Abstract
Objective
In this paper, we review the conceptual basis, definitions, and evolution of cognitive training (CT) approaches for the treatment of mental disorders.
Method
We review the current state of the knowledge on CT in psychiatric illnesses, and its neural and behavioral targets, and summarize the factors that appear to relate to a successful response to CT, including learner characteristics that influence clinical outcome. We also discuss methodological issues relevant to the development and testing of CT approaches, with the goal of creating maximally efficient and effective approaches to training. Finally, we identify gaps in existing knowledge, and outline key research directions for the future.
Results
While much of the early work has been conducted in schizophrenia, CT has more recently been applied to a widening range of neuropsychiatric illnesses, including attention deficit disorder, mood disorders, and substance use disorders. CT harnesses the inherent neuroplastic capacities of the brain, targeting neural system function across psychiatric disorders, and thus improving cognitive processes that play a role in emotion regulation, clinical symptoms, and adaptive community functioning.
Conclusions
CT offers considerable promise, especially given the limited efficacy of pharmacological interventions in ameliorating cognitive deficits. However, more work is needed to understand mechanisms underlying CT, predictors of response, generalization and real-world applicability, and approaches to dissemination in practice settings.
Introduction and Overview
On April 9-10, 2012, NIMH convened a group of experts in cognitive training (CT) to review the current state of evidence for the efficacy of current and emerging CT approaches for mental disorders, identify challenges as well as research gaps, and learn of efforts to adopt CT interventions in clinical practice. CT methods that harness neuroplasticity mechanisms for cognitive enhancement in impaired neural systems show promise as evidence-based interventions in psychiatry. Meeting participants expressed optimism that one day in the not-too-distant future, we will be able to identify the key neural system impairments unique to individual patients, and prescribe personalized CT programs in order to enhance cognition, improve community functioning, and optimize well-being.
CT is one of a range of behavioral interventions for cognitive enhancement (Figure 1), which also includes exercise, mindfulness-based meditation practice, and other approaches (including the more “non-specific” cognitive and socio-affective engagement that can occur in psychotherapy). CT in psychiatry uses diverse approaches (paper-and-pencil, computer-administered or guided behavioral exercises) to enhance cognitive function and optimize well-being in mental disorders.
Figure 1. Taxonomy of approaches to cognitive enhancements in mental illness.

Cognitive training is but one of an array of potential interventions to enhance cognitive functioning. TMS= Transcranial Magnetic Stimulation. tDCS= Transcranial Direct Current Stimulation.
How is Cognitive Training Defined?
The terms cognitive training, cognitive remediation, and cognitive rehabilitation are used both interchangeably and inconsistently in the literature and in clinical practice. We herein broadly define CT as an intervention that uses specifically designed, behaviorally constrained cognitive or socio-affective learning events delivered in a scalable and reproducible manner to potentially improve neural system operations. The eventual goal of CT is to target known neural mechanisms of behavioral impairment, to affect clinical change.
CT seeks to drive learning and adaptive neuroplastic changes in an individual's neural representational systems through the use of specifically-defined, neuroscience-based, and controlled learning events. The more specifically-defined, controlled learning events delivered in CT differ from the relatively unconstrained, uncontrolled, and unpredictable approaches to learning associated with cognitive behavioral therapy or with the use of a psychoeducational video tutorial. We also distinguish CT from the broad and non-specific (though therapeutically important) forms of cognitive and socio-affective stimulation that occur, for example, from participating in a 12-step program, or joining a community organization such as a church group. CT defined as above is typically embedded in a larger therapeutic context that makes use of therapist and participant expectancy, instillation of hope, and other psychosocial ingredients. These factors are themselves all potent agents of neuro-behavioral change. Indeed, CT, like many successful treatment programs (such as group therapies, vocational rehabilitation, and psychosocial skills training) explicitly harnesses multiple non-specific and contextual therapeutic factors, in order to maximize overall functional gains for participants.
Another term that is applied in the field is Cognitive Remediation, which has been defined as “a behavioural training-based intervention that aims to improve cognitive processes with the general aim of durability and generalization to community functioning” (Cognitive Remediation Expert Working Group, cited in (1). In this paper, we emphasize the term “training” as opposed to “remediation”, since the former carries less stigma and implies the improvement or restoration of physiologic mechanisms in individuals at all levels of functioning, while the latter implies the correction of a fault or deficiency and may also include the development of compensatory or “work-around” skills.
Mental Illness, Neuroplasticity, and CT
Clinical interest in “brain remediation” dates back to World War I (2) when methods were developed to treat soldiers with war-related brain injuries. However, emerging basic and clinical research has motivated a theory-driven design of CT approaches, with an emphasis on harnessing the brain's inherent capacities for change in order to promote or restore adaptive cognitive and socio-affective processes. How might this principle be applied in mental illnesses? First, mental illnesses can be broadly viewed as resulting from inefficient, maladaptive, and/or biased distributed neural representations underlying critical cognitive and emotional processes necessary for successful community functioning (Figure 2). Second, experimental neuroscience has unequivocally shown that the brain changes with the introduction of new experiences and with the training of new perceptual, cognitive, or motor skills—a process termed neuroplasticity.
Figure 2. Translational hierarchy of outcomes with CT.

Carefully targeted improvements in neural system function in mental illness should translate into better community functioning via their effects on cognition.
Neuroplasticity occurs in at least two (not mutually exclusive) developmental contexts (Figure 3). Very early in development, experience and its resulting neuronal activity can shape neuronal response properties irrespective of an organism's attention to a stimulus. This process of experience-expectant neuroplasticity (3) shapes neural representations to reflect statistical regularities in inputs (e.g. from one eye vs. another, (4)) and in the environment (5). Such plasticity is often conceptualized to occur within a finite window, a so-called “critical period.” Maladaptive experiences or insults to the developing brain during these critical periods can have lasting behavioral consequences.
Figure 3. Critical windows of neuroplasticity during human life.

Cognitive training makes use of experience-dependent plasticity that is present throughout the human lifespan.
A qualitatively different process, experience-dependent neuroplasticity, occurs throughout development. Also termed “learning,” this process involves changes in neuronal activity that represents meaningful stimuli and behaviors, particularly those associated with reward; such activity in turn effects lasting neural representations (e.g.,(6–10), see also (11,12) for reviews). Maladaptive or distorted learning about behaviorally important events or stimuli (particularly during the extended critical period for socio-affective development), followed by enduring alterations in neural representations, can serve as a model for how psychiatric symptoms first emerge precipitously or insidiously, then stabilize and become chronic.
Neuroplastic processes related to learning can be intentionally harnessed for therapeutic purposes across a wide range of disorders. CT focuses on identifying the impaired representational systems underlying critical cognitions, and then training such processes via implicit or explicit learning mechanisms in order to improve the speed and accuracy of task-relevant information processing and distributed neural responses, and thereby improve community functioning.. Growing knowledge of the specific processing anomalies, developmental features, and distributed neural circuits that characterize various psychiatric illnesses will inform the next generation of CT approaches, including their content and timing. This requires a constant iterative process bridging cognitive, affective, and developmental neuroscience with clinical treatment development.
Neural Targets for Cognitive Training in Mental Illness
Cognitive/socio-affective deficits are found in all major neuropsychiatric disorders, often predate the symptoms, and may determine functional outcomes (13–15). Such disturbances may reflect aberrant neural representations involving affect regulation, motivation, incentive salience, self and other, and social perception (15). Emerging evidence shows that targeted intensive CT can induce more normal and efficient neural system operations and potentially harness inherent plasticity processes (e.g., 16). Improving neural functioning at the macro-circuit level may de facto impact on cellular and synaptic-level functioning at the micro-circuit level, including neurotransmitter and neurotrophin systems (17).
Relevant distributed neural system targets are being identified for such intensive CT approaches in mental illness. Successful CT can harness “bottom-up” or “feed-forward” processes (such as perception and pre-attentive perceptual biasing) and/or “top-down” or “feed-back”processes (e.g. attention, cognitive control, metacognitive appraisal), as will be discussed later. In schizophrenia, for example, these range from impairments in sensory encoding to inefficient prefrontal operations that have an impact on working memory, episodic memory, and social cognition (16). In major depression, impairments in executive function and processing speed can be observed, associated with aberrant activity in prefrontal and limbic system networks associated with affect dysregulation (18)(19)(20) as well as abnormal biasing of attention to negative cognitions (21). Thus, disorders are characterized by both distinct and cross-cutting patterns of neural dysfunction, offering unique combinations of cognitive and socio-affective targets for training . However, the ultimate goal is to identify target processes in which training will induce the widest range of generalized clinical and behavioral improvement. For example, inefficiencies or biases in prefrontal predictive and cognitive control operations may represent a fundamental or “common denominator” underlying a range of mental illnesses, resulting in disorder-specific or cross-diagnostic impairments or biases in perception, affect and cognition (22); Such processes may represent promising CT targets, not only as a treatment, but potentially as a preventive or pre-emptive intervention. However, challenges to this approach include the fact that no current assessment or intervention approaches address single neural system targets, nor is this likely to ever be the case; changes in one brain system (e.g. attention) inevitably affect other systems (such as working memory).
CT in Mental Illnesses: Current State of Knowledge
CT approaches have been applied across various mental illnesses, have used a variety of methods, and study designs, making it currently difficult to integrate findings, draw definitive conclusions, or suggest best practices. Study outcomes have also shown varying efficacy in the targeted cognitive domains, while others have shown generalization or transfer to untrained domains and to functional outcome; only a few have examined issues of durability beyond the end of treatment. We briefly review the current state of knowledge, beginning with the areas with the strongest evidence base.
Schizophrenia
Several CT approaches have been studied in schizophrenia, ranging from repetitive practice to therapist-guided strategy coaching; these approaches have targeted a wide range of cognitive processes, including perception, working memory, attention, and social cognition, or combinations of these functions. A recent meta-analysis of 40 studies conducted between 1973 and 2009 found modest efficacy, with a mean effect size on cognition of 0.45 (1); some CT benefits appear durable after the end of treatment (23,24). Functional outcomes were significantly better in studies where cognitive remediation was combined with other forms of rehabilitation and when it included strategy coaching. Age, pre-treatment cognitive function, motivation, clinician expertise, therapeutic alliance, and measures of ‘brain reserve’, may in certain cases be predictors of a positive treatment response (25–28). Initial neuroimaging findings suggest that CT response is associated with structural and functional changes in key prefrontal brain regions (16,26,27). Overall, the evidence thus far supports the neurobiological rationale and the efficacy of CT in schizophrenia, but replication of positive results is needed; many questions remain in regard to therapeutic mechanisms, key therapeutic ingredients, and approaches to dissemination in routine clinical settings.
Attention Deficit Hyperactivity Disorder (ADHD)
CT, focused on executive functioning and working memory, may benefit individuals with ADHD (31–33). Klingberg et al (34) showed that CT improved working memory as well as nontrained functions of response inhibition and reasoning; parent-rated inattention and hyperactivity/impulsivity symptoms also decreased after CT, and the positive effects were maintained at 3 months. Such improvements appear to be related to increased activity in frontoparietal and striatal regions mediating working memory functions in ADHD (35). In an open-label study of preschool-age children with ADHD and their parents, who participated in group sessions, Halperin et al.(36) used motor activities/games designed to enhance inhibitory control, working memory, attention, visuo-spatial abilities, planning and motor skills. Parental involvement facilitated the implementation of this play-based intervention at home. Both parent and teacher-ratings indicated significant improvement in ADHD symptoms. Similarly, an open trial of metacognitive executive function training for ADHD resulted in improved attention, working memory, and cognitive flexibility (37). CT targets for ADHD may need to be broadened to include self-control or delay aversion (38). Taken together, CT appears effective in ADHD, but well-powered and well-controlled randomized trials with precisely defined targets and outcome measures, as well as longitudinal follow-up to examine durability, are needed.
Anxiety Disorders
Anxiety disorders are characterized by implicit attentional biases to threat-related stimuli (39). This has led investigators to develop attention bias modification (ABM) strategies, where response targets are repetitively presented at the location of neutral, rather than threat, stimuli with the goal of inducing an implicit bias away from threat in order to reduce overall anxiety levels. Two recent meta-analyses of ABM training in adults with anxiety disorders show moderate to large effect sizes (0.8-1.4) for changes in attentional bias, and an effect size of .61 for clinical symptoms, comparable to what is seen with cognitive-behavioral therapy and selective serotonin reuptake inhibitors (40,41). Anxiety reductions were also seen in a large randomized controlled trial of children with anxiety disorders (42). Critical next steps include: determining the components of successful training (stimuli, optimal task design, training duration) and the neural mechanisms that support behavioral change;demonstrating the robustness and durability of the effects of ABM on subjective experiences and real-world functioning; and developing approaches to dissemination in routine clinical settings.
Mood Disorders
Emerging research indicates that both major depressive disorder and bipolar disorder are associated with persistent cognitive impairment in domains such as processing speed and executive functioning. In addition to these impairments in “cold” cognition ((43)), , depression is associated with attentional bias to negative emotional stimuli, even at a level below awareness (40–43,). Emerging evidence suggests (18)) that CT can be effective in mood disorders. Deckersbach et al. (48) found that a 14-session remediation program targeting residual depressive symptoms and cognitive functioning was associated with decreased depression ratings and improved executive functioning scores that were related to better work functioning. A recent meta-analysis (49) found an effect size of .32 for CT in studies with patients with affective or schizoaffective disorders. Attentional bias training in depression seems to potentially reduce relapse risk in depression (50). Further studies need to examine specificity, durability, mechanisms and generalization of CT effects in mood disorders.
Substance Use Disorders
Substance use disorders are associated with a range of cognitive impairments-- particularly in attention, working memory, response inhibition, and delay discounting (i.e. preference for immediate versus delayed rewards)—which predict poor outcome and adherence to treatment. Although the nature, course, and significance of these deficits is unclear at present, CT is under active investigation in this field. For instance, CT training on computerized working memory tasks has been shown to reduce impulsivity and delay discounting among stimulant users (51). Cognitive Bias Modification (brief training to automatically avoid alcohol cues) has also shown promise in treating alcohol addiction patients during a one year follow-up (52). While promising, the field is in its early stages and many questions still need to be addressed. For example, it remains unclear whether CT approaches might work generally across individuals with substance use disorders, or whether they need to be reserved for those with demonstrable cognitive impairments (53). In addition, the cognitive targets that will result in the highest yield clinical outcomes are as of yet unknown.
Autism Spectrum Disorders (ASD)
There is an emerging trend toward the use of social cognition training in autism spectrum disorders (ASD), though the field is in its infancy (54). Eack et al. (55) observed significant improvement (effect size >1) in cognition, social cognition and overall functioning in a preliminary study of individuals with ASD treated with cognitive enhancement therapy (CET), which combines computerized neurocognitive and social-cognitive remediation with social skills group therapy. Another approach has focused on computerized methods that promote a holistic approach to face processing, known to be abnormal in ASD (56). Overall, CT research in children and adults with ASD is currently very limited and needs to capitalize on emerging findings on neurocognitive and neural system impairments in these illnesses.
Other Disorders
CT applications in other brain disorders will be briefly mentioned here, and details are beyond the scope of this review. CT directed at enhancing phonological awareness and auditoryprocessing, related to impaired to left parietotemporal cortex engagement, is effective in treatment of dyslexia ((57)). Several studies have examined effects of CT interventions in mild cognitive impairments in older adults, and have found promising, but inconclusive results; it is unclear if CT interventions have an impact on the conversion rates from MCI to dementia (58) There is evidence for benefits of CT in traumatic brain injury and stroke ((59) however, overall, at present the evidence remains insufficient on the efficacy and utility of CT approaches in stroke and other acquired brain disease ((60)).
Predictors and Moderators of Response to Cognitive Training
Learner Characteristics
Age and neurodevelopmental effects
Few studies have systematically examined the effects of age or neurodevelopmental stage on the response to CT. “Sensitive periods”, when specific neural systems are undergoing rapid change, may provide windows of opportunity during which CT could have a particularly potent effect. Early adolescence, characterized by a heightened sensitivity to reward and rapid development of cognitive control systems, may be one such period (38).
CT delivered as a preventive/pre-emptive intervention may diminish the cascading effects of psychopathology, both at neural and environmental levels, potentially avoiding increasing deterioration in role functioning as well as the development of comorbidities. Such potential effects have been shown with early (childhood) interventions in anxiety (42), ADHD (61), and depression (62). This view is being tested in animal models as well; a recent study showed that “adolescent” cognitive training in juveniles prevented adult cognitive control impairment in rats with neonatal ventral hippocampus lesions, an established neurodevelopmental model of schizophrenia (63). Clearly, once effective CT approaches have been developed, they will prove highly useful if delivered pre-emptively across a broad range of psychopathologies.
Genotype
Given that genetic factors regulate plasticity and cognition, genotype will likely moderate response to CT in people with mental illnesses. Genetic variability related to the catechol-O-methyl transferase (COMT) gene (Val158Met polymorphism), a gene regulating prefrontal dopamine levels, has been investigated as one such factor. Bosia et al. (64) reported a greater response to CT in schizophrenia patients who were COMT Met carriers, though no such relationship was observed by Greenwood et al. (65). However, using an intensive computerized CT paradigm in schizophrenia patients, Panizzuttii et al. showed an association between 8 SNPs at the 3′ end of the COMT gene and global cognition improvement (66). BDNF polymorphisms have been associated with greater gains in response to CT in older adults (67). Genetic factors might interact with one another; in healthy adults, the interaction of BDNF with COMT polymorphisms demonstrates a clear role in human cortical plasticity and the genotype-related differences in neurophysiology translate into behavioral differences (68). Given the large sample sizes needed from well-defined clinical trials to determine the relationship of genotype to behavioral outcome, it will be many years before we have definitive answers on the role of specific genes and gene interactions in CT response.
Cognitive and brain reserve
Baseline cognitive function may be a moderator of outcome with CT ((69);, (70)). Keshavan et al. (28) have shown that pretreatment neurobiologic reserve, operationalized as whole brain surface area and gray matter volume, significantly moderated the effects of Cognitive Enhancement Therapy (CET, computerized cognitive training plus group-based social skills practice) in early-course schizophrenia. Greater reserve predicted a more rapid social-cognitive response to CET in the first year of treatment, while those with less reserve responded at a slower rate. The lower grey matter volume at baseline may itself reflect an underlying pathophysiologic process with an adverse impact on plasticity, and/or previous environmental insults and stressors that have affected neural substrate. The ability to improve basic auditory processing speed during CT in schizophrenia may be related to the overall cognitive gains made by individuals., (71), suggesting that an inherent psychophysical “learning capacity” influences the response to training. Baseline cognitive and/or “neuroplastic potential” may thus predict treatment response; lower reserve may require adjunctive physical exercise interventions, pharmacologic enhancement (72), and/or neuromodulation (73), as discussed below.
Motivation and emotional state
Factors linked to motivation, self-efficacy, and emotional state clearly play an important role in treatment response to CT. For example, it is known in healthy individuals that motivational state exerts both local and global neural effects on cognitive control operations, while a positive mood enhances prefrontal activation and facilitates creative problem-solving (74,75). Intrinsic motivation can best be fostered by providing a personalized context that links CT to goals of everyday life, and by fostering autonomy so that aspects of the training can be tailored to the learning style and goals of each participant (76). In addition, internalized beliefs influence cortical responses to feedback during learning (77) and are likely to moderate response to CT. Finally, inter-individual variations in reward sensitivity predict rates of learning, and will need to be the focus of future studies (78).
Features of Training
Training approaches
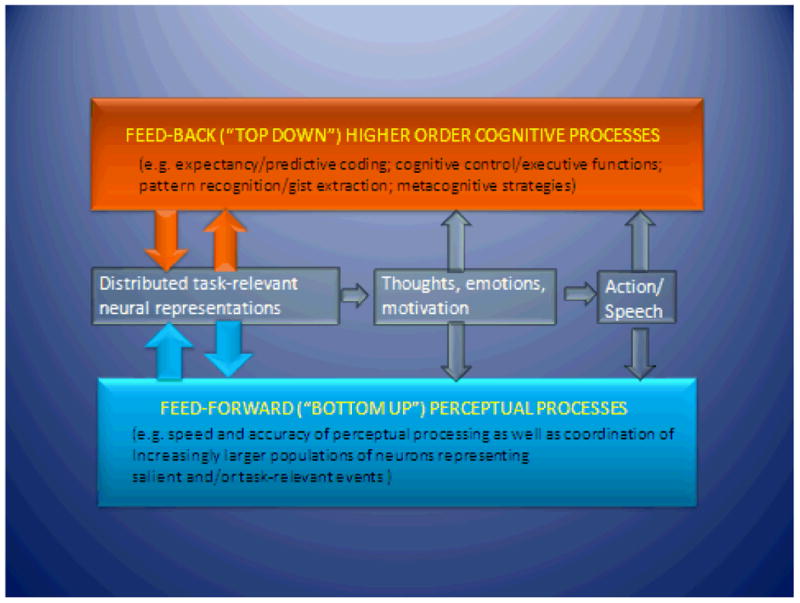
The field is barely at the threshold of understanding the key “active ingredients” for the development of training methods that maximize cognitive and functional gains in patients with mental illness. As discussed earlier, CT involves varying combinations of “top-down” or feed-back approaches (training of higher-order meta-cognitive skills, executive functioning, or strategic techniques) and “bottom-up” or feed-forward approaches (training of perceptual and attentional skills) (Figure 4). Neurocognitive operations rely on both feed-forward and feed-back operations in the brain, as these processes may not be as distinct as was once thought; their synergistic interactions may be especially critical in impaired brains (e.g., impaired perception influences higher-order cognition, and perceptual learning shows generalization in the impaired brain (79)). Thus, field may benefit from describing CT methods in terms of their relative emphasis on these levels of operations as well as their emphasis on implicit vs. explicit learning methods (15). ”Bridge” groups ((19) are an example of a “top-down” approach often combined with computerized CT in order to help patients apply newly acquired cognitive skills to real-world situations and promote socialization. As the field moves forward in the developing targeted CT methods, the key challenge will be to identify and engineer precisely defined learning trials relevant to the neural system impairments of interest, and to elucidate their behavioral and neural effects in both healthy and impaired brains.
Figure 4. Schematic representation of the interaction between feed-back and feed-forward information processing operations.
Intensity and progression of training
Several principles for achieving robust and specific integration of neural representations have been proposed and supported (15). A desired skill has to be practiced through repetitive trials on an intensive and frequent schedule. Close engagement of attention and reward systems is needed; with a high proportion of the learning trials being attended to, performed correctly, and immediately rewarded. Individualized adaptation of task difficulty helps to drive learning, so that accurate performance on initial “easy” trials is gradually led toward accurate performance on systematically more difficult trials (scaffolding), while maintaining a high trial-by-trial reward schedule. Learning tasks should drive progressively refined and detailed neural representations of task-relevant stimuli. Finally, learning approaches need to support generalization of improved function to real-world environments (such as through the use of bridging groups ((19). Learning-driven improvements in cognitive and socio-affective operations must generalize to untrained stimuli, tasks, and most importantly, to cognitively demanding real-world situations.
Adjunctive Cognitive-enhancing Interventions
Physical exercise
Physical exercise (PE) has important benefits for patients with mental disorders (80). In addition to its important metabolic effects, aerobic exercise has been associated with increased neurogenesis, angiogenesis, as well as production of neurotrophic factors such as brain-derived neurotrophic factor (BDNF) involved in neuroprotection and synaptic plasticity (81). A recent study (82) reported increased hippocampal volume in schizophrenia and healthy subjects following three months of aerobic exercise (cycling) with no change in a control condition (table football). Schizophrenia patients, but not healthy controls, showed a corresponding increase in hippocampal N-acetylaspartate, a marker of neuronal integrity. PE may promote neurocognitive function and development in ADHD (83). Combining CT with PE programs may particularly help psychiatric patients who have been sedentary. However, the field needs a more precise understanding of the effects of specific types of exercise on cognition in mental illness, and its potential interactive effects with CT.
Pharmacological agents
In schizophrenia, the field now agrees that pro-cognitive agents need to be developed and tested in combination with CT in order to obtain maximal behavioral effects (84); these issuesl also apply to other psychiatric disorders. Examples of pro-cognitive agents include the glycine agonist D-serine, the GlyT1 inhibitor sarcosine, ampakines (85), metabotropic mGlu2/3 agonists, the GABAergic agent MK-0777 (86), modafinil (which has complex effects on multiple neurotransmitter systems), and d-amphetamine (87,88). Given these various mechanisms of action, drugs with different molecular targets may potentiate CT effects in diverse clinical populations. For example, cholinergic enhancement with donepezil has been found to improve perceptual learning in healthy humans (89), but its effects in clinical groups is unknown. Dopaminergic and cholinergic agents may potentially enhance reward related learning and thereby augment cognitive enhancement (90). A great deal of preclinical and clinical work lies ahead.
Brain neuromodulation
Neuromodulation approaches, such as Transcranial Magnetic Stimulation (TMS), Transcranial Direct Current Stimulation (tDCS), and Vagal Nerve Stimulation (VNS), can potentially enhance cognition by modulating neuronal excitability (91). Effects of brain stimulation may be determined by the initial neural activation state (92); thus, manipulating neural activation states may allow one to selectively enhance activity in a given neural circuit. Andrews et al. (93) showed that in healthy controls, tDCS applied while performing an n-back working memory task resulted in better performance, compared with tDCS applied while at rest and sham tDCS. Ditye et al. (94) reported that anodal tDCS combined with cognitive training was able to improve performance on the stop signal task. Animal work indicates that pairing VNS with specific learning events increases the cortical representations of those events (95). Systematic studies of specific neuromodulatory interventions combined with CT are needed in various clinical populations. It is unclear whether adjunctive neuromodulation will be most useful in illnesses characterized by widespread neural deficits and cortical inefficiency (e.g., schizophrenia), or as a “learning accelerator” in illnesses characterized by more circumscribed representational impairments (e.g., ADHD or anxiety disorders).
Trial Design Issues
Several critical methodological issues must be addressed as the field moves forward in the development and testing of CT approaches for psychiatric illness (see Table 1)(96).
Table 1. Points for consideration in the design, conduct and review of CT Intervention research.
Participant Characterization:
|
Intervention Targets:
|
Outcome assessment:
|
Concomitant treatments:
|
Comparison condition:
|
Scalability/potential for dissemination:
|
Design Considerations
|
First, the etiologic and phenotypic heterogeneity of mental disorders makes it unlikely that particular domain-specific neurocognitive deficits will be universal to all individuals within a diagnostic category. Different cognitive phenotypes may have a similar clinical presentation; not all individuals will demonstrate specific cognitive deficits of interest; and specific cognitive deficits may cut across disorders and healthy states (97). This raises the question of how to operationally define “caseness” in selecting individuals for intervention studies. Thus, if the entry criteria consider only diagnostic criteria without addressing baseline neurocognitive heterogeneity, individuals for whom the proposed CT intervention is not relevant could inadvertently be included in the sample.
Second, if we are to develop prescriptive, personalized intervention strategies, we must design CT studies that include dense multi-modal baseline assessments of participants so that we can determine both predictors of treatment response and factors associated with successful target engagement. In this manner, the feasibility of assessing putative moderators, as suggested by theory and research, could be explored in pilot studies. These putative moderators could then be examined in formal moderator analyses in subsequent studies with larger samples. Robust moderators thus identified could then be used as tailoring variables for more prescriptive treatment assignment. For example, neurobiologic measures derived from imaging, electrophysiology or TMS (98) might be used to assess neural reserve or neuroplastic capacity. Genotype, reward sensitivity, motivational state, and internalized beliefs might also be important baseline predictive factors.
Third, understanding the mechanisms driving behavioral change requires us to define the treatment target of the CT intervention under study and to demonstrate target engagement. Accordingly, preliminary proof of concept studies would seek to demonstrate that the intervention results in hypothesized changes in the hypothesized proximal target (e.g., a specific brain circuit, or a psychological process, such as working memory or attention bias),. Larger studies would then formally interrogate mediational pathways and examine whether changes in the presumed target mediate or translate into clinical benefit.
We must also control for non-specific factors such as concomitant therapies that can influence behavioral outcomes unrelated to CT. In schizophrenia (1), meta-analyses suggest that adjunctive psychosocial rehabilitation enhancesCT's impact on functional outcomes, but the specificity of this effect is unclear. Future studies need to quantify extra-protocol interventions, and assess outcomes to identify synergistic benefits and to develop optimized interventions.
A final but highly important consideration involves the dissemination of CT into practice. Many factors contribute to delays in the research-to-practice translation (99) including fundamental differences between efficacy studies and the usual care context. This research-to-practice “gap” has prompted calls to re-think the intervention development and testing process. For example, a “deployment-focused” model of intervention development and testing (100), emphasizes incorporating information about typical patients, providers, and settings and stakeholder perspectives (e.g., consumers, family members, providers, administrators, insurers and payers) much earlier in the intervention testing process. Careful consideration of patient characteristics (e.g., common comorbidities), candidate interventions (e.g., scalability, complexity, patient burden, costs), potential providers (e.g., current competencies, training needs) and settings (e.g., capacity, competing demands, supervision infrastructure, reimbursement structure) will also likely facilitate the development of more practice-ready, scalable, cost-efficient interventions.
Conclusions and Future Directions
Current evidence indicates that CT interventions can result in significant, albeit modest, improvements in specific cognitive functions (e.g., memory, attention, problem-solving) across a range of mental illnesses. In parallel, the mechanisms underlying neuroplasticity as well as neural circuitry alterations in psychiatric disorders are increasingly well understood. These developments are promising, especially given the limited efficacy of pharmacological interventions in improving cognitive and socio-affective processing. However, effects of CT (as currently studied) on symptoms and everyday functioning, and their durability vary across clinical populations and need further study.
The field is in its infancy and rigorously-designed, adequately powered, randomized controlled trials are needed that, in addition to testing efficacy, also investigate variability of treatment response, and determine mediators and moderators of CT effects. Future studies must also incorporate measures of the hypothesized mechanisms of action and of target engagement, as well as determine the time course and time scale of exposure needed for enduring changes in neurocognition and functioning. The synergistic effects of combining CT with other interventions is also a highly promising area of inquiry (e.g., CT plus physical exercise in ADHD, CT plus group social skills training for schizophrenia and autism).
CT's promise as a safe preventive and early intervention for individuals at younger ages and at earlier stages of illness highlights the need to design engaging and developmentally-appropriate methods, which may or may not be computer-based. Even more compelling is the prospect of early “prodromal” detection and intervention for impaired neural systems in order to pre-empt the onset of full-blown illness. There may even be the possibility of primary prevention of mental disorders if neural biomarkers of vulnerability can be found and improved through CT, although ethical issues related to “labeling” of individuals without overt symptoms will need to be addressed. Finally, our field must develop models to disseminate efficacious CT approaches into community settings, and to emphasize “deployment-focused” models of intervention development.
Acknowledgments
Matcheri Keshavan has been supported by NIH grants MH092440 and MH060902. He has also received a grant from Sunovion. Dr. Vinogradov has been supported by NIH grants MH068725, MH082818, MH068725, MH081051, MH081807 and the Stanley Medical Research Institute.
Footnotes
Disclosures: This work is based on a workshop sponsored by the National Institute of Mental Health, National Institutes of Health, and held on April 9 & 10, 2012. The opinions and assertions contained in this article are the private views of the authors and are not to be considered as official or as reflecting the views of the Department of Health and Human Services, the National Institutes of Health, or the NIMH.
Workshop Participants: Matcheri Keshavan, Harvard University (co-chair); Ann Wagner, NIMH (co-chair); Yair Bar-Haim, Tel Aviv University; Cameron Carter, University of California Davis; David Chambers, NIMH; Bruce Cuthbert, NIMH; Daniel Dickstein, Bradley Hospital/Brown University; Amy Dorin, FEGS Health and Human Services System, New York; Shaun Eack, University of Pittsburgh; Amit Etkin, Stanford University; Adam Gazzaley, University of California San Francisco; Russ Glasgow, National Cancer Institute; Adam Haim, NIMH; Jeffrey Halperin, Queens College; Courtenay Harding, The Coalition of Behavioral Health Agencies, New York; Robert Heinssen, NIMH; Thomas Insel, NIMH; Wendy Kates, SUNY Upstate Medical Center Ellen Leibenluft, NIMH; Susan McGurk, Boston University; Alice Medalia, Columbia University; Sarah Morris, NIMH; Alvaro Pascual-Leone, Harvard Medical School; Daniel Pine, NIMH; Judith Rumsey, NIMH; Christopher Sarampote, NIMH; Suzy Scherf, Pennsylvania State University; Joel Sherrill, NIMH; David Sommers, NIMH; Leanne Tamm, Cincinnati Children's Hospital Medical Center; Carol Tamminga, University of Texas Southwestern Medical Center; Sophia Vinogradov, University of California San Francisco; Bruce Wexler, Yale University; Til Wykes, University of London Institute of Psychiatry.
Sophia Vinogradov is a consultant to Brain Plasticity Institute, a company with a commercial interest in cognitive training software. She is also a consultant to Genentech, EnVIvo, and Hoffman La Roche. Other authors do not report any disclosures.
Contributor Information
Matcheri S. Keshavan, Department of Psychiatry, Beth Israel Deaconess Medical Center and Harvard Medical School, Boston MA.
Sophia Vinogradov, Department of Psychiatry, University of California, San Francisco, San Francisco VA Medical Center, San Francisco CA.
Judith Rumsey, Clinical Neuroscience Research Branch, Division of Adult Translational Research and Treatment Development, National Institute of Mental Health, Bethesda, MD.
Joel Sherrill, Treatment & Preventive Intervention Research Branch, Division of Services and Intervention Research, National Institute of Mental Health, Bethesda, MD
Ann Wagner, Neurobehavioral Mechanisms of Mental Disorders Branch, Division of Developmental Translational Research, National Institute of Mental Health, Bethesda, MD.
Bibliography
- 1.Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes. The American journal of psychiatry. 2011;168(5):472–85. doi: 10.1176/appi.ajp.2010.10060855. [DOI] [PubMed] [Google Scholar]
- 2.Boake C. Paul H Brookes Pub Co. 1991. History of cognitive rehabilitation following head injury Cognitive rehabilitation for persons with traumatic brain injury: A functional approach. [Google Scholar]
- 3.Greenough WT, Black JE, Wallace CS. Experience and brain development. Child Dev. 1987 Jun;58(3):539–59. [PubMed] [Google Scholar]
- 4.Shatz CJ, Stryker MP. Ocular dominance in layer IV of the cat's visual cortex and the effects of monocular deprivation. J Physiol (Lond) 1978 Aug;281:267–83. doi: 10.1113/jphysiol.1978.sp012421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppola DM, Purves HR, McCoy AN, Purves D. The distribution of oriented contours in the real world. Proceedings of the National Academy of Sciences. 1998;95(7):4002–6. doi: 10.1073/pnas.95.7.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jenkins WM, Merzenich MM, Ochs MT, Allard T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63(1):82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 7.Recanzone GH, Merzenich MM, Jenkins WM, Grajski KA, Dinse HR. Topographic reorganization of the hand representation in cortical area 3b owl monkeys trained in a frequency-discrimination task. Journal of Neurophysiology. 1992;67(5):1031–56. doi: 10.1152/jn.1992.67.5.1031. [DOI] [PubMed] [Google Scholar]
- 8.Recanzone GH, Schreiner C, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. The Journal of Neuroscience. 1993;13(1):87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X, Merzenich MM, Sameshima K, Jenkins WM. Remodelling of hand representation in adult cortex determined by timing of tactile stimulation. Nature. 1995;378(6552):71–5. doi: 10.1038/378071a0. [DOI] [PubMed] [Google Scholar]
- 10.Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16(2):785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cruikshank SJ, Weinberger NM. Evidence for the Hebbian hypothesis in experience-dependent physiological plasticity of neocortex: a critical review. Brain Res Brain Res Rev. 1996;22(3):191–228. doi: 10.1016/s0165-0173(96)00015-x. [DOI] [PubMed] [Google Scholar]
- 12.Buonomano DV, Merzenich MM. CORTICAL PLASTICITY: From Synapses to Maps. Annual Review of Neuroscience. 1998;21(1):149–86. doi: 10.1146/annurev.neuro.21.1.149. [DOI] [PubMed] [Google Scholar]
- 13.Spaulding WD, Reed D, Sullivan M, Richardson C, Weiler M. Effects of cognitive treatment in psychiatric rehabilitation. Schizophr Bull. 1999;25(4):657–76. doi: 10.1093/oxfordjournals.schbul.a033409. [DOI] [PubMed] [Google Scholar]
- 14.Kleim JA, Jones TA. Principles of Experience-Dependent Neural Plasticity: Implications for Rehabilitation After Brain Damage. J Speech Lang Hear Res. 2008 Feb 1;51(1):S225–239. doi: 10.1044/1092-4388(2008/018). [DOI] [PubMed] [Google Scholar]
- 15.Vinogradov S, Fisher M, de Villers-Sidani E. Cognitive training for impaired neural systems in neuropsychiatric illness. Neuropsychopharmacology. 2012;37:43–76. doi: 10.1038/npp.2011.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Subramaniam K, Luks TL, Fisher M, Simpson GV, Nagarajan S, Vinogradov S. Computerized cognitive training restores neural activity within the reality monitoring network in schizophrenia. Neuron. 2012 Feb 23;73(4):842–53. doi: 10.1016/j.neuron.2011.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNab F, Varrone A, Farde L, Jucaite A, Bystritsky P, Forssberg H, et al. Changes in cortical dopamine D1 receptor binding associated with cognitive training. Science Signalling. 2009;323(5915):800. doi: 10.1126/science.1166102. [DOI] [PubMed] [Google Scholar]
- 18.Bowie C, Gupta M, Holshausen K. Cognitive remediation therapy for mood disorders: rationale, early evidence, and future directions. Can J Psychiatry. 2013 Jun;58(6):319–25. doi: 10.1177/070674371305800603. [DOI] [PubMed] [Google Scholar]
- 19.Medalia A, Choi J. Cognitive Remediation in Schizophrenia. Neuropsychology Review. 2009 May 15;19(3):353–64. doi: 10.1007/s11065-009-9097-y. [DOI] [PubMed] [Google Scholar]
- 20.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhé HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev. 2013 Aug 6; doi: 10.1016/j.neubiorev.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 21.Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011 Sep;168(9):968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- 22.Arguello PA, Gogos JA. Genetic and cognitive windows into circuit mechanisms of psychiatric disease. Trends in neurosciences. 2011 doi: 10.1016/j.tins.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Wykes T, Reeder C, Williams C, Corner J, Rice C, Everitt B. Are the effects of cognitive remediation therapy (CRT) durable? Results from an exploratory trial in schizophrenia. Schizophr Res. 2003 Jun 1;61(2-3):163–74. doi: 10.1016/s0920-9964(02)00239-6. [DOI] [PubMed] [Google Scholar]
- 24.Eack SM, Greenwald DP, Hogarty SS, Keshavan MS. One-year durability of the effects of cognitive enhancement therapy on functional outcome in early schizophrenia. Schizophr Res. 2010 Jul;120(1-3):210–6. doi: 10.1016/j.schres.2010.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurtz MM. Cognitive remediation for schizophrenia: current status, biological correlates and predictors of response. Expert Rev Neurother. 2012 Jul;12(7):813–21. doi: 10.1586/ern.12.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huddy V, Reeder C, Kontis D, Wykes T, Stahl D. The effect of working alliance on adherence and outcome in cognitive remediation therapy. J Nerv Ment Dis. 2012 Jul;200(7):614–9. doi: 10.1097/NMD.0b013e31825bfc31. [DOI] [PubMed] [Google Scholar]
- 27.Kontis D, Huddy V, Reeder C, Landau S, Wykes T. Effects of Age and Cognitive Reserve on Cognitive Remediation Therapy Outcome in Patients With Schizophrenia. Am J Geriatr Psychiatry. 2012 Mar 9; doi: 10.1016/j.jagp.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Keshavan MS, Eack SM, Wojtalik JA, Prasad KMR, Francis AN, Bhojraj TS, et al. A broad cortical reserve accelerates response to cognitive enhancement therapy in early course schizophrenia. Schizophr Res. 2011 Aug;130(1-3):123–9. doi: 10.1016/j.schres.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eack SM, Hogarty GE, Cho RY, Prasad KMR, Greenwald DP, Hogarty SS, et al. Neuroprotective effects of cognitive enhancement therapy against gray matter loss in early schizophrenia: results from a 2-year randomized controlled trial. Arch Gen Psychiatry. 2010 Jul;67(7):674–82. doi: 10.1001/archgenpsychiatry.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haut KM, Lim KO, MacDonald A. Prefrontal cortical changes following cognitive training in patients with chronic schizophrenia: effects of practice, generalization, and specificity. Neuropsychopharmacology. 2010;35(9):1850–9. doi: 10.1038/npp.2010.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beck SJ, Hanson CA, Puffenberger SS, Benninger KL, Benninger WB. A controlled trial of working memory training for children and adolescents with ADHD. J Clin Child Adolesc Psychol. 2010;39(6):825–36. doi: 10.1080/15374416.2010.517162. [DOI] [PubMed] [Google Scholar]
- 32.Holmes J, Gathercole SE, Dunning DL. Adaptive training leads to sustained enhancement of poor working memory in children. Developmental Science. 2009;12(4):F9–F15. doi: 10.1111/j.1467-7687.2009.00848.x. [DOI] [PubMed] [Google Scholar]
- 33.Karbach J, Kray J. How useful is executive control training? Age differences in near and far transfer of task-switching training. Developmental science. 2009;12(6):978–90. doi: 10.1111/j.1467-7687.2009.00846.x. [DOI] [PubMed] [Google Scholar]
- 34.Klingberg T, Fernell E, Olesen PJ, Johnson M, Gustafsson P, Dahlstrom K, et al. Computerized training of working memory in children with ADHD--a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry. 2005;44(2):177–86. doi: 10.1097/00004583-200502000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Klingberg T. Training and plasticity of working memory. Trends Cogn Sci. 2010;14(7):317–24. doi: 10.1016/j.tics.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Halperin JM, Marks DJ, Bedard ACV, Chacko A, Curchack JT, Yoon CA, et al. Training executive, attention, and motor skills: a proof-of-concept study in preschool children with ADHD. Journal of attention disorders. 2012 doi: 10.1177/1087054711435681. [DOI] [PubMed] [Google Scholar]
- 37.Tamm L, Epstein JN, Peugh JL, Nakonezny PA, Hughes CW. Preliminary data suggesting the efficacy of attention training for school-aged children with ADHD. Dev Cogn Neurosci. 2012 Nov 15; doi: 10.1016/j.dcn.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutledge KJ, van den Bos W, McClure SM, Schweitzer JB. Training cognition in ADHD: current findings, borrowed concepts, and future directions. Neurotherapeutics. 2012 Jul;9(3):542–58. doi: 10.1007/s13311-012-0134-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-Related Attentional Bias in Anxious and Nonanxious Individuals: A Meta-Analytic Study. Psychological Bulletin. 2007;133(1):1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- 40.Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, et al. Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biological psychiatry. 2010;68(11):982–90. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: a meta-analytic review. Behav Ther. 2012 Dec;43(4):724–40. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, et al. Attention Bias Modification Treatment for Pediatric Anxiety Disorders: A Randomized Controlled Trial. Am J Psychiatry. 2012 Feb 1;169(2):213–20. doi: 10.1176/appi.ajp.2011.11060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bowie CR, McGurk SR, Mausbach B, Patterson TL, Harvey PD. Combined Cognitive Remediation and Functional Skills Training for Schizophrenia: Effects on Cognition, Functional Competence, and Real-World Behavior. Am J Psychiatry. 2012 May 11; doi: 10.1176/appi.ajp.2012.11091337. [DOI] [PubMed] [Google Scholar]
- 44.Lee RSC, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. J Affect Disord. 2012 Oct;140(2):113–24. doi: 10.1016/j.jad.2011.10.023. [DOI] [PubMed] [Google Scholar]
- 45.Xu G, Lin K, Rao D, Dang Y, Ouyang H, Guo Y, et al. Neuropsychological performance in bipolar I, bipolar II and unipolar depression patients: a longitudinal, naturalistic study. J Affect Disord. 2012 Feb;136(3):328–39. doi: 10.1016/j.jad.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 46.Yang W, Zhu X, Wang X, Wu D, Yao S. Time course of affective processing bias in major depression: An ERP study. Neuroscience letters. 2011;487(3):372–7. doi: 10.1016/j.neulet.2010.10.059. [DOI] [PubMed] [Google Scholar]
- 47.Victor TA, Furey ML, Fromm SJ, Bellgowan PSF, Öhman A, Drevets WC. The extended functional neuroanatomy of emotional processing biases for masked faces in major depressive disorder. PLoS ONE. 2012;7(10):e46439. doi: 10.1371/journal.pone.0046439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Deckersbach T, Nierenberg AA, Kessler R, Lund HG, Ametrano RM, Sachs G, et al. RESEARCH: Cognitive rehabilitation for bipolar disorder: An open trial for employed patients with residual depressive symptoms. CNS Neurosci Ther. 2010 Oct;16(5):298–307. doi: 10.1111/j.1755-5949.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anaya C, Martinez Aran A, Ayuso-Mateos JL, Wykes T, Vieta E, Scott J. A systematic review of cognitive remediation for schizo-affective and affective disorders. J Affect Disord. 2012 Dec 15;142(1-3):13–21. doi: 10.1016/j.jad.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 50.Browning M, Grol M, Ly V, Goodwin GM, Holmes EA, Harmer CJ. Using an experimental medicine model to explore combination effects of pharmacological and cognitive interventions for depression and anxiety. Neuropsychopharmacology. 2011 Dec;36(13):2689–97. doi: 10.1038/npp.2011.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bickel WK, Yi R, Landes RD, Hill PF, Baxter C. Remember the future: working memory training decreases delay discounting among stimulant addicts. Biol Psychiatry. 2011;69(3):260–5. doi: 10.1016/j.biopsych.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J. Retraining automatic action tendencies changes alcoholic patients' approach bias for alcohol and improves treatment outcome. Psychol Sci. 2011 Apr;22(4):490–7. doi: 10.1177/0956797611400615. [DOI] [PubMed] [Google Scholar]
- 53.Sofuoglu M, DeVito EE, Waters AJ, Carroll KM. Cognitive enhancement as a treatment for drug addictions. Neuropharmacology. 2013 Jan;64:452–63. doi: 10.1016/j.neuropharm.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bishop-Fitzpatrick L, Minshew NJ, Eack SM. A Systematic Review of Psychosocial Interventions for Adults with Autism Spectrum Disorders. J Autism Dev Disord. 2014 Jul 24; doi: 10.1007/s10803-012-1615-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Eack SM, Greenwald DP, Hogarty SS, Bahorik AL, Litschge MY, Mazefsky CA, et al. Cognitive Enhancement Therapy for Adults with Autism Spectrum Disorder: Results of an 18-month Feasibility Study. J Autism Dev Disord. 2013 Apr 26; doi: 10.1007/s10803-013-1834-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Damiano C, Churches O, Ring H, Baron-Cohen S. The development of perceptual expertise for faces and objects in autism spectrum conditions. Autism Res. 2011 Aug;4(4):297–301. doi: 10.1002/aur.205. [DOI] [PubMed] [Google Scholar]
- 57.Gabrieli JDE. Dyslexia: a new synergy between education and cognitive neuroscience. Science. 2009 Jul 17;325(5938):280–3. doi: 10.1126/science.1171999. [DOI] [PubMed] [Google Scholar]
- 58.Huckans M, Hutson L, Twamley E, Jak A, Kaye J, Storzbach D. Efficacy of cognitive rehabilitation therapies for mild cognitive impairment (MCI) in older adults: working toward a theoretical model and evidence-based interventions. Neuropsychol Rev. 2013 Mar;23(1):63–80. doi: 10.1007/s11065-013-9230-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011 Apr;92(4):519–30. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 60.Chung CSY, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non-progressive acquired brain damage. Cochrane Database Syst Rev. 2013;4:CD008391. doi: 10.1002/14651858.CD008391.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klingberg T. Computerized training of working memory in children with ADHD. European Neuropsychopharmacology. 2007;17:S192–S193. [Google Scholar]
- 62.Browning M, Holmes EA, Charles M, Cowen PJ, Harmer CJ. Using Attentional Bias Modification as a Cognitive Vaccine Against Depression. Biological Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee H, Dvorak D, Kao HY, Duffy ÁM, Scharfman HE, Fenton AA. Early cognitive experience prevents adult deficits in a neurodevelopmental schizophrenia model. Neuron. 2012 Aug 23;75(4):714–24. doi: 10.1016/j.neuron.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bosia M, Bechi M, Marino E, Anselmetti S, Poletti S, Cocchi F, et al. Influence of catechol-O-methyltransferase Val158Met polymorphism on neuropsychological and functional outcomes of classical rehabilitation and cognitive remediation in schizophrenia. Neurosci Lett. 2007 May 7;417(3):271–4. doi: 10.1016/j.neulet.2007.02.076. [DOI] [PubMed] [Google Scholar]
- 65.Greenwood K, Hung CF, Tropeano M, McGuffin P, Wykes T. No association between the Catechol-O-Methyltransferase (COMT) val158met polymorphism and cognitive improvement following cognitive remediation therapy (CRT) in schizophrenia. Neurosci Lett. 2011 Jun 1;496(2):65–9. doi: 10.1016/j.neulet.2011.03.075. [DOI] [PubMed] [Google Scholar]
- 66.Panizzutti R, Hamilton SP, Vinogradov S. Genetic correlate of cognitive training response in schizophrenia. Neuropharmacology. 2013 Jan;64:264–7. doi: 10.1016/j.neuropharm.2012.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Colzato LS, van Muijden J, Band GPH, Hommel B. Genetic Modulation of Training and Transfer in Older Adults: BDNF ValMet Polymorphism is Associated with Wider Useful Field of View. Front Psychol. 2011;2:199. doi: 10.3389/fpsyg.2011.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witte AV, Kürten J, Jansen S, Schirmacher A, Brand E, Sommer J, et al. Interaction of BDNF and COMT polymorphisms on paired-associative stimulation-induced cortical plasticity. J Neurosci. 2012 Mar 28;32(13):4553–61. doi: 10.1523/JNEUROSCI.6010-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kurtz MM, Wexler BE, Fujimoto M, Shagan DS, Seltzer JC. Symptoms versus neurocognition as predictors of change in life skills in schizophrenia after outpatient rehabilitation. Schizophrenia research. 2008;102(1-3):303–11. doi: 10.1016/j.schres.2008.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fiszdon JM, Cardenas AS, Bryson GJ, Bell MD. Predictors of remediation success on a trained memory task. J Nerv Ment Dis. 2005;193(9):602–8. doi: 10.1097/01.nmd.0000177790.23311.ba. [DOI] [PubMed] [Google Scholar]
- 71.Fisher M, Holland C, Merzenich MM, Vinogradov S. Using neuroplasticity-based auditory training to improve verbal memory in schizophrenia. Am J Psychiatry. 2009 Jul;166(7):805–11. doi: 10.1176/appi.ajp.2009.08050757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Floel A, Garraux G, Xu B, Breitenstein C, Knecht S, Herscovitch P, et al. Levodopa increases memory encoding and dopamine release in the striatum in the elderly. Neurobiology of Aging. 2008 Feb;29(2):267–79. doi: 10.1016/j.neurobiolaging.2006.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hummel F, Cohen LG. Improvement of Motor Function with Noninvasive Cortical Stimulation in a Patient with Chronic Stroke. Neurorehabilitation and Neural Repair. 2005 Mar 1;19(1):14–9. doi: 10.1177/1545968304272698. [DOI] [PubMed] [Google Scholar]
- 74.Subramaniam K, Kounios J, Parrish TB, Jung-Beeman M. A brain mechanism for facilitation of insight by positive affect. J Cogn Neurosci. 2009 Mar;21(3):415–32. doi: 10.1162/jocn.2009.21057. [DOI] [PubMed] [Google Scholar]
- 75.Savine AC, Braver TS. Local and global effects of motivation on cognitive control. Cogn Affect Behav Neurosci. 2012 Dec;12(4):692–718. doi: 10.3758/s13415-012-0113-y. [DOI] [PubMed] [Google Scholar]
- 76.Medalia A, Saperstein A. The role of motivation for treatment success. Schizophr Bull. 2011 Sep;37(Suppl 2):S122–128. doi: 10.1093/schbul/sbr063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mangels JA, Butterfield B, Lamb J, Good C, Dweck CS. Why do beliefs about intelligence influence learning success? A social cognitive neuroscience model. Soc Cogn Affect Neurosci. 2006;1(2):75–86. doi: 10.1093/scan/nsl013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Flagel SB, Clark JJ, Robinson TE, Mayo L, Czuj A, Willuhn I, et al. A selective role for dopamine in stimulus-reward learning. Nature. 2011 Jan 6;469(7328):53–7. doi: 10.1038/nature09588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wong YK, Folstein JR, Gauthier I. The nature of experience determines object representations in the visual system. J Exp Psychol Gen. 2012 Nov;141(4):682–98. doi: 10.1037/a0027822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Knöchel C, Oertel-Knöchel V, O'Dwyer L, Prvulovic D, Alves G, Kollmann B, et al. Cognitive and behavioural effects of physical exercise in psychiatric patients. Prog Neurobiol. 2012 Jan;96(1):46–68. doi: 10.1016/j.pneurobio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- 81.Kramer AF, Erickson KI. Capitalizing on cortical plasticity: influence of physical activity on cognition and brain function. Trends in Cognitive Sciences. 2007 Aug;11(8):342–8. doi: 10.1016/j.tics.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 82.Pajonk FG, Wobrock T, Gruber O, Scherk H, Berner D, Kaizl I, et al. Hippocampal plasticity in response to exercise in schizophrenia. Arch Gen Psychiatry. 2010;67(2):133–43. doi: 10.1001/archgenpsychiatry.2009.193. [DOI] [PubMed] [Google Scholar]
- 83.Berwid OG, Halperin JM. Emerging support for a role of exercise in attention-deficit/hyperactivity disorder intervention planning. Curr Psychiatry Rep. 2012 Oct;14(5):543–51. doi: 10.1007/s11920-012-0297-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chou HH, Twamley E, Swerdlow NR. Towards medication-enhancement of cognitive interventions in schizophrenia. Handb Exp Pharmacol. 2012;(213):81–111. doi: 10.1007/978-3-642-25758-2_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Arai AC, Kessler M. Pharmacology of ampakine modulators: from AMPA receptors to synapses and behavior. Curr Drug Targets. 2007 May;8(5):583–602. doi: 10.2174/138945007780618490. [DOI] [PubMed] [Google Scholar]
- 86.Buchanan RW, Keefe RSE, Lieberman JA, Barch DM, Csernansky JG, Goff DC, et al. A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry. 2011 Mar 1;69(5):442–9. doi: 10.1016/j.biopsych.2010.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Minzenberg MJ, Carter CS. Developing treatments for impaired cognition in schizophrenia. [cited 2011 Dec 22];Trends in Cognitive Sciences [Internet] 2011 Dec; doi: 10.1016/j.tics.2011.11.017. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1364661311002506. [DOI] [PubMed]
- 88.Barch DM, Carter CS. Amphetamine improves cognitive function in medicated individuals with schizophrenia and in healthy volunteers. Schizophr Res. 2005 Sep 1;77(1):43–58. doi: 10.1016/j.schres.2004.12.019. [DOI] [PubMed] [Google Scholar]
- 89.Rokem A, Silver MA. Cholinergic enhancement augments magnitude and specificity of visual perceptual learning in healthy humans. Curr Biol. 2010 Oct 12;20(19):1723–8. doi: 10.1016/j.cub.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Acheson DT, Twamley EW, Young JW. Reward learning as a potential target for pharmacological augmentation of cognitive remediation for schizophrenia: a roadmap for preclinical development. Front Neurosci. 2013;7:103. doi: 10.3389/fnins.2013.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Miniussi C, Cappa SF, Cohen LG, Floel A, Fregni F, Nitsche MA, et al. Efficacy of repetitive transcranial magnetic stimulation/transcranial direct current stimulation in cognitive neurorehabilitation. Brain Stimul. 2008 Oct;1(4):326–36. doi: 10.1016/j.brs.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 92.Silvanto J, Muggleton N, Walsh V. State-dependency in brain stimulation studies of perception and cognition. Trends Cogn Sci (Regul Ed) 2008 Dec;12(12):447–54. doi: 10.1016/j.tics.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Andrews SC, Hoy KE, Enticott PG, Daskalakis ZJ, Fitzgerald PB. Improving working memory: the effect of combining cognitive activity and anodal transcranial direct current stimulation to the left dorsolateral prefrontal cortex. Brain Stimul. 2011 Apr;4(2):84–9. doi: 10.1016/j.brs.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 94.Ditye T, Jacobson L, Walsh V, Lavidor M. Modulating behavioral inhibition by tDCS combined with cognitive training. Exp Brain Res. 2012 Jun;219(3):363–8. doi: 10.1007/s00221-012-3098-4. [DOI] [PubMed] [Google Scholar]
- 95.Porter BA, Khodaparast N, Fayyaz T, Cheung RJ, Ahmed SS, Vrana WA, et al. Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb Cortex. 2012 Oct;22(10):2365–74. doi: 10.1093/cercor/bhr316. [DOI] [PubMed] [Google Scholar]
- 96.Sherrill JT, Sommers DI, Nierenberg AA, Leon AC, Arndt S, Bandeen-Roche K, et al. Integrating statistical and clinical research elements in intervention-related grant applications: summary from an NIMH workshop. Acad Psychiatry. 2009 Jun;33(3):221–8. doi: 10.1176/appi.ap.33.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Constantino JN, Todd RD. Autistic traits in the general population: a twin study. Archives of General Psychiatry. 2003;60(5):524. doi: 10.1001/archpsyc.60.5.524. [DOI] [PubMed] [Google Scholar]
- 98.Pascual-Leone A, Freitas C, Oberman L, Horvath JC, Halko M, Eldaief M, et al. Characterizing Brain Cortical Plasticity and Network Dynamics Across the Age-Span in Health and Disease with TMS-EEG and TMS-fMRI. Brain Topography. 2011 Aug 14;24(3-4):302–15. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Balas EA, Weingarten S, Garb CT, Blumenthal D, Boren SA, Brown GD. Improving preventive care by prompting physicians. Arch Intern Med. 2000 Feb 14;160(3):301–8. doi: 10.1001/archinte.160.3.301. [DOI] [PubMed] [Google Scholar]
- 100.Weisz JR, Ugueto AM, Cheron DM, Herren J. Evidence-based youth psychotherapy in the mental health ecosystem. J Clin Child Adolesc Psychol. 2013;42(2):274–86. doi: 10.1080/15374416.2013.764824. [DOI] [PubMed] [Google Scholar]


