Abstract
Thymic dendritic cells (DC) mediate self-tolerance by presenting self-peptides to and depleting autoreactive thymocytes. Despite a significant role in negative selection, the events regulating thymic DC maturation and function under steady-state conditions are poorly understood. We report that crosstalk with thymocytes regulates thymic conventional DC (cDC) numbers, phenotype, and function. In mice lacking TCR-expressing thymocytes, thymic cDC were reduced and exhibited a less mature phenotype. Furthermore, thymic cDC in TCR transgenic mice lacking cognate antigen expression in the thymus were also immature; notably, however, thymic cDC maturation was reestablished by an Ag-specific cognate interaction with CD4+ or CD8+ single-positive thymocytes (SP). Blockade of CD40 ligand during Ag-specific interactions with CD4SP but not CD8SP limited the effect on cDC maturation. Together, these novel findings demonstrate that homeostatic maturation and function of thymic cDC is regulated by feedback delivered by CD4SP and CD8SP via distinct mechanisms during a cognate Ag-specific interaction.
Keywords: Cell Activation, Dendritic Cells, Thymus, Tolerance, Transgenic/Knockout Mice
INTRODUCTION
Thymocyte maturation and self-tolerance is induced by interactions with thymus-resident APC. Cortical thymic epithelial cells promote the positive selection of CD4+CD8+ double-positive thymocytes (DP), while medullary thymic epithelial cells (mTEC), which express multiple peripheral-tissue Ag, drive negative selection of autoreactive single-positive thymocytes (SP) (1). Thymic dendritic cells (DC) also induce negative selection of self-reactive thymocytes (2–5) as well as promote natural regulatory T cell development (5–8). Moreover, ablation of DC in mice has been shown to lead to either autoimmunity due to aberrant thymic tolerance (9) or dysregulation of peripheral immune homeostasis (10, 11), highlighting the broad regulatory function of DC.
DC in the thymus include three major subsets: CD8α+ conventional DC (cDC), signal regulatory protein alpha/CD172a+ (SIRPα+) cDC, and plasmacytoid DC (pDC) (12). Migratory SIRPα+ cDC and pDC ferry peripheral self-Ag to the thymus to mediate negative selection (3, 13, 14). Additionally, SIRPα+ and intrathymically-developed CD8α+ cDC uptake soluble blood-borne Ag and subsequently process and present self-epitopes to thymocytes (12, 15–17). Furthermore, thymic DC can acquire Ag expressed by mTEC (18–21), which expands the self-Ag pool presented by thymic DC. Interestingly, thymic DC express elevated levels of MHC and costimulatory molecules, which correlate with enhanced T cell stimulatory capacity relative to resting peripheral (e.g. splenic) DC (5, 7, 22, 23). The latter is expected to enhance the efficacy of thymic negative selection. Little is known, however, about the factors that regulate thymic DC homeostasis. Herein we show that increased activation and maturation of steady-state thymic DC required Ag-specific interactions with CD4SP or CD8SP. CD4SP- but not CD8SP-derived feedback was abrogated by blockade of CD40L. Thus, DC:thymocyte crosstalk is critical for the maintenance of thymic DC phenotypic activation and function.
MATERIALS AND METHODS
Mice
NOD/LtJ (NOD), NOD/BDC2.5, NOD.TCRα−/−, NOD/BDC2.5 × NOD.TCRα−/− (BDC2.5/TCRα−/−), and NOD.Clone 4 TCR transgenic × NOD.scid (CL4.scid) mice have been described (24, 25) and were housed in specific pathogen-free facilities at the University of North Carolina at Chapel Hill (UNC-CH). All procedures were approved by the UNC-CH Institutional Animal Care and Use Committee.
Cell isolation and culture
Thymi from 3- to 6-wk old mice were digested with 1 mg/mL collagenase D and 20 µg/mL DNAse I (Roche) for 30 min at room temperature. EDTA was included for the final 5 min. DC were enriched using an OptiPrep gradient (Axis-Shield) and purified (>95%) via FACS. Splenic T cells were purified using a CD4+ Isolation Kit (Miltenyi Biotec).
To measure T cell proliferation, T cells were labeled with 5 µM CellTrace Violet (Invitrogen), co-cultured with peptide-pulsed DC at a 1:10 DC:T cell ratio for 3 d, and assessed by FACS. Division Index was calculated with FlowJo (TreeStar).
FACS
mAb and streptavidin were purchased from BD Biosciences, eBioscience, BioLegend, or Invitrogen. Cells were incubated with αCD16/32 (2.4G2) to block FcR prior to Ab staining. Dead cells were excluded using propidium iodide, DAPI, or LIVE/DEAD stain (Invitrogen). For IL-12 (p40) staining, DC were cultured for 2–4 h with 10 µg/mL Brefeldin A (Sigma-Aldrich), fixed and permeabilized with BD Cytofix/Cytoperm solutions. Data were acquired on a BD LSR II and analyzed with FlowJo.
DC localization
Thymic sections (7-µm) were stained with rabbit αKeratin 5 (Covance), biotin αDEC-205 (BioLegend), and Alexa Fluor 647 αCD11c (eBioscience) followed by Alexa Fluor 488 goat αrabbit IgG (Invitrogen) and Alexa Fluor 594 streptavidin (Invitrogen). Images (10×) were acquired using a LSM 710 spectral laser-scanning confocal microscope and ZEN software (Zeiss). Image analysis was performed in ImageJ: channels were split and converted to binary with automatic thresholding (negative = 0; positive = 255). Mean intensity of CD11c was measured for a given area of Keratin 5+ medulla or DEC-205+ cortex. This was performed for 3 separate medulla and cortex zones per image, averaged, and recorded as 1 data point.
mAb production and peptides
The αCD40L blocking mAb MR1 (ATCC CRL-2580) and αCD40 agonist mAb 1C10 were produced in-house. Control animals received either PBS or rat IgG2a isotype control (2A3, BioXCell). The super BDC agonist (sBDC, RTRPLWVRME) and influenza hemagglutinin (HA, IYSTVASSL) peptides were produced at ≥95% purity by the UNC High-Throughput Peptide Synthesis and Array Facility.
Statistical analysis
Statistical analyses were performed using GraphPad Prism software. Unless otherwise indicated, significance was calculated by ANOVA with Bonferroni posttest.
RESULTS AND DISCUSSION
SP are necessary for normal thymic cDC numbers, phenotype, and function
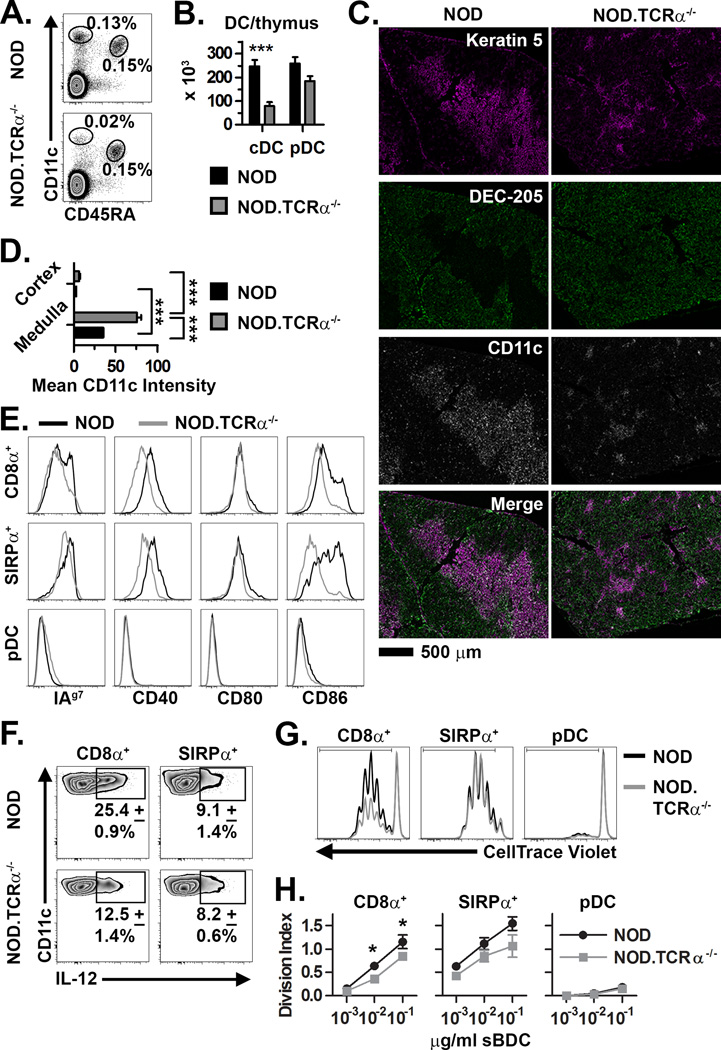
Similar to C57BL/6 (B6) mice (5, 7, 22, 23), we found NOD mouse thymic DC expressed significantly higher levels of MHC I, MHC II, CD40 and CD86, but not CD80, compared to resting splenic DC, and also more efficiently stimulated CD4+ and CD8+ T cell proliferation (Supplemental Fig. 1). We hypothesized that the increased activation and maturation status of thymic DC is regulated by cognate interactions with SP. Accordingly, thymic DC from NOD.TCRα−/− mice, in which CD4SP and CD8SP development is blocked were studied. While overall thymus cellularity is unaffected by TCRα deficiency (26, 27), the frequency and number of thymic CD11chi cDC were reduced 3- to 5-fold in NOD.TCRα−/− versus NOD mice (Fig. 1A,B); pDC were unaffected, however. We also investigated whether DC localization was disrupted in the NOD.TCRα−/− thymus, which lacks an orderly medulla (27–29). As expected, the majority of CD11c+ thymic DC resided in the well-organized medulla of NOD thymi. In comparison, NOD.TCRα−/− thymi contained only small, disorganized medullary “islands” in which CD11c+ cells were enriched (Fig. 1C,D). Further analyses confirmed that both cDC and pDC predominantly localized to the medulla in NOD and NOD.TCRα−/− thymi (unpublished observations). Thus, the organization of DC in NOD.TCRα−/− thymi mirrors that of the decreased and disrupted medulla, suggesting that thymic DC cellularity is linked to medulla size.
Figure 1. Dysregulation of thymic DC in NOD mice lacking SP.
(A) Frequency and (B) absolute number (±SEM) of thymic cDC and pDC in NOD and NOD.TCRα−/− thymi (n=8 each). (C) Staining of thymic sections for Keratin 5+ mTEC, DEC-205+ cTEC, and CD11c+ DC. (D) Quantification of mean CD11c intensity per unit area (±SEM) in the thymic medulla and cortex (n=12 sections each). (E) Analysis of MHC and costimulatory molecule expression by NOD and NOD.TCRα−/− thymic DC. Data are representative of 4 experiments. (F) Constitutive intracellular IL-12 expression (±SD from 3 experiments) by thymic cDC from NOD and NOD.TCRα−/− mice. (G) DC subsets were FACS-sorted from NOD and NOD.TCRα−/− thymi and BDC2.5 CD4+ T cell proliferation measured. Histograms are gated on live/Thy1.2+/CD4+ cells from representative co-cultures with 10−2 µg/ml sBDC-pulsed DC subsets. (H) Division Index (±SEM) calculated from cells proliferating in Panel G. Data represent 3 pooled experiments. *, P<0.05; ***, P<0.001.
Next, the activation and functional status of thymic DC in NOD.TCRα−/− mice were assessed. NOD.TCRα−/− versus NOD thymic cDC expressed decreased MHC II, CD40 and CD86 whereas CD80 levels remained unchanged (Fig. 1E). Interestingly, constitutive expression of IL-12, a cytokine implicated in the deletion of CD4SP (30, 31), was readily detected in NOD thymic cDC, especially CD8α+ cDC; however the frequency of IL-12-producing thymic CD8α+ cDC was decreased 2-fold in NOD.TCRα−/− mice (Fig. 1F). ELISA confirmed that IL-12p70 was secreted by thymic DC (unpublished observations). To test DC stimulatory capacity, CD8α+ cDC, SIRPα+ cDC, and pDC were FACS-sorted from NOD and NOD.TCRα−/− thymi, pulsed with sBDC agonist peptide, and then co-cultured with BDC2.5 CD4+ T cells. NOD CD8α+ cDC induced significantly more BDC2.5 CD4+ T cell proliferation than NOD.TCRα−/− CD8α+ cDC (Fig. 1G,H). BDC2.5 CD4+ T cell proliferation was also increased by NOD versus NOD.TCRα−/− SIRPα+ cDC although this trend did not reach statistical significance. Thymic pDC from either NOD and NOD.TCRα−/− mice induced only low levels of proliferation. Overall, these data indicate that thymic cDC but not pDC number, phenotype, and function are significantly altered in the absence of SP. With the latter in mind our subsequent efforts focused on thymic cDC.
Ag-specific feedback regulates homeostatic thymic cDC maturation
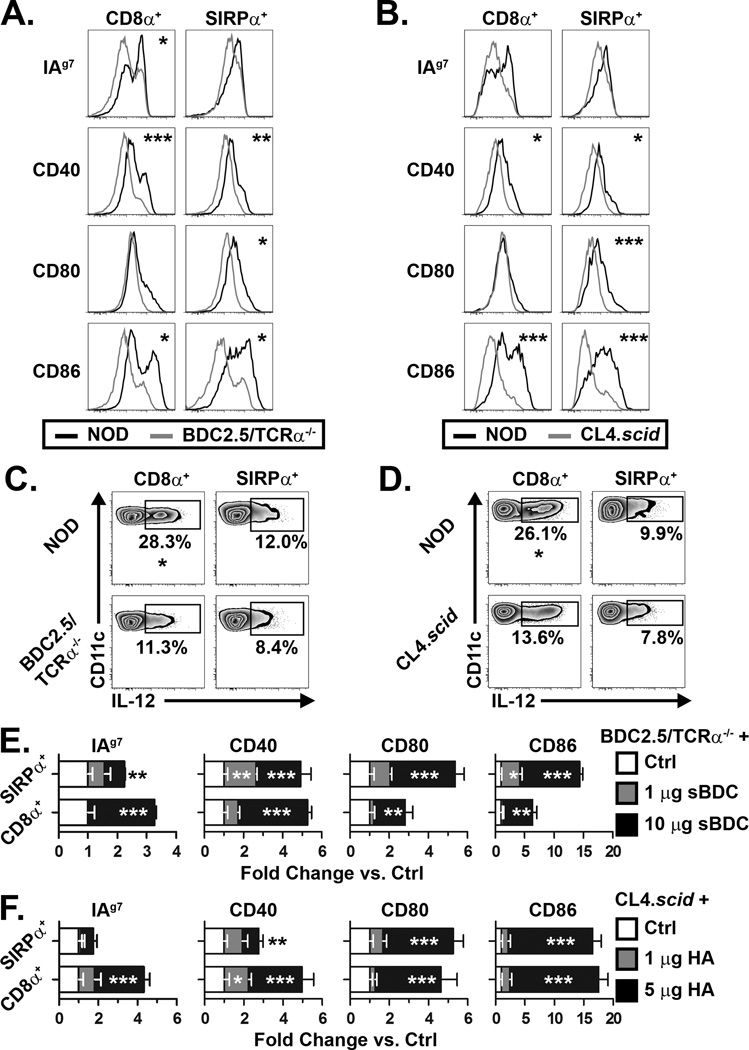
Mature CD4+ and CD8+ T cells provide distinct modes of feedback to regulate peripheral DC maturation and effector function during a cognate immune response. Accordingly, whether CD4SP and CD8SP have distinct effects on thymic cDC homeostasis was studied using BDC2.5/TCRα−/− and CL4.scid mice in which only CD4+ T cells or CD8+ T cells develop, respectively. Despite a significant SP thymocyte pool, thymic cDC from either BDC2.5/TCRα−/− or CL4.scid mice resembled NOD.TCRα−/− thymic cDC (Fig. 1), marked by a less mature phenotype (Fig. 2A,B) and reduced frequency of IL-12+ cells (Fig. 2C,D) compared to wild-type NOD thymic cDC.
Figure 2. Ag-specific interactions with SP regulate thymic DC activation status.
(A–B) MHC and costimulatory molecule expression by thymic cDC isolated from NOD and (A) BDC2.5/TCRα−/− or NOD and (B) CL4.scid mice. (C–D) Constitutive IL-12 production by thymic cDC from NOD and (C) BDC2.5/TCRα−/− or (D) CL4.scid mice. (E) BDC2.5/TCRα−/− or (F) CL4.scid mice were injected i.v. with sBDC or HA, respectively, or PBS (Ctrl), and 16–18 h later MHC and costimulatory molecule expression by thymic DC measured. Values are expressed as fold change in mean fluorescence intensity versus Ctrl (normalized to 1). Inset asterisks represent comparison to Ctrl. Data are representative of 3–5 experiments. *, P<0.05; **, P<0.01; ***, P<0.001.
In BDC2.5/TCRα−/− and CL4.scid mice thymocytes undergo positive but minimal (if any) negative selection due to the lack of thymic expression of the cognate Ag. This suggested that thymic cDC phenotype was regulated by Ag-dependent interactions with thymocytes. To test this BDC2.5/TCRα−/− and CL4.scid mice were injected with cognate peptide, sBDC and HA, respectively, to facilitate direct interaction between DC and thymocytes. As expected, thymocyte apoptosis and activation were detected after peptide injection (unpublished observations). Thymic cDC from BDC2.5/TCRα−/− mice injected with 10 µg sBDC exhibited enhanced activation relative to controls (Fig. 2E). Interestingly, at a reduced dose of 1 µg sBDC, only SIRPα+ cDC in BDC2.5/TCRα−/− mice upregulated MHC and costimulatory molecules (Fig. 2E), suggesting increased sensitivity to CD4SP feedback and/or enhanced uptake and presentation of soluble Ag. Similarly, thymic cDC expression of MHC and costimulatory molecules was increased in an HA dose-dependent manner in CL4.scid mice (Fig. 2F). Importantly, we confirmed that activation of peripheral T cells is not responsible for thymic cDC activation using an adoptive transfer model wherein Ag-specific T cells were present in peripheral tissues but not the thymus (Supplemental Fig. 2A,B). No effect on thymic cDC activation was observed when peripheral T cells were activated by administration of cognate Ag (Supplemental Fig. 2C,D). Notably, similar if not greater levels of serum IFNγ were detected in T cell-recipient NOD.TCRα−/− mice compared to BDC2.5/TCRα−/− and CL4.scid mice after Ag injection (Supplemental Fig. 2E), indicating comparable levels of peripheral T cell stimulation. Overall these findings, coupled with a report indicating that thymic DC, not mTEC, primarily acquire i.v. injected peptide (15), demonstrate that robust peripheral T cell activation has no effect on thymic cDC, and that thymic cDC activation and maturation are dependent on an Ag-specific cognate interaction with CD4SP or CD8SP.
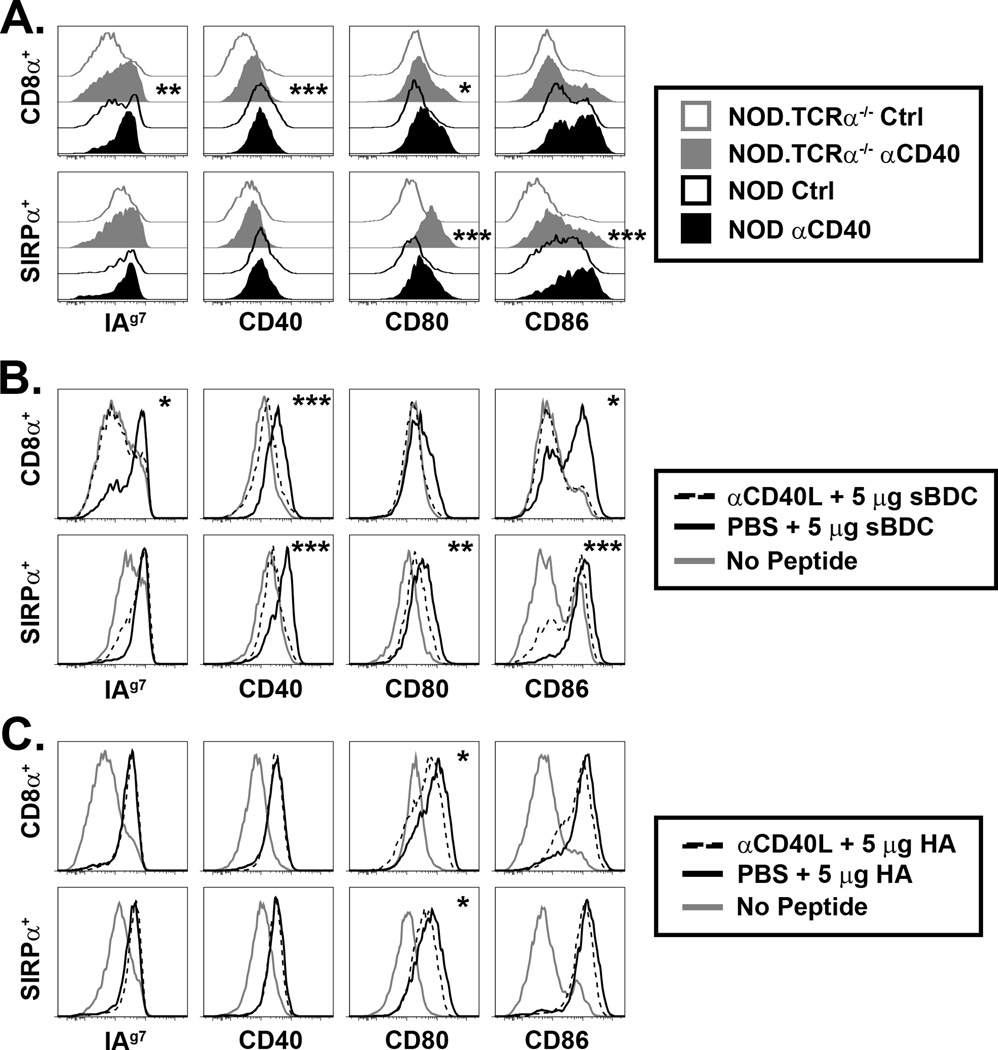
A role for CD40/CD40L in CD4SP feedback to thymic cDC
Peripheral DC activation and maturation are regulated in part by CD40 signaling induced by binding to CD40L expressed by activated T cells. We hypothesized that CD40/CD40L play a similar role in thymic DC:thymocyte crosstalk. First, NOD.TCRα−/− mice were injected with an agonist αCD40 mAb. Expression of MHC II, CD40, CD80, and CD86 by thymic cDC was increased in αCD40 mAb-treated NOD.TCRα−/− mice (Fig. 3A). Thymic cDC from NOD mice treated with αCD40 exhibited little increase in maturation despite higher initial CD40 expression (Fig. 1E, 3A). Thus, ligation of CD40 alone was sufficient to induce thymic cDC activation to some extent. To test whether CD4SP regulated thymic cDC maturation via CD40L during Ag-specific interactions, BDC2.5/TCRα−/− mice were injected with a blocking αCD40L mAb prior to peptide administration. CD40L blockade strongly limited upregulation of MHC and costimulatory molecules by CD8α+ cDC and, to a lesser degree, by SIRPα+ cDC (Fig. 3B). In contrast, CD40L blockade had little effect on MHC and costimulatory molecule upregulation by thymic cDC in HA-injected CL4.scid mice (Fig. 3C). Overall, these data show a role for CD40 ligation by CD4SP but not CD8SP, and indicate additional feedback mechanisms contribute to murine thymic DC function.
Figure 3. CD40/CD40L partially regulates thymic DC phenotype.
(A) NOD and NOD.TCRα−/− mice were injected i.p. with 200 µg agonist αCD40 or isotype control (Ctrl) mAb, and MHC and costimulatory molecule expression by thymic DC assessed 16–18 h later. Inset asterisks represent analysis of Ctrl vs. αCD40. (B) BDC2.5/TCRα−/− or (C) CL4.scid mice were treated daily i.p. for 3 d with 250 µg blocking αCD40L mAb or PBS then, at the time of the final αCD40L treatment, injected i.v. with 5 µg sBDC (B) or 5 µg HA (C), and thymic DC expression of MHC and costimulatory molecules measured 16–18 h later. Inset asterisks represent analysis of PBS + peptide vs. αCD40L + peptide. Data are representative of 3–5 experiments. *, P<0.05; **, P<0.01; ***, P<0.001.
Our study demonstrates that cognate interactions with SP are critical for regulating thymic cDC homeostasis, including thymic cDC abundance (Fig. 1A,B). Notably, analyses of BrdU incorporation revealed no difference in the rate of thymic DC turnover in NOD.TCRα−/− versus NOD mice (unpublished observations). This suggests that reduced thymic cDC cellularity in NOD.TCRα−/− mice is due to a developmental defect in the absence of SP, which may be especially relevant for intrathymically-derived CD8α+ cDC. It is possible that mTEC, which are also reduced in TCRα−/− mice (28, 29, 32), contribute to thymic cDC cellularity and maturation. mTEC may provide a niche through production of chemokines such as XCL1 and CCL8 or other factors that regulate thymic cDC localization, recruitment, and/or maturation (17, 21, 33). However, thymic DC numbers are normal in β2M- or MHC II-deficient mice, the latter exhibiting a reduced mature mTEC pool (29, 32). These findings illustrate 2 key points: 1) either SPCD4 or SPCD8 are sufficient to sustain DC homeostasis, consistent with our findings in peptide-treated BDC2.5/TCRα−/− and CL4.scid mice (Fig. 2), and 2) thymic cDC homeostasis is maintained in the absence of mature mTEC. This second scenario is supported by findings demonstrating that thymic cDC maturation is unaffected in NOD (unpublished observations) and B6 (21) mice lacking Aire expression and thus mature mTEC. Therefore, mTEC appear to have only a limited effect on thymic cDC homeostasis. On the other hand, these observations support our model that thymocyte feedback is the key factor regulating thymic cDC numbers and maturation.
We propose that SP regulate thymic cDC homeostasis based in part on our observations obtained with TCRα−/− mice (Fig. 1). TCRα−/− mice though, also lack TCRαβ+DP that may contribute to thymic cDC feedback. While the inability of DC to induce positive selection in vivo (2) likely precludes feedback from the majority (>95%) of DP, post-positive selection CD69hi/TCRαβ+ DP may provide feedback to thymic cDC. Positive selection induces the migration of CD69hi/TCRαβ+ DP into the medulla (34), which then may interact with thymic cDC. CD69hi DP express CD40L mRNA (28, 29), which we show is associated with feedback mediated by MHC II-restricted thymocytes (Fig. 3B). However, levels of CD40L mRNA expression are reduced ~10-fold compared to CD4SP (28), and <4% of CD69hi DP express surface CD40L, compared to nearly 30% of CD4SP (35). Consequently, we favor a dominant role for SP in feedback to thymic cDC due to a numerical advantage, uniform medullary localization, and significantly higher frequency of CD40L+ cells relative to TCRαβ+ DP. Nevertheless, future work is needed to address if TCRαβ+ DP indeed contribute (or not) to the maintenance of thymic cDC homeostasis.
Whereas cDC were regulated by SP, thymic pDC were not. Little is known about the factors regulating thymic pDC homeostasis. For example, CCL25, which regulates CCR9-dependent pDC migration to the murine thymus (14), is produced by thymic stroma in an AIRE-independent manner (21), and may explain the normal pDC numbers in the NOD.TCRα−/− thymus (Fig. 1A,B). Regulation of migration may be a primary means of thymic pDC regulation. Migration of activated versus immature pDC to the thymus is significantly reduced (14), which may prevent tolerance induction against foreign Ag during infection. Despite an immature phenotype and poor ex vivo stimulatory capacity of thymic pDC (Fig. 1E,G,H), peptide-loaded pDC transferred i.v. have been shown to delete Ag-specific SP (14). One interesting scenario is that pDC ferry peripheral self-Ag to the thymus, which is then “transferred” to cDC that stimulate negative selection. A similar process of Ag transfer occurs from mTEC to DC (18–21).
Analyses of NOD.TCRα−/− thymic cDC revealed decreased activation status, IL-12 production, and T cell stimulatory capacity compared to NOD thymic cDC (Fig. 1E–H). DC maturation was regulated by a cognate Ag-specific interaction with either CD4SP or CD8SP (Fig. 2E,F). Similarly, SP thymocyte feedback is critical for mTEC differentiation, but is mediated exclusively by CD4SP (29). Intriguingly, CD4SP and CD8SP regulated thymic cDC homeostasis by distinct mechanisms; CD4SP- but not CD8SP-mediated effects were CD40L-dependent (Fig. 3). The latter is not surprising since CD8SP express low levels of CD40L mRNA relative to CD4SP (28, 29). Of keen interest is defining the nature of CD8SP-mediated feedback, as well as determining if thymic cDC subsets are regulated by distinct mechanisms. Full characterization of the molecular interactions occurring during thymocyte:DC crosstalk will help define the events that influence the efficacy of thymic negative selection, and may reveal novel mechanisms by which DC subsets and thymic stromal cells contribute to thymocyte development.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. C. Robert Bagnell, Jr. for assistance with analysis of microscopy images.
This study was supported by funding from the National Institutes of Health (1R01AI083269) and Juvenile Diabetes Research Foundation (33-2008-412) to R.T. N.A.S. was supported by a National Institutes of Health Training Grant (T32 AI007273).
Abbreviations used in this study
- AIRE
autoimmune regulator; (c/p)
- (c/p)DC
(conventional/plasmacytoid) dendritic cell
- DP
double-positive thymocyte
- HA
influenza hemagglutinin peptide
- SIRPα
signal regulatory protein alpha
- SP
single-positive thymocyte
- (m/c)TEC
(medullary/cortical) thymic epithelial cell
REFERENCES
- 1.Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009;9:833–844. doi: 10.1038/nri2669. [DOI] [PubMed] [Google Scholar]
- 2.Brocker T, Riedinger M, Karjalainen K. Targeted expression of major histocompatibility complex (MHC) class II molecules demonstrates that dendritic cells can induce negative but not positive selection of thymocytes in vivo. J Exp Med. 1997;185:541–550. doi: 10.1084/jem.185.3.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonasio R, Scimone ML, Schaerli P, Grabie N, Lichtman AH, von Andrian UH. Clonal deletion of thymocytes by circulating dendritic cells homing to the thymus. Nat Immunol. 2006;7:1092–1100. doi: 10.1038/ni1385. [DOI] [PubMed] [Google Scholar]
- 4.McCaughtry TM, Baldwin TA, Wilken MS, Hogquist KA. Clonal deletion of thymocytes can occur in the cortex with no involvement of the medulla. J Exp Med. 2008;205:2575–2584. doi: 10.1084/jem.20080866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guerri L, Peguillet I, Geraldo Y, Nabti S, Premel V, Lantz O. Analysis of APC Types Involved in CD4 Tolerance and Regulatory T Cell Generation Using Reaggregated Thymic Organ Cultures. J Immunol. 2013;190:2102–2110. doi: 10.4049/jimmunol.1202883. [DOI] [PubMed] [Google Scholar]
- 6.Watanabe N, Wang Y-H, Lee HK, Ito T, Wang Y-H, Cao W, Liu Y-J. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–1185. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 7.Proietto AI, van Dommelen S, Zhou P, Rizzitelli A, D'Amico A, Steptoe RJ, Naik SH, Lahoud MH, Liu Y, Zheng P, Shortman K, Wu L. Dendritic cells in the thymus contribute to T-regulatory cell induction. Proc Natl Acad Sci U S A. 2008;105:19869–19874. doi: 10.1073/pnas.0810268105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin-Gayo E, Sierra-Filardi E, Corbi AL, Toribio ML. Plasmacytoid dendritic cells resident in human thymus drive natural Treg cell development. Blood. 2010;115:5366–5375. doi: 10.1182/blood-2009-10-248260. [DOI] [PubMed] [Google Scholar]
- 9.Ohnmacht C, Pullner A, King SB, Drexler I, Meier S, Brocker T, Voehringer D. Constitutive ablation of dendritic cells breaks self-tolerance of CD4 T cells and results in spontaneous fatal autoimmunity. J Exp Med. 2009;206:549–559. doi: 10.1084/jem.20082394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birnberg T, Bar-On L, Sapoznikov A, Caton ML, Cervantes-Barragan L, Makia D, Krauthgamer R, Brenner O, Ludewig B, Brockschnieder D, Riethmacher D, Reizis B, Jung S. Lack of conventional dendritic cells is compatible with normal development and T cell homeostasis, but causes myeloid proliferative syndrome. Immunity. 2008;29:986–997. doi: 10.1016/j.immuni.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Bar-On L, Birnberg T, Kim KW, Jung S. Dendritic cell-restricted CD80/86 deficiency results in peripheral regulatory T-cell reduction but is not associated with lymphocyte hyperactivation. Eur J Immunol. 2011;41:291–298. doi: 10.1002/eji.201041169. [DOI] [PubMed] [Google Scholar]
- 12.Wu L, Shortman K. Heterogeneity of thymic dendritic cells. Semin Immunol. 2005;17:304–312. doi: 10.1016/j.smim.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Park J, Foss D, Goldschneider I. Thymus-homing peripheral dendritic cells constitute two of the three major subsets of dendritic cells in the steady-state thymus. J Exp Med. 2009;206:607–622. doi: 10.1084/jem.20082232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadeiba H, Lahl K, Edalati A, Oderup C, Habtezion A, Pachynski R, Nguyen L, Ghodsi A, Adler S, Butcher EC. Plasmacytoid dendritic cells transport peripheral antigens to the thymus to promote central tolerance. Immunity. 2012;36:438–450. doi: 10.1016/j.immuni.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Atibalentja DF, Byersdorfer CA, Unanue ER. Thymus-blood protein interactions are highly effective in negative selection and regulatory T cell induction. J Immunol. 2009;183:7909–7918. doi: 10.4049/jimmunol.0902632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atibalentja DF, Murphy KM, Unanue ER. Functional redundancy between thymic CD8alpha+ and Sirpalpha+ conventional dendritic cells in presentation of blood-derived lysozyme by MHC class II proteins. J Immunol. 2011;186:1421–1431. doi: 10.4049/jimmunol.1002587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baba T, Nakamoto Y, Mukaida N. Crucial contribution of thymic Sirp alpha+ conventional dendritic cells to central tolerance against blood-borne antigens in a CCR2-dependent manner. J Immunol. 2009;183:3053–3063. doi: 10.4049/jimmunol.0900438. [DOI] [PubMed] [Google Scholar]
- 18.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–1049. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Millet V, Naquet P, Guinamard RR. Intercellular MHC transfer between thymic epithelial and dendritic cells. Eur J Immunol. 2008;38:1257–1263. doi: 10.1002/eji.200737982. [DOI] [PubMed] [Google Scholar]
- 20.Koble C, Kyewski B. The thymic medulla: a unique microenvironment for intercellular self-antigen transfer. J Exp Med. 2009;206:1505–1513. doi: 10.1084/jem.20082449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubert FX, Kinkel SA, Davey GM, Phipson B, Mueller SN, Liston A, Proietto AI, Cannon PZ, Forehan S, Smyth GK, Wu L, Goodnow CC, Carbone FR, Scott HS, Heath WR. Aire regulates the transfer of antigen from mTECs to dendritic cells for induction of thymic tolerance. Blood. 2011;118:2462–2472. doi: 10.1182/blood-2010-06-286393. [DOI] [PubMed] [Google Scholar]
- 22.Proietto AI, Lahoud MH, Wu L. Distinct functional capacities of mouse thymic and splenic dendritic cell populations. Immunol Cell Biol. 2008;86:700–708. doi: 10.1038/icb.2008.63. [DOI] [PubMed] [Google Scholar]
- 23.Dresch C, Ackermann M, Vogt B, de Andrade Pereira B, Shortman K, Fraefel C. Thymic but not splenic CD8(+) DCs can efficiently cross-prime T cells in the absence of licensing factors. Eur J Immunol. 2011;41:2544–2555. doi: 10.1002/eji.201041374. [DOI] [PubMed] [Google Scholar]
- 24.Katz JD, Benoist C, Mathis D. T helper cell subsets in insulin-dependent diabetes. Science. 1995;268:1185–1188. doi: 10.1126/science.7761837. [DOI] [PubMed] [Google Scholar]
- 25.Pang S, Zhang L, Wang H, Yi Z, Li L, Gao L, Zhao J, Tisch R, Katz JD, Wang B. CD8(+) T cells specific for beta cells encounter their cognate antigens in the islets of NOD mice. Eur J Immunol. 2009;39:2716–2724. doi: 10.1002/eji.200939408. [DOI] [PubMed] [Google Scholar]
- 26.Mombaerts P, Clarke AR, Rudnicki MA, Iacomini J, Itohara S, Lafaille JJ, Wang L, Ichikawa Y, Jaenisch R, Hooper ML, et al. Mutations in T-cell antigen receptor genes alpha and beta block thymocyte development at different stages. Nature. 1992;360:225–231. doi: 10.1038/360225a0. [DOI] [PubMed] [Google Scholar]
- 27.Philpott KL, Viney JL, Kay G, Rastan S, Gardiner EM, Chae S, Hayday AC, Owen MJ. Lymphoid development in mice congenitally lacking T cell receptor alpha beta-expressing cells. Science. 1992;256:1448–1452. doi: 10.1126/science.1604321. [DOI] [PubMed] [Google Scholar]
- 28.Hikosaka Y, Nitta T, Ohigashi I, Yano K, Ishimaru N, Hayashi Y, Matsumoto M, Matsuo K, Penninger JM, Takayanagi H, Yokota Y, Yamada H, Yoshikai Y, Inoue J, Akiyama T, Takahama Y. The cytokine RANKL produced by positively selected thymocytes fosters medullary thymic epithelial cells that express autoimmune regulator. Immunity. 2008;29:438–450. doi: 10.1016/j.immuni.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 29.Irla M, Hugues S, Gill J, Nitta T, Hikosaka Y, Williams IR, Hubert FX, Scott HS, Takahama Y, Hollander GA, Reith W. Autoantigen-specific interactions with CD4+ thymocytes control mature medullary thymic epithelial cell cellularity. Immunity. 2008;29:451–463. doi: 10.1016/j.immuni.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 30.Godfrey DI, Kennedy J, Gately MK, Hakimi J, Hubbard BR, Zlotnik A. IL-12 influences intrathymic T cell development. J Immunol. 1994;152:2729–2735. [PubMed] [Google Scholar]
- 31.Ludviksson BR, Ehrhardt RO, Strober W. Role of IL-12 in intrathymic negative selection. J Immunol. 1999;163:4349–4359. [PubMed] [Google Scholar]
- 32.Irla M, Guerri L, Guenot J, Serge A, Lantz O, Liston A, Imhof BA, Palmer E, Reith W. Antigen recognition by autoreactive CD4(+) thymocytes drives homeostasis of the thymic medulla. PLoS One. 2012;7:e52591. doi: 10.1371/journal.pone.0052591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lei Y, Ripen AM, Ishimaru N, Ohigashi I, Nagasawa T, Jeker LT, Bosl MR, Hollander GA, Hayashi Y, Malefyt Rde W, Nitta T, Takahama Y. Aire-dependent production of XCL1 mediates medullary accumulation of thymic dendritic cells and contributes to regulatory T cell development. J Exp Med. 2011;208:383–394. doi: 10.1084/jem.20102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi YI, Duke-Cohan JS, Ahmed WB, Handley MA, Mann F, Epstein JA, Clayton LK, Reinherz EL. PlexinD1 glycoprotein controls migration of positively selected thymocytes into the medulla. Immunity. 2008;29:888–898. doi: 10.1016/j.immuni.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Desanti GE, Cowan JE, Baik S, Parnell SM, White AJ, Penninger JM, Lane PJ, Jenkinson EJ, Jenkinson WE, Anderson G. Developmentally regulated availability of RANKL and CD40 ligand reveals distinct mechanisms of fetal and adult cross-talk in the thymus medulla. J Immunol. 2012;189:5519–5526. doi: 10.4049/jimmunol.1201815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.