Abstract
Glycosylation engineering is used to generate glycoproteins, glycolipids or proteoglycans with a more defined complement of glycans on their glycoconjugates. For example, a mammalian cell glycosylation mutant lacking a specific glycosyltransferase generates glycoproteins, and/or glycolipids, and/or proteoglycans, with truncated glycans missing the sugar transferred by that glycosyltransferase, and also missing those sugars that would be added subsequently. In some cases, an alternative glycosyltransferase may then use the truncated glycans as acceptors, thereby generating a new or different glycan subset in the mutant cell. Another type of glycosylation mutant arises from gain-of-function mutations that, for example, activate a silent glycosyltransferase gene. In this case, glycoconjugates will have glycans with additional sugar(s) that are more elaborate than the glycans of wild type cells. Mutations in other genes that affect glycosylation, such as nucleotide sugar synthases or transporters, will alter the glycan complement in more general ways that usually affect several types of glycoconjugates. There are now many strategies for generating a precise mutation in a glycosylation gene in a mammalian cell. Large-volume cultures of mammalian cells may also give rise to spontaneous mutants in glycosylation pathways. This article will focus on how to rapidly characterize mammalian cells with an altered glycosylation activity. The key reagents for the protocols described are plant lectins that bind mammalian glycans with varying avidities, depending on the specific structure of those glycans. Cells with altered glycosylation generally become resistant or hypersensitive to lectin toxicity, and have reduced or increased lectin or antibody binding. Here we describe rapid assays to compare the cytotoxicity of lectins in a lectin resistance test, and the binding of lectins or antibodies by flow cytometry in a glycan-binding assay. Based on these tests, glycosylation changes expressed by a cell can be revealed, and glycosylation mutants classified into phenotypic groups that may reflect a loss-of-function or gain-of-function mutation in a specific gene involved in glycan synthesis.
Keywords: lectin toxicity, glycosylation mutants, mammalian cells, engineer glycans, glycan binding, lectins, antibodies, CHO cells
INTRODUCTION
The ability to manipulate the glycan complement of mammalian glycoproteins has revolutionized basic research into the biological functions of individual sugars and classes of glycan, and allowed the production of tailored glycoproteins with desirable properties. The general name for this field is glycosylation engineering (Jefferis, 2013; Stanley, 1992). Thus, many recombinant glycoproteins for therapeutic use require an optimal glycan content to be most efficacious. For example, erythropoietin (EPO) has a long half-life in the circulation if it is fully sialylated on its three N-glycans and single O-glycan (Debeljak and Sytkowski, 2012; Fukuda et al., 1989; Zhang et al., 2010); antibodies designed to kill immune or cancer cells by antigen-dependent cellular cytotoxicity (ADCC), are markedly improved if the N-glycan in their Fc region lacks fucose (Kanda et al., 2006; Natsume et al., 2005; Ogorek et al., 2012; Shinkawa et al., 2003); and lysosomal enzymes used for enzyme replacement therapy are most effective if their N-glycans terminate in mannose, which causes them to be targeted to mannose receptors on reticuloendothelial cells (Tekoah et al., 2013). Each requirement can be achieved by producing the recombinant therapeutic in a mammalian cell engineered for optimal glycosylation (Zhang et al., 2013). For EPO, cells that synthesize highly branched, completely sialylated glycans can be facilitated by the introduction of glycosyltransferase transgenes or the introduction of additional N-glycan glycosylation sites (Su et al., 2010); for antibodies lacking fucose, inactivating mutations in one or more fucosyltransferase genes, or genes encoding the enzymes or transporters necessary for the synthesis or transport of GDP-fucose into the Golgi compartment, can be engineered (Lux and Nimmerjahn, 2011); and to obtain N-glycans that terminate solely in mannose, the Mgat1 glycosyltransferase gene responsible for the synthesis of complex N-glycans, may be inactivated or silenced (Grabowski et al., 1995). Alternatively, other organisms such as yeast or plants that produce the desired glycosylation of a therapeutic may be used (Shaaltiel et al., 2007). Glycosylation mutants have also been widely used to address roles for glycans in selectin recognition (Phillips et al., 1990), pathogen binding (Ravdin et al., 1989), growth factor signaling (Song et al., 2010), and Notch signaling (Chen et al., 2001a; Hou et al., 2012; Stahl et al., 2008).
Engineering glycosylation genes in mammalian cells began with the isolation of lectin-resistant cell mutants (reviewed in (Stanley, 1983; Stanley, 1984)). While selection for survivors of lectin toxicity or screening for altered glycans is quite simple, characterization of the biochemical and genetic bases of different mutants is extremely time consuming (Esko and Stanley, 2009). Nevertheless, there is an extensive panel of well-characterized mutants of CHO and other mammalian cells for which the altered glycosylation activity and the structural consequences for glycan synthesis are known, and in which the glycosylation gene mutation has been identified (Patnaik and Stanley, 2006) (Table 1). To isolate new mutations, general selection methods or screens are no longer efficient. Rather, modern technologies such as zinc finger nucleases, TALENs or the Crisper/CAS system of genome engineering to delete or mutate a specific glycosylation gene, should be employed (Steentoft et al., 2011; Yang et al., 2013). While transgenes can be used to knockdown or overexpress a gene, mammalian cells tend to silence transgenes in a random manner, and in addition, transgene expression level is highly variable. In order to facilitate stable expression and uniform transcription of a transgene, it is desirable to engineer a specific genomic DNA locus in the host cell for the introduction of transgenes (Turan et al., 2013). Regardless of which approach is taken to generate a glycosylation mutant, or if a mutant arises spontaneously during population expansion in a bioreactor, the protocols described here will aid in rapidly identifying and classifying a cloned mutant isolate that differs in glycosylation from the parental population. For example, the Chinese hamster ovary (CHO) double mutant Lec15.Lec1 was easily generated from Lec15 CHO cells by selecting for resistance to the leuko-agglutinin from Phaseolus vulgaris (L-PHA) and determining the lectin-resistance phenotype of surviving colonies (Aguilan et al., 2009), as described below.
Table 1.
Lectin resistance phenotype of commonly used CHO glycosylation mutants.
| Cell Line | Biochemical Change | Gene Mutated | Lectin Resistance Relative to Parent CHO | Key ref. | ||||
|---|---|---|---|---|---|---|---|---|
| L-PHA | WGA | ConA | RIC | LCA | ||||
| Lec1 | No complex N-glycans | Mgat1 | R>1000 | R30 | S5 | R100 | R>200 | (Chen and Stanley, 2003) |
| Lec2 | Little NeuAc on all GCs | Slc35a1 | S<2 | R11 | -- | S100 | S2 | (Eckhardt et al., 1998) |
| Lec4 | No β1,6 GlcNAc branch on N-glycans | Mgat5 | R>1000 | R<2 | S<2 | S<2 | S<2 | (Weinstein et al., 1996) |
| Lec8 | Little Gal & NeuAc on GCs | Slc35a2 | R10 | R100 | S<2 | R<2 | S10 | (Oelmann et al., 2001) |
| LEC10 | β(1,4)GlcNAc added to N-glycan core | Active Mgat3 | S2 | S<2 | -- | R20 | -- | (Campbell and Stanley, 1984) |
| LEC11 | α(1,3)Fuc added to form LeX, SLeX | Active Fut6 | R4 | R8 | -- | S25 | R3 | (Zhang et al., 1999) |
| LEC12 | Add α(1,3)Fuc to form LeX | Active Fut9 | R3 | R50 | -- | S4 | R2 | (Patnaik et al., 2000) |
| Lec13 | Little Fuc on all GCs | Gmds | -- | -- | -- | -- | R27 | (Ripka et al., 1986) |
D10 values for each CHO mutant are shown compared to the D10 for parental Pro-5 CHO cells. R, fold-resistant; S, fold-sensitive; --, unchanged. GC, glycoconjugate.
Basic Protocol 1 describes a test for resistance to the toxicity of different plant lectins that are commercially available. Each lectin kills 90% CHO cells at a concentration less than 20 μg/ml and must bind to cell surface glycans in order to enter the cell and exert its toxicity. Resistant cells may arise from altered glycosylation that adds, removes or alters the glycan binding site(s) of one or more lectins. If selection for resistance to one lectin generates resistance or hypersensitivity to one or more distinct lectins concomitantly, it is an excellent indication that a glycosylation mutation underlies the lectin-resistance phenotype. Lack of cross-resistance or –hypersensitivity to one or more lectins not used in selection may indicate that a mutant is resistant to some aspect of lectin toxicity that occurs subsequent to lectin binding. The lectin-resistance assay described in Basic Protocol 1 determines the concentration of each lectin that kills 90% of cells growing on a 96-well tissue culture dish, by staining surviving cells with Methylene Blue. For cells that do not detach from the plate when they die, Alternative Protocol 1 describes a viability assay that determines the relative proportion of viable cells in a well based on their ability to metabolize a dye.
Basic Protocol 2 directly determines the binding of a lectin or anti-glycan antibody to cells using fluorescence-assisted flow cytometry. Lectins or antibodies that are conjugated to fluorphores or biotinylated and detected by streptavidin are incubated with fresh or fixed cells, unbound lectin or antibody is removed by washing, and flow cytometry is performed. Bound antibodies are detected by anti-Ig secondary antibodies conjugated to a fluorphore or biotin. While the flow cytometry assay has the advantage of measuring lectin-glycan binding directly, it requires the use of a flow cytometer, is more time-consuming to set up a comparison of numerous lectin concentrations, and is less sensitive than the lectin toxicity assay. On the other hand, if few lectins or antibodies need to be compared at a single concentration, flow cytometry is quantitative and graphic.
STRATEGIC PLANNING
Choice of Lectins
Plant lectins are excellent reagents for rapidly surveying the glycosylation state of a cell. Plant lectins bind to a specific subset of glycans on cell surface glycoconjugates (GCs) with different avidity depending on their concentration. Subsequent to binding, several plant lectins are toxic to mammalian cells. For some, including ricin, abrin and modeccin, the mechanism of toxicity is known (Olsnes, 2004; Walsh et al., 2013). Although the mechanism of cell killing is not known for most lectins, all must bind to glycans on the cell surface prior to entering a cell and, thus may be used to reflect changes in cell surface glycan complement. The affinity, avidity, degree of binding, and therefore toxicity, of a lectin will depend on the glycan complement of the cell. Mammalian lectins such as galectins are not generally cytotoxic but may be used in glycan binding assays (Patnaik et al., 2006). Antibodies that recognize specific glycan epitopes are also useful in binding assays (Patnaik et al., 2004; Zhang et al., 1999). For rapid characterization of a glycosylation change, both the lectin resistance test and glycan-binding assay, are easy and fast. The protocols below focus on CHO glycosylation mutants in comparison to parental CHO cell lines. CHO cells represent the most widely used cell line in the biotechnology industry, and CHO cell lines, as well as a female Chinese hamster genome, have been sequenced (Hammond et al., 2012; Lewis et al., 2013). Other cell lines almost certainly vary in the nature of their binding of lectins and antibodies. However, the general strategies and approaches developed for the characterization of CHO glycosylation mutants may be empirically applied.
BASIC PROTOCOL 1: LECTIN RESISTANCE TEST
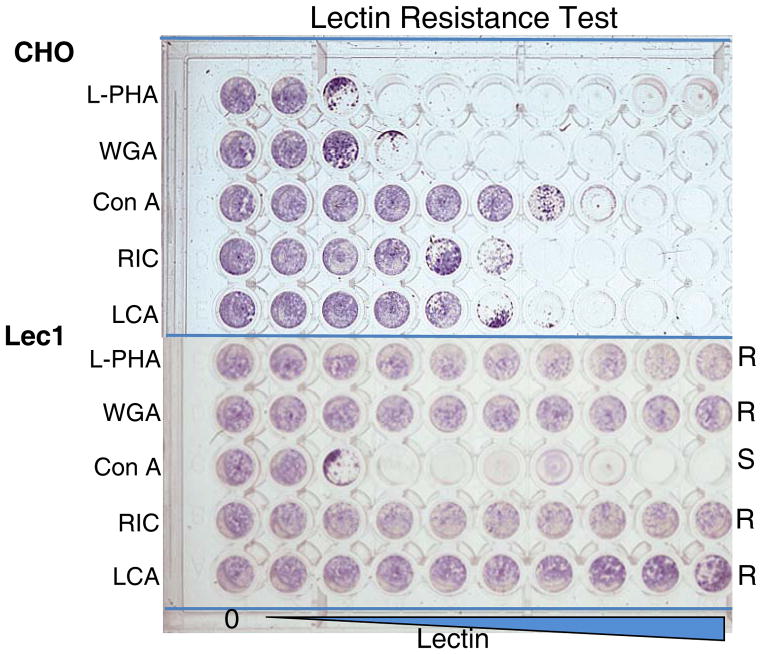
The lectin resistance test is a rapid method for determining if cell lines differ in the complement of glycans they express at the cell surface. Differences are determined by culturing cells in the presence of a range of concentrations of several different plant lectins that are cytotoxic at relatively low concentrations. In order to exert a cytotoxic effect, the lectin must bind to glycans at the cell surface, and thus resistance to lectin toxicity is a measure of an altered complement of cell surface glycans. Since the synthesis of N-glycans involves many glycosylation genes (Fig. 1), lectins that bind to N-glycans are most helpful for an initial screen of lectin toxicity. Determining the relative resistance of different cell isolates to a panel of plant lectins allows cell lines to be classified. Many of the mutations that affect N-glycan synthesis may also affect O-GalNAc glycan (mucin), glycosphingolipid (GSL) and/or glycosaminoglycan (GAG) synthesis. Thus, the lectin resistance test is a method to quickly determine whether glycosylation pathways differ between cell lines. Five lectins that are toxic to CHO cells are useful for a quick survey. They are: the leuko-agglutinin from Phaseolus vulgaris (L-PHA); wheat germ agglutinin (WGA); concanavalin A (Con A); the toxin from Ricinus communis (RIC); and the agglutinin from Lens culinaris (LCA). Companies that specialize in lectin purification such as Vector Labs (Burlingame, CA) or EY Labs (San Mateo, CA) are recommended sources. They also describe in some detail the nature of the sugars and glycans recognized by each lectin. A brief summary of lectin specificities is given in Table 2 and the results of a lectin resistance test are shown in Fig. 2.
Figure 1. Glycans expressed by CHO Cells and altered in the Lec1 CHO mutant.
The CHO cells from which the glycosylation mutants described in Table 1 were derived express the major classes of glycans depicted in the figure (North et al., 2010) using a broadly accepted symbol nomenclature (Varki et al., 2009). Minor glycans found on particular glycoproteins at very low levels such that they were not readily detected by mass spectrometry, include O-fucose, O-glucose, O-xylose and O-GlcNAc glycans at particular consensus sites on EGF repeats in extracellular domains of certain membrane proteins such as Notch receptors (Rana and Haltiwanger, 2011), and O-mannose glycans on alpha-dystroglycan and a few other proteins (Aguilan et al., 2009). The synthesis of N-glycans requires the participation of many glycosyltransferases, nucleotide sugar synthases and transporters, as well as factors that regulate the integrity of the secretory pathway. Thus, activating or inactivating mutations in a large number of genes that alter glycosylation will be reflected in altered N-glycan synthesis and structure. The lectins used in the assays described here all bind to N-glycans. Lectin-resistant Lec1 CHO mutants lack the glycosyltransferase MGAT1 and are blocked in N-glycan synthesis so that the substrate of MGAT1 replaces all complex N-glycans (dashed line). Complex N-glycans are not synthesized in Lec1 CHO cells but no other glycans are altered.
Table 2.
CHO Glycans Bound by Plant Lectins and Simple Inhibitors of Binding
| Lectin | Major CHO Cell Surface Glycans Recognized | Simple Inhibitors |
|---|---|---|
| L-PHA | Gal in branched N-glycans, particularly in poylactosamine on the α(1,6)Manβ(1,6) GlcNAc branch | Gal, Galβ(1,4)GlcNAc |
| WGA | Terminal sialic acid or GlcNAc in complex N-glycans; GSLs with sialic acid, O-GalNAc glycans with sialic acid | GlcNAc, GlcNAc oligomers |
| Con A | Mannose on high mannose N-glycans and in the core of biantennary complex N-glycans | Mannose, glucose |
| RIC | Terminal Gal or Gal substituted with α(2,3) sialic acid on complex N-glycans, O-GalNAc glycans, GSLs. Not Gal substituted with α(2,6) sialic acid. | Gal, Galβ(1,4)GlcNAc |
| LCA | Biantennary and β(1,6) GlcNAc-triantennary N-glycans with a core fucose, mannose | Mannose, glucose |
Figure 2. Lectin resistance test.
Resistance to a panel of increasing concentrations of plant lectins for CHO cells (Pro-5) versus Lec1 mutant cells derived from Pro-5. Lec1 cells lack MGAT1 activity. When the first well of each row was confluent, all wells were scored by inverted light microscopy for their degree of confluence, and the cells were then stained using Methylene Blue. Lec1 cells are more resistant than CHO cells to L-PHA, WGA, RIC and LCA, but more sensitive than CHO cells to Con A.
Materials
Lectins from Vector Labs (Burlingame, CA) or EY Labs (San Mateo, CA).
Tissue culture medium (e. g. Dulbecco’s modified Eagle’s medium, DMEM) containing 10% fetal calf serum (FCS)
Cells growing exponentially, preferably in suspension culture that can also grow in monolayer culture. If cells grow only in monolayer, use enzyme-free cell dissociation buffer (Millipore) to make a suspension for cell counting. Trypsinization releases glycoproteins from the membrane and may alter the lectin-resistance phenotype. If cells grow in suspension and cannot grow in monolayer, use Alternate Protocol 1 or Basic Protocol 2.
Clear-capped, sterile plastic tissue culture tubes (5 ml) to make lectin dilutions
96-well flat-bottom tissue culture dish with lid
Methylene Blue (50 g per100 ml in 50% methanol in distilled water)
Chlorine bleach
Two 2L plastic beakers
Low speed bench centrifuge
Repeater Plus Pipettor with Combitip (Eppendorf)
Particle cell counter (Coulter) or hemocytometer
Tissue culture incubator humidified, atmosphere 5% CO2
Tissue Culture Hood
Inverted phase light microscope
Protocol Steps
Preparation of 96-well plate with lectins
-
1
Label the lid of the 96-well plate with the lectin and concentrations for each row of 12 wells.
The lid should be of the type that can be placed correctly in only one orientation. If only the lid is labeled, it is important to always keep it associated with its 96-well plate base. -
2
Under sterile conditions, make a stock of the highest concentration of lectin to be used in tissue culture medium containing 10% FCS. A volume of 0.1 ml should contain twice (2X) the final concentration of lectin needed. Make sufficient stock so that serial dilutions can be made to cover all lectin concentrations.
The first well should contain medium alone and is the control for zero killing; the next wells should contain lectin of increasing concentration. Care should be taken to prepare the highest concentration of lectin very precisely. The lectin titration should be designed so that ~90% of the cells are killed at approximately the titration mid-point. This endpoint is referred to as the D10 value, i. e. the concentration of lectin at which only 10% cells survive. The subsequent 5–7 wells should be of increasing concentration so that the degree of resistance of a test line is clear. In this manner, cells that are more sensitive to lectin killing than the parent have a D10 value lower than the parent, and those that are resistant have a D10 value higher than the parent. -
3Prepare the following range of concentrations for the lectins shown as the final concentration in a final volume of 0.2 ml. This range is based on knowledge of the concentration of each lectin that kills 90% wild type CHO cells, defined as the D10 value and shown bolded. If a glycosylation change causes a mutant cell to be resistant to a lectin compared to CHO cells, the D10 will occur at a higher lectin concentration. Conversely, if a glycosylation change causes a mutant to more sensitive to a lectin, the D10 will occur before that of CHO cells.
Lectin Final Lectin Concentration L-PHA (μg/ml) 0 10 15 20 22.5 25 30 35 40 60 75 100 WGA (μg/ml) 0 1 2 3 4 5 7.5 10 15 20 25 40 Con A (μg/ml) 0 1 3 5 7.5 10 15 20 25 30 40 50 RIC (ng/ml) 0 0.2 0.4 0.6 0.8 1 1.25 1.5 2.5 5 7.5 10 LCA (μg/ml) 0 5 7.5 10 15 20 25 30 40 50 75 100 The bolded concentrations are those at which ~10% wild-type CHO cells survive. This is defined as the D10 value. Note that RIC is ~1000-fold more toxic than the other lectins.Ricin is a very toxic lectin and should be handled with utmost care. The concentration that kills mammalian cells is in the ng/ml range. It is important to use a repeat pipetting device and blunt tips rather than conventional tips that can puncture the skin.Note that the lectin concentration given is the final concentration. Since the final volume per well is 0.2 ml, each lectin added to a well in 0.1 ml must be at twice the concentration shown in the table. A repeating pipetting device works well for aliquoting lectins. The ranges of lectin concentrations shown are for CHO cells derived in Toronto, Canada (Pro-5 or Gat-2; see Stanley et al., 1975), with the bolded concentration denoting the D10 value for these parental CHO cells. Cells with altered glycans should be either more sensitive (lower D10 value) or more resistant (higher D10 value) than parental cells. The commonly used CHO-K1 cell line may have a different lectin resistance profile that should be empirically determined. Other cell lines such as MDBK or HEK-293T are sensitive to ricin, but may not be killed by all lectins that are toxic to CHO cells. -
4
Return the lid to the 96-well dish and place it in a tissue culture incubator at 37ºC in an atmosphere of 5% CO2 for at least 30 min or up to several hr.
Preparation of cells and addition to 96-well plate
-
5
For cells growing in suspension culture, count an aliquot and determine the number of cells per ml. For cells growing in monolayer, make a single cell suspension using commercially available enzyme-free cell dissociation buffer (Millipore), count and determine cells/ml.
Trypsinization should not be used as it may cleave membrane glycoproteins and reduce the complement of glycans to bind lectins. CHO cells derived from parental lines isolated in Toronto grow in suspension or monolayer culture in the same medium interchangeably (Stanley et al., 1975). CHO-K1 cells need to be adapted to suspension culture. Other cell types such as HeLa cells may need different cell culture medium for growth in suspension versus monolayer culture. -
6
Dilute to 2 X 104 cells per ml in medium containing 10% FCS.
-
7
Remove 96-well plate containing lectins from the incubator.
-
8
Add 0.1 ml cells per well that already contains 0.1 ml lectin, to give 0.2 ml final volume.
-
9
Replace lid on 96-well plate and return to tissue culture incubator.
Determining D10 values
-
10
After 3 days observe each well of the 96-well plate by light microscopy using an inverted microscope.
It is important that the cells in the first well do not go beyond confluence since all other wells are judged in relation to the degree of confluence of the first well. Arrange cell lines so that all control wells with no lectin become confluent at the same time. This may mean using a different plate for each cell line if cells have different growth rates. Alternatively, different numbers of cells may be added at the beginning so all lines will become confluent on the same day. -
11
If cells in the first well, that contains no lectin, have formed a confluent monolayer, examine each well by light microscopy, record the confluence of each well and make a note of the well in which only ~10% cells remain adherent to the plate. If the first well is not confluent, return the plate to the incubator until confluence occurs in the first well that has no lectin.
-
12
Stain cells with Methylene Blue as follows. Tip the medium from the 96-well plate into a 2 L beaker containing 500 ml ~50% chlorine bleach in tap water.
-
13
Tap the 96-well plate to remove all liquid.
-
14
Add 0.2 ml Methylene Blue solution to fill each well.
-
15
After 30 min at room temperature, decant the Methylene Blue into a funnel on top of a 500 ml glass bottle through 3MM Whatman paper. Screw bottle cap on tightly and store at room temperature.
-
16
Immerse the 96-well plate gently into 1.5 L warm tap water in a 2 L plastic beaker to rinse away remaining dye.
If the first well was overconfluent, cells may be easily dislodged from the surface at this stage. Washing away dye by the immersion method is very gentle and allows loosely adherent cells to remain attached. -
17
Invert at room temperature with labeled lid resting on top, secured with a rubber band, until dry.
-
18
Using an inverted microscope and a 10X lens, determine the D10 value as the concentration of lectin in the well that has only ~10% blue-stained cells compared to the control well that contains no lectin and is 100% confluent.
ALTERNATE PROTOCOL 1: LECTIN SURVIVAL ASSAY BASED ON VIABILITY DETERMINED USING MTT
For cells that do not detach from a well when they die, Methylene Blue cannot be used since it stains cells whether they are dead or alive. Therefore, dead cells that remain attached will be stained and there will be few differences between wells. In this case, the MTT assay for cell viability can be used to determine the concentration of lectin which kills ~90% of the cells. The titration of lectins in a 96-well tissue culture plate is performed as described above. When the first well containing no lectin is confluent, as determined by light microscopy using an inverted microscope, it is time to perform the MTT viability assay. This assay detects NAD(P)H-dependent oxidoreductase activity which is a reflection of viable cells present. Oxidoreductases reduce MTT, the tetrazolium dye 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), converting the yellow MTT solution to formazan, a deep purple precipitate. This may be viewed by the naked eye for a qualitative result, or the precipitate may be solubilized in dimethylsulfoxide (DMSO) and the absorbance read at 500–600 nm, compared to solvent alone, in an ELISA plate reader or a spectrophotometer. A standard curve is unnecessary since the relative viability of cells exposed to different concentrations of lectin versus a control well with no lectin gives the result.
Materials
MTT (many commercial sources)
Phosphate buffered saline (PBS) containing 1 mM CaCl2 and 1 mM MgCl2, pH 7.2 (PBS/CaMg) DMSO
Paper towel
ELISA plate reader
Protocol steps
Add 10 μl MTT reagent to each well containing 0.2 ml cell culture medium with 10% FCS and a cell monolayer.
Incubate 3 hr at 37ºC
Invert the plate over a beaker containing water with ~ 50% chlorine bleach.
Tap dry on paper towel to blot liquid from the top of each well.
Fill each well with 200 μl PBS/CaMg
Tip into beaker and blot away excess liquid on paper towel.
MTT forms a purple precipitate that can be solubilized in DMSO and quantitated.
Add 200 μl DMSO to each well.
Pipet up and down twice to mix well.
Read OD at 540 nm and 650 nm in an ELISA plate reader
Subtract the reading at 650 nm (background) from that at 540 nm.
Graph lectin concentration versus OD540 – OD650 to determine lectin concentration at 10% viability relative to 100% viability of cells in the well with no lectin.
BASIC PROTOCOL 2: LECTIN OR ANTIBODY BINDING ASSAY USING FLOW CYTOMETRY
The binding of lectins or antibodies to cells is most readily determined by flow cytometry. Binding assays using a flow cytometer, while comparatively high throughput, are not as sensitive as cytotoxicity assays. Binding profiles are plotted on a log scale and thus lectin concentrations cannot be titrated very tightly. Generally, binding is determined using a saturating concentration of ligand or antibody, and this may also mask subtle differences that are revealed by a tight series of dilutions in the Lectin Resistance Assay. Nevertheless, lectin and antibody binding assays can be very informative, and will readily detect major glycan binding changes (Fig. 3). They also have the advantage that the specificity of binding may be determined by including a simple sugar, glycan, peptide antigen or non-fluorescent lectin as a competitive inhibitor during the binding incubation. In addition, several lectins or antibodies that do not compete with each other may be compared simultaneously using different fluorescent conjugates. Antibodies may also be conjugated to a fluorescent probe or detected with unlabeled primary antibody, by a fluorescently labeled secondary antibody.
Figure 3. Lectin and antibody binding test.
Comparison of lectin and antibody binding to CHO cells and LEC11 CHO cells with an activated Fut6 gene that encodes α(1,3)fucosyltransferase VI (Zhang et al., 1999). The lectins are Pisum sativum (PSA) that recognizes the Fuc in the complex N-glycan core GlcNAc and Aleuria aurentia lectin (AAL) that binds to any terminal fucose residue. The antibodies are anti-SSEA-1, which binds to the glycan epitope shown, also the Lewis X (LeX) blood group, and anti-CSLEX-1, which binds to sialylated LeX (SLeX). This research was originally published in The Journal of Biological Chemistry. North, S. J., Huang, H-H., Sundaram, S., Jang-Lee, J., Etienne, A. T., Trollope, A., Al-Chalabi, S., Dell, A., Stanley P. and Haslam, S. M. (2010) Glycomics profiling of Chinese hamster ovary (CHO) cell glycosylation mutants reveals N-glycans of a novel size and complexity. J. Biol. Chem., 285, 5759–5775 © the American Society for Biochemistry and Molecular Biology. Permission from The Journal of Biological Chemistry is automatically given and referred to as they request. See Fig. 3 legend.
Materials
Lectins conjugated to a fluorescent probe such as fluorescein or phycoerythrin from Vector Labs (Burlingame, CA) or EY Labs (San Mateo, CA).
Antibodies with or without a conjugated probe (commercially available)
Binding buffer (BB) based on Hank’s balanced salts solution (HBSS) pH 7.2, containing 2% (weight/volume) bovine serum albumin (Fraction V) and 0.1% sodium azide.
7-actinomycin D (7-AAD; 0.5 μg/ml in PBS/CaMg
Cells in suspension
Cell counter (Coulter) or hemocytometer
Low speed clinical centrifuge
Conical centrifuge tubes (15 or 50 ml)
Eppendorf tubes (1.5 ml)
Rotator for Eppendorf tubes
Flow cytometer
Software to analyze the flow cytometry data (e. g. FlowJo, Tree Star Inc.)
Protocol steps
Preparing the cells
-
1
Generate a cell suspension either from cells already growing in suspension culture or following treatment of a cell monolayer with enzyme-free cell dissociation buffer (Millipore)
-
2
Count and determine the number of cells per ml.
-
3
Transfer to a 15 or 50 ml conical centrifuge tube (Corning)
-
4
Centrifuge at least twice as many cells as will finally be needed, at 1200 rpm for 10 min at room temperature.
Final cell number needed is 107 cells/ml. The number of ml will depend on the number of lectins or antibodies to be tested. For each reaction, 50 μl cells containing 5 X 105 cells is used. -
5
Remove the supernatant by vacuum aspiration
-
6
Resuspend cells in 10 ml cold BB and centrifuge as above (first wash)
-
7
Remove the supernatant by vacuum aspiration
-
8
Resuspend in 10 ml cold BB (second wash)
-
9
Count and determine cells/ml
-
10
Transfer a volume containing the desired number of cells for the entire assay to a 15 ml centrifuge tube
The desired number of cells is calculated based on the number of binding reactions to be performed with different lectins or antibodies. Each binding reaction uses 50 μl cells containing 5 X 105 cells. The volume of cell suspension needed to give a final concentration of 107 cells/ml is the volume that should be centrifuged at this step. -
11
Centrifuge at 1200 rpm for 10 min at room temperature
-
12
Aspirate the supernatant
-
13
Resuspend the cells in cold BB to give 107 cells/ml and place tube in ice
Binding assay
-
14
To 1.5 ml Eppendorf tubes on ice, add 150 μl cold BB
-
15
Add 50 μl lectin or antibody conjugated to a fluorescent probe and dissolved in BB, to give a final concentration of 5–20 μg/ml (lectin) or 1–5 μg/ml (antibody) in 250 μl final volume.
For a new mutant with a suspected glycosylation defect, use both the low and high concentration of lectin or antibody to determine which gives you the most difference between mutant and control cells. Titrate lectin or antibody to determine the lowest concentration that will give maximal binding to wild type cells. Alternatively, if a test cell line is suspected of having a greater binding ability than wild type, determine the lowest concentration of lectin at which binding to wild type may be detected. The same strategy is applied to primary antibody dilutions. -
16
To control for background binding, add 50 μl BB instead of lectin in a separate tube
Simple sugars or oligosaccharides may also be used to inhibit binding to obtain insight into lectin binding specificity (Table 2). However, since complex glycans on an array of glycoconjugates are present on the cell surface, simple sugars may not be effective inhibitors. Sugars should be used at a concentration of 5–10 mM. If the antibody to be tested is not available as an antibody conjugated to a fluorescent probe, an unlabeled antibody may be used and detected using a secondary antibody that is conjugated to a probe. Such secondary antibodies are widely available commercially. In this case, the background control is secondary antibody alone to detect background binding. -
17
Add 50 μl cells containing 5 X 105 cells to each tube
-
18
Mix and incubate on ice (or on a rotator) at 4ºC for 30 – 60 min
-
19
Mix and add 1 ml cold BB
-
20
Centrifuge in a microfuge at 1500 rpm for 5 min at RT
-
21
Aspirate or tip off supernatant
-
22
Resuspend in 1.5 ml BB and centrifuge again as in step 17 (wash)
-
23
Aspirate or tip off supernatant
-
24
Resuspend in 0.5 ml BB
-
25
Add 3 μl 7-AAD (0.5 μg/ml)
7-actinomycin D (7-AAD) enters dying cells that have a compromised membrane and fluoresces when it binds to DNA. Dying cells will take up lectin or antibody non-specifically and thus need to be removed from the analysis of live cells that do not bind 7-AAD. This is achieved during flow cytometry by gating out cells containing 7-AAD. -
26
Perform flow cytometry
Depending on the flow cytometer available, several different probes that fluoresce in different channels at different wavelengths may be used simultaneously. Thus, binding of several lectins and/or antibodies may be determined in one tube. In this case, appropriate controls must be performed to calibrate the flow cytometer for optimal detection of the separate probes. Only reagents that do not interact with each other, or inhibit binding of each other to the cell surface, may be used together. As this is often unknown, mixing of lectins and antibodies that detect cell surface glycans is seldom performed. -
27
Analyze data, gating out dead cells that stained with 7-AAD.
REAGENTS AND SOLUTIONS
Use double-distilled water or MilliQ filtered water for all solutions.
Lectin solutions
For the lectin resistance test, make a solution of each lectin at 1–2 mg/ml as described below.
All the following lectins are very stable and remain active in solution for months or years at 4ºC.
L-PHA
Add 2.5 ml of sterile distilled water to a vial containing 5 mg of L-PHA. Mix well to dissolve. Filter through a 0.22 micron syringe filter into a sterile plastic tube. Take 25 μl of this solution and add to 975 μl of phosphate buffered saline (PBS), pH 7.2. Mix well and measure the OD280. The extinction coefficient of L-PHA is 11.6, the reading for a solution of 10 mg/ml. Adjust volume to 1 mg/ml in sterile distilled water. Store at 4ºC for months to years.
WGA
Add 4 ml sterile distilled water to a vial of 10 mg of WGA and mix well to dissolve. Filter through a 0.22 micron syringe filter into a sterile plastic tube. Take 25 μl of this solution and add to 975 μl of phosphate buffered saline (PBS), pH 7.2. Mix well and measure the OD280. The extinction coefficient of WGA is 15, the reading for solution of 10 mg/ml. Adjust volume to 1 mg/ml in sterile distilled water. Store at 4ºC for months to years.
Con A
Add 4 ml sterile distilled water to a vial of 10 mg of Con A and mix well to dissolve. Filter through a 0.22 micron syringe filter into a sterile plastic tube. Take 25 μl of this solution and add to 975 μl of phosphate buffered saline (PBS), pH 7.2. Mix well and measure the OD280. The extinction coefficient of Con A is 12.4, the reading for a solution of 10 mg/ml. Adjust volume to 1 mg/ml in sterile distilled water. Store at 4ºC for months to years.
RIC
Ricin is a potent toxin and should be handled with utmost care. Use only plastic dispensers. Add 5 ml sterile PBS containing 1 mM CaCl2 and 1 mM MgCl2 to a vial of 10 mg of ricin and mix well to dissolve. Filter through a 0.22 micron syringe filter into a sterile plastic tube. Take 25 μl of this solution and add to 975 μl of phosphate buffered saline (PBS), pH 7.2. Mix well and measure the OD280. The extinction coefficient of Ricin is 11.8, the reading for a solution of 10 mg/ml. Adjust volume to 2 mg/ml in PBS containing 1 mM CaCl2 and 1 mM MgCl2. Since Ricin is cytotoxic at very low concentrations (ng/ml), also dilute 5 μl of the 2 mg/ml stock solution into 10 ml of PBS to give a 1 μg/ml stock. Store at 4ºC.
LCA
Add 2.5 ml of sterile PBS containing 1 mM CaCl2 and 1 mM MgCl2 to a 10 mg vial of LCA and mix well. Filter through a 0.22 micron syringe filter into a sterile plastic tube. Take 25 μl of this solution and add to 975 μl of phosphate buffered saline (PBS), pH 7.2. Mix well and measure the OD280. The extinction coefficient of LCA is 12.6, the reading of a solution of 10 mg/ml. Adjust volume to 1 mg/ml in PBS containing 1 mM CaCl2 and 1 mM MgCl2. Store at 4ºC for months or years.
For lectins or antibodies conjugated to a fluorescent probe, store at the concentration provided by the manufacturer. Store at 4ºC for months or years but keep completely protected from light.
Cell culture medium
Dulbecco’s modified Eagle’s medium (DMEM) or alpha medium from GIBCO Life Technologies. To 900 ml medium add 100 ml fetal calf serum. Store at 4ºC for up to 3 months.
PBS/CaMg
To 900 ml distilled water in a beaker with a stir bar add: 8 g NaCl, 0.2 g KCl, 1.44 g Na2 HPO4, 0.25 g KH2 PO4, 0.2 g MgCl2 hexahydrate. Allow to dissolve completely with stirring. Add 0.55 g CaCl2 (anhydrous). Allow to dissolve completely. Adjust volume to 1 L with distilled water. Adjust pH to 7.2 with drops of 2N HCl. Filter through a 0.22 micron filter to sterilize. Store at 4ºC indefinitely.
Methylene Blue
Methylene Blue 50 g/100 ml in 50% methanol in distilled water. The solution should be made in a 500 ml glass bottle, capped loosely to avoid alcohol fume build up, and mixed overnight with a magnetic stir bar at room temperature. Next morning, cap the bottle tightly and store at room temperature for up to 6 months.
MTT reagent
MTT is dissolved in PBS/CaMg at 5 mg/ml. It forms a bright yellow solution. Store in aliquots at −20ºC. It may be stored for several years.
Binding Buffer (BB) for glycan binding assay
To 900 ml distilled water in a beaker with a stir bar add: 8 g NaCl, 0.4 g KCl, 1.0 g glucose, 0.36 g Na2PO4, 0.6 g KH2PO4, 0.2 g MgCl2 hexahydrate. Allow to dissolve completely with stirring. Add 0.72 g CaCl2. Allow to dissolve completely. Adjust volume to 1 L with distilled water. Adjust pH to 7.2 with drops of 2N HCl. Add 20 g BSA (Fraction V) and dissolve. Add 0.1 g Na azide and dissolve. Filter through a 0.22 micron filter and store at 4ºC indefinitely.
7-actinomycin D
7-AAD is very toxic and should be handled with care. It can be obtained commercially as a solution for use in flow cytometry assays. Alternatively, if bought as a powder, it should be prepared as follows: to 1 mg 7-AAD, add 50 μl DMSO to dissolve, then add 950 μl PBS/CaMg for a 1 mg/ml solution. Protect from light. Store at 4ºC for 6 months.
COMMENTARY
Background Information
The assays described above were developed to rapidly determine if a putative glycosylation mutant expresses an altered complement of glycans on cell surface glycoproteins reflecting a mutation causing altered glycan synthesis (Stanley, 1983; Stanley et al., 1975). The lectin resistance test was designed to distinguish different types of mutant cells that had been selected for resistance to a plant lectin as shown in Figure 2. Since resistance to one lectin often confers increased resistance or hypersensitivity to another lectin, a panel of 5 lectins was able to distinguish between CHO glycosylation mutants that arose from mutations in different glycosylation genes (Stanley et al., 1975). Table 1 shows the lectin resistance phenotypes of CHO mutants that are commonly used for glycosylation engineering of organisms or recombinant proteins. A more complete list of the CHO glycosylation mutants derived by this laboratory has been previously published (Patnaik and Stanley, 2006). More recently, several additional CHO lines designed for glycosylation engineering have been reported (Zhang et al., 2013). For cells with a qualitatively different lectin resistance phenotype from other mutants, it is extremely likely that the cell carries a mutation in a different glycosylation gene. On the other hand, if the lectin resistance phenotype is qualitatively similar and only quantitatively different, the mutation may be in the same gene as in the related mutant with a similar phenotype, but inactivate the glycosylation activity to a different degree. This was observed for Lec1 and Lec1A that carry inactivating versus weakening mutations in the Mgat1 gene (Chaney and Stanley, 1986; Chen et al., 2001b; Stanley and Chaney, 1985), and Lec4 versus Lec4A CHO mutants which carry null versus point mutations in the Mgat5 gene (Chaney et al., 1989; Weinstein et al., 1996).
Lectin and antibody binding assays are also very useful for revealing a change in glycosylation (Fig. 3). However, the sensitivity of flow cytometry is less than the lectin resistance assay. Flow cytometry cannot readily distinguish differences of less than 2-fold, whereas lectins can be titrated so that differences of much less than 2-fold can be teased out using the Lectin Resistance Test.
Critical Parameters and Troubleshooting
For the lectin resistance assay, the toxicity of lectins may vary depending on their source or even their batch number. This is not usually a major problem because the assay is always comparing relative resistance to a lectin. However, if lectin toxicity decreases to such an extent that wild type cells are not killed at a reasonable concentration, an alternative source should be sought. It is a good idea to buy a good supply of a lectin once initial characterization identifies it as causing the expected toxicity. Lectins are very stable, either in powder form or in solution, and thus storage for long periods without loss of toxicity is possible.
Cells must be able to adhere to plastic for the lectin resistance assay, but not for the MTT assay or the lectin-binding assay.
If no cells are present in occasional wells before the lectin becomes very toxic, it is likely to be due to bacterial or fungal contamination that killed the cells, or caused them to detach. Before staining with Methylene Blue, observe the 96-well plate by eye from underneath to detect wells in which the medium is less clear compared to other wells. Lack of clarity would indicate contamination. Bacteria should be visible, especially if motile, when determining the confluency of each well using the inverted phase microscope at a magnification of 40 X.
For the lectin binding assay, lectin batches may vary in binding ability. Titration is necessary to obtain the best differential in lectin binding between a wild type and mutant cell line.
Anticipated Results and Time Considerations
Typical results of a lectin resistance test for CHO wild type and the CHO mutant Lec1 are shown in Fig. 2. This assay takes about 2 hr to set up and requires 3–4 days of incubation until cell monolayers in the wells with no lectin reaches confluence. Staining with Methylene Blue takes about 30–60 min. A similar time frame is required for the MTT assay. The relative resistance or sensitivity of cells to a panel of lectins identifies its lectin resistance phenotype (Table 1).
Typical results for flow cytometry assays using lectins and antibodies are shown in Fig. 3. The washing, counting and incubation of cells with fluorescent conjugate takes 2–3 hr. The flow cytometry should take 30–60 min, including setting machine parameters.
Acknowledgments
We thank Barry Potvin for development of the MTT assay and Santosh Patnaik and Huang-Hsiang Huang for developing the lectin and antibody binding protocols. This work was supported by NIGMS grant RO1 GM105399 to PS.
Footnotes
INTERNET RESOURCES
This site has the latest information on the sequencing of the CHO genome and transcriptome and is useful for glycosylation mutations that arise in CHO cells.
http://www.functionalglycomics.org/static/consortium/consortium.shtml
This site provides excellent information on resources and assays for characterizing glycans.
KEY REFERENCE
This reference contains a more extensive description of the panel of CHO glycosylation mutants that have been characterized and can be used for glycosylation engineering.
LITERATURE CITED
- Aguilan JT, Sundaram S, Nieves E, Stanley P. Mutational and functional analysis of Large in a novel CHO glycosylation mutant. Glycobiology. 2009;19:971–986. doi: 10.1093/glycob/cwp074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C, Stanley P. A dominant mutation to ricin resistance in Chinese hamster ovary cells induces UDP-GlcNAc:glycopeptide beta-4-N-acetylglucosaminyltransferase III activity. J Biol Chem. 1984;259:13370–13378. [PubMed] [Google Scholar]
- Chaney W, Stanley P. Lec1A Chinese hamster ovary cell mutants appear to arise from a structural alteration in N-acetylglucosaminyltransferase I. Journal of Biological Chemistry. 1986;261:10551–10557. [PubMed] [Google Scholar]
- Chaney W, Sundaram S, Friedman N, Stanley P. The Lec4A CHO glycosylation mutant arises from miscompartmentalization of a Golgi glycosyltransferase. Journal of Cell Biology. 1989;109:2089–2096. doi: 10.1083/jcb.109.5.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Moloney DJ, Stanley P. Fringe modulation of Jagged1-induced Notch signaling requires the action of beta 4galactosyltransferase-1. Proc Natl Acad Sci U S A. 2001a;98:13716–13721. doi: 10.1073/pnas.241398098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Stanley P. Five Lec1 CHO cell mutants have distinct Mgat1 gene mutations that encode truncated N-acetylglucosaminyltransferase I. Glycobiology. 2003;13:43–50. doi: 10.1093/glycob/cwg003. [DOI] [PubMed] [Google Scholar]
- Chen W, Unligil UM, Rini JM, Stanley P. Independent Lec1A CHO Glycosylation Mutants Arise from Point Mutations in N-Acetylglucosaminyltransferase I That Reduce Affinity for Both Substrates. Molecular Consequences Based on the Crystal Structure of GlcNAc-TI(,) Biochemistry. 2001b;40:8765–8772. doi: 10.1021/bi015538b. [DOI] [PubMed] [Google Scholar]
- Debeljak N, Sytkowski AJ. Erythropoietin and erythropoiesis stimulating agents. Drug testing and analysis. 2012;4:805–812. doi: 10.1002/dta.1341. [DOI] [PubMed] [Google Scholar]
- Eckhardt M, Gotza B, Gerardy-Schahn R. Mutants of the CMP-sialic acid transporter causing the Lec2 phenotype. J Biol Chem. 1998;273:20189–20195. doi: 10.1074/jbc.273.32.20189. [DOI] [PubMed] [Google Scholar]
- Esko JD, Stanley P. Glycosylation Mutants of Cultured Cells. In: Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME, editors. Essentials of Glycobiology. 2. Cold Spring Harbor (NY): 2009. [PubMed] [Google Scholar]
- Fukuda MN, Sasaki H, Lopez L, Fukuda M. Survival of recombinant erythropoietin in the circulation: the role of carbohydrates. Blood. 1989;73:84–89. [PubMed] [Google Scholar]
- Grabowski GA, Barton NW, Pastores G, Dambrosia JM, Banerjee TK, McKee MA, Parker C, Schiffmann R, Hill SC, Brady RO. Enzyme therapy in type 1 Gaucher disease: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Intern Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- Hammond S, Kaplarevic M, Borth N, Betenbaugh MJ, Lee KH. Chinese hamster genome database: an online resource for the CHO community. Biotechnol Bioeng. 2012;109:1353–1356. doi: 10.1002/bit.24374. at http://www.CHOgenome.org. [DOI] [PubMed] [Google Scholar]
- Hou X, Tashima Y, Stanley P. Galactose differentially modulates lunatic and manic fringe effects on Delta1-induced NOTCH signaling. J Biol Chem. 2012;287:474–483. doi: 10.1074/jbc.M111.317578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferis R. Review of Glycosylation Engineering of Biopharmaceuticals: Methods and Protocols: A book edited by Alain Beck. mAbs. 2013;5:638–640. [Google Scholar]
- Kanda Y, Yamane-Ohnuki N, Sakai N, Yamano K, Nakano R, Inoue M, Misaka H, Iida S, Wakitani M, Konno Y, Yano K, Shitara K, Hosoi S, Satoh M. Comparison of cell lines for stable production of fucose-negative antibodies with enhanced ADCC. Biotechnol Bioeng. 2006;94:680–688. doi: 10.1002/bit.20880. [DOI] [PubMed] [Google Scholar]
- Lewis NE, Liu X, Li Y, Nagarajan H, Yerganian G, O’Brien E, Bordbar A, Roth AM, Rosenbloom J, Bian C, Xie M, Chen W, Li N, Baycin-Hizal D, Latif H, Forster J, Betenbaugh MJ, Famili I, Xu X, Wang J, Palsson BO. Genomic landscapes of Chinese hamster ovary cell lines as revealed by the Cricetulus griseus draft genome. Nat Biotechnol. 2013;31:759–765. doi: 10.1038/nbt.2624. [DOI] [PubMed] [Google Scholar]
- Lux A, Nimmerjahn F. Impact of differential glycosylation on IgG activity. Adv Exp Med Biol. 2011;780:113–124. doi: 10.1007/978-1-4419-5632-3_10. [DOI] [PubMed] [Google Scholar]
- Natsume A, Wakitani M, Yamane-Ohnuki N, Shoji-Hosaka E, Niwa R, Uchida K, Satoh M, Shitara K. Fucose removal from complex-type oligosaccharide enhances the antibody-dependent cellular cytotoxicity of single-gene-encoded antibody comprising a single-chain antibody linked the antibody constant region. J Immunol Methods. 2005;306:93–103. doi: 10.1016/j.jim.2005.07.025. [DOI] [PubMed] [Google Scholar]
- North SJ, Huang HH, Sundaram S, Jang-Lee J, Etienne AT, Trollope A, Chalabi S, Dell A, Stanley P, Haslam SM. Glycomics profiling of Chinese hamster ovary cell glycosylation mutants reveals N-glycans of a novel size and complexity. J Biol Chem. 2010;285:5759–5775. doi: 10.1074/jbc.M109.068353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmann S, Stanley P, Gerardy-Schahn R. Point mutations identified in lec8 chinese hamster ovary glycosylation mutants that inactivate both the udp-galactose and cmp-sialic acid transporters. J Biol Chem. 2001;276:26291–26300. doi: 10.1074/jbc.M011124200. [DOI] [PubMed] [Google Scholar]
- Ogorek C, Jordan I, Sandig V, von Horsten HH. Fucose-targeted glycoengineering of pharmaceutical cell lines. Methods Mol Biol. 2012;907:507–517. doi: 10.1007/978-1-61779-974-7_29. [DOI] [PubMed] [Google Scholar]
- Olsnes S. The history of ricin, abrin and related toxins. Toxicon : official journal of the International Society on Toxinology. 2004;44:361–370. doi: 10.1016/j.toxicon.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Potvin B, Carlsson S, Sturm D, Leffler H, Stanley P. Complex N-glycans are the major ligands for galectin-1, -3, and -8 on Chinese hamster ovary cells. Glycobiology. 2006;16:305–317. doi: 10.1093/glycob/cwj063. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Potvin B, Stanley P. LEC12 and LEC29 gain-of-function chinese hamster ovary mutants reveal mechanisms for regulating VIM-2 antigen synthesis and E-selectin binding. J Biol Chem. 2004;279:49716–49726. doi: 10.1074/jbc.M408755200. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Stanley P. Lectin-resistant CHO glycosylation mutants. Methods Enzymol. 2006;416:159–182. doi: 10.1016/S0076-6879(06)16011-5. [DOI] [PubMed] [Google Scholar]
- Patnaik SK, Zhang A, Shi S, Stanley P. alpha(1,3)fucosyltransferases expressed by the gain-of-function Chinese hamster ovary glycosylation mutants LEC12, LEC29, and LEC30. Arch Biochem Biophys. 2000;375:322–332. doi: 10.1006/abbi.1999.1693. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Nudelman E, Gaeta FC, Perez M, Singhal AK, Hakomori S, Paulson JC. ELAM-1 mediates cell adhesion by recognition of a carbohydrate ligand, sialyl-Lex. Science. 1990;250:1130–1132. doi: 10.1126/science.1701274. [DOI] [PubMed] [Google Scholar]
- Rana NA, Haltiwanger RS. Fringe benefits: functional and structural impacts of O-glycosylation on the extracellular domain of Notch receptors. Curr Opin Struct Biol. 2011;21:583–589. doi: 10.1016/j.sbi.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravdin JI, Stanley P, Murphy CF, Petri WA., Jr Characterization of cell surface carbohydrate receptors for Entamoeba histolytica adherence lectin. Infect Immun. 1989;57:2179–2186. doi: 10.1128/iai.57.7.2179-2186.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripka J, Adamany A, Stanley P. Two Chinese hamster ovary glycosylation mutants affected in the conversion of GDP-mannose to GDP-fucose. Arch Biochem Biophys. 1986;249:533–545. doi: 10.1016/0003-9861(86)90031-7. [DOI] [PubMed] [Google Scholar]
- Shaaltiel Y, Bartfeld D, Hashmueli S, Baum G, Brill-Almon E, Galili G, Dym O, Boldin-Adamsky SA, Silman I, Sussman JL, Futerman AH, Aviezer D. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant biotechnology journal. 2007;5:579–590. doi: 10.1111/j.1467-7652.2007.00263.x. [DOI] [PubMed] [Google Scholar]
- Shinkawa T, Nakamura K, Yamane N, Shoji-Hosaka E, Kanda Y, Sakurada M, Uchida K, Anazawa H, Satoh M, Yamasaki M, Hanai N, Shitara K. The absence of fucose but not the presence of galactose or bisecting N-acetylglucosamine of human IgG1 complex-type oligosaccharides shows the critical role of enhancing antibody-dependent cellular cytotoxicity. J Biol Chem. 2003;278:3466–3473. doi: 10.1074/jbc.M210665200. [DOI] [PubMed] [Google Scholar]
- Song Y, Aglipay JA, Bernstein JD, Goswami S, Stanley P. The bisecting GlcNAc on N-glycans inhibits growth factor signaling and retards mammary tumor progression. Cancer Res. 2010;70:3361–3371. doi: 10.1158/0008-5472.CAN-09-2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl M, Uemura K, Ge C, Shi S, Tashima Y, Stanley P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J Biol Chem. 2008;283:13638–13651. doi: 10.1074/jbc.M802027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley P. Selection of lectin-resistant mutants of animal cells. Methods in Enzymology. 1983;96:157–184. doi: 10.1016/s0076-6879(83)96015-9. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation mutants of animal cells. Annu Rev Genet. 1984;18:525–552. doi: 10.1146/annurev.ge.18.120184.002521. [DOI] [PubMed] [Google Scholar]
- Stanley P. Glycosylation engineering. Glycobiology. 1992;2:99–107. doi: 10.1093/glycob/2.2.99. [DOI] [PubMed] [Google Scholar]
- Stanley P, Caillibot V, Siminovitch L. Selection and characterization of eight phenotypically distinct lines of lectin-resistant Chinese hamster ovary cell. Cell. 1975;6:121–128. doi: 10.1016/0092-8674(75)90002-1. [DOI] [PubMed] [Google Scholar]
- Stanley P, Chaney W. Control of carbohydrate processing: the lec1A CHO mutation results in partial loss of N-acetylglucosaminyltransferase I activity. Mol Cell Biol. 1985;5:1204–1211. doi: 10.1128/mcb.5.6.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steentoft C, Vakhrushev SY, Vester-Christensen MB, Schjoldager KT, Kong Y, Bennett EP, Mandel U, Wandall H, Levery SB, Clausen H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat Methods. 2011;8:977–982. doi: 10.1038/nmeth.1731. [DOI] [PubMed] [Google Scholar]
- Su D, Zhao H, Xia H. Glycosylation-modified erythropoietin with improved half-life and biological activity. Int J Hematol. 2010;91:238–244. doi: 10.1007/s12185-010-0496-x. [DOI] [PubMed] [Google Scholar]
- Tekoah Y, Tzaban S, Kizhner T, Hainrichson M, Gantman A, Golembo M, Aviezer D, Shaaltiel Y. Glycosylation and Functionality of Recombinant ss-Glucocerebrosidase from Various Production Systems. Biosci Rep. 2013 doi: 10.1042/BSR20130081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan S, Zehe C, Kuehle J, Qiao J, Bode J. Recombinase-mediated cassette exchange (RMCE) - a rapidly-expanding toolbox for targeted genomic modifications. Gene. 2013;515:1–27. doi: 10.1016/j.gene.2012.11.016. [DOI] [PubMed] [Google Scholar]
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Marth JD, Bertozzi CR, Hart GW, Etzler ME. Symbol nomenclature for glycan representation. Proteomics. 2009;9:5398–5399. doi: 10.1002/pmic.200900708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh MJ, Dodd JE, Hautbergue GM. Ribosome-inactivating proteins: Potent poisons and molecular tools. Virulence. 2013:4. doi: 10.4161/viru.26399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein J, Sundaram S, Wang X, Delgado D, Basu R, Stanley P. A point mutation causes mistargeting of Golgi GlcNAc-TV in the Lec4A Chinese hamster ovary glycosylation mutant. J Biol Chem. 1996;271:27462–27469. doi: 10.1074/jbc.271.44.27462. [DOI] [PubMed] [Google Scholar]
- Yang H, Wang H, Shivalila CS, Cheng AW, Shi L, Jaenisch R. One-Step Generation of Mice Carrying Reporter and Conditional Alleles by CRISPR/Cas-Mediated Genome Engineering. Cell. 2013;154:1370–1379. doi: 10.1016/j.cell.2013.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang A, Potvin B, Zaiman A, Chen W, Kumar R, Phillips L, Stanley P. The gain-of-function Chinese hamster ovary mutant LEC11B expresses one of two Chinese hamster FUT6 genes due to the loss of a negative regulatory factor. J Biol Chem. 1999;274:10439–10450. doi: 10.1074/jbc.274.15.10439. [DOI] [PubMed] [Google Scholar]
- Zhang P, Chan KF, Haryadi R, Bardor M, Song Z. CHO Glycosylation Mutants as Potential Host Cells to Produce Therapeutic Proteins with Enhanced Efficacy. Adv Biochem Eng Biotechnol. 2013;131:63–87. doi: 10.1007/10_2012_163. [DOI] [PubMed] [Google Scholar]
- Zhang P, Tan DL, Heng D, Wang T, Mariati, Yang Y, Song Z. A functional analysis of N-glycosylation-related genes on sialylation of recombinant erythropoietin in six commonly used mammalian cell lines. Metabolic engineering. 2010;12:526–536. doi: 10.1016/j.ymben.2010.08.004. [DOI] [PubMed] [Google Scholar]