genes. Science. 2010;330:641–646. doi: 10.1126/science.1197005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nuttle X, et al. Rapid and accurate large scale genotyping of duplicated genes and discovery of interlocus gene conversions. Nat Methods. 2013;10:903–909. doi: 10.1038/nmeth.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dennis MY, et al. Evolution of human-specific neural SRGAP2 genes by incomplete segmental duplication. Cell. 2012;149:912–922. doi: 10.1016/j.cell.2012.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bovee D, et al. Closing gaps in the human genome with fosmid resources generated from multiple individuals. Nat Genet. 2008;40:96–101. doi: 10.1038/ng.2007.34. [DOI] [PubMed] [Google Scholar]
- 26.Genovese G, et al. Using population admixture to help complete maps of the human genome. Nat Genet. 2013;45:406–414. doi: 10.1038/ng.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carlson C, et al. Molecular definition of 22q11 deletions in 151 velo-cardio-facial syndrome patients. Am J Hum Genet. 1997;61:620–629. doi: 10.1086/515508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitzman JO, et al. Haplotype-resolved genome sequencing of a Gujarati Indian individual. Nat Biotech. 2011;29:59–63. doi: 10.1038/nbt.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peters BA, et al. Accurate whole-genome sequencing and haplotyping from 10 to 20 human cells. Nature. 2012;487:190–195. doi: 10.1038/nature11236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Porreca GJ, et al. Multiplex amplification of large sets of human exons. Nat Methods. 2007;4:931–936. doi: 10.1038/nmeth1110. [DOI] [PubMed] [Google Scholar]
- 31.Turner EH, et al. Massively parallel exon capture and library-free resequencing across 16 genomes. Nat Methods. 2009;6:315–316. doi: 10.1038/nmeth.f.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Roak BJ, et al. Multiplex targeted sequencing identifies recurrently mutated genes in autism spectrum disorders. Science. 2012;338:1619–1622. doi: 10.1126/science.1227764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tuzun E, Bailey JA, Eichler EE. Recent segmental duplications in the working draft assembly of the brown Norway rat. Genome Res. 2004;14:493–506. doi: 10.1101/gr.1907504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kidd JM, et al. Characterization of missing human genome sequences and copy number polymorphic insertions. Nat Methods. 2010;7:365–371. doi: 10.1038/nmeth.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chin CS, et al. Nonhybrid, finished microbial genome assemblies from long-read SMRT sequencing data. Nat Methods. 2013;10:563–569. doi: 10.1038/nmeth.2474. [DOI] [PubMed] [Google Scholar]
- 36.Renshaw MA, Giresi M, Adams JO. Microsatellite fragment analysis using the ABI PRISM ® 377 DNA sequencer. In: Kantartzi SK, editor. Methods in Molecular Biology, vol. 1006: Microsatellites. Humana Press; Totowa, NJ, USA: 2013. pp. 181–196. [DOI] [PubMed] [Google Scholar]
- 37.Alkan C, et al. Personalized copy number and segmental duplication maps using next-generation sequencing. Nat Genet. 2009;41:1061–1067. doi: 10.1038/ng.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broman KW, Murray JC, Sheffield VC, White RL, Weber JL. Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet. 1998;63:861–869. doi: 10.1086/302011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.