Abstract
Introduction:
Hepatitis B infection is an important health problem all over the world, and according to the studies, Iran is a country with intermediate prevalence, so vaccination is a cost-benefit approach. In this study, evidence about the efficacy of hepatitis B vaccine was collected by systematic review methods and its amount was estimated by a meta-analysis.
Materials and Methods:
In this study, documents and literature search were performed using valid key words in Information Sciences Institute, PubMed, Scientific Information Data base, and Iranmedex databases from 1997 to 2010 in different regions of Iran. All cross-sectional studies about the efficacy of hepatitis B vaccine in Iran which fulfilled the inclusion criteria entered the study. Antibody titer (Anti-hemoglobins > 10 IU/L) was considered as the desired efficacy. In order to present the results, prevalence and Forest plot were used and for evaluation of the inconsistency meta-regression model and I2 index were used. We used R.15.3.2 software for analysis.
Results:
Totally 64 studies (52 studies in general population and 12 studies among specific populations) including 12,575 subjects with age range from 8 months to 55 years entered the meta-analysis. The efficacy was 86.3% (confidence interval [CI]: 83.9-88.7%) in the general population and 59.62% (CI: 47.9-71.29%) in specific patient populations. Also the efficacy was significantly related to the year of publication, age and gender (P < 0.05).
Conclusion:
Prevention is an important issue in general health. Hepatitis B vaccination is one of the methods used to prevent hepatitis B infection. According to this study, the efficacy of hepatitis B vaccination was more than 80% in general population, so injection of full course of hepatitis B vaccinationis enough and booster dose is not required.
Keywords: Hepatitis B, meta-analysis, meta-regression, systematic review, vaccine
INTRODUCTION
All over the world, hepatitis B virus (HBV) is a common cause of hepatic disease.[1] This virus is present in all body fluids and secretions. It is transmitted via sexual contact, intravenous (IV) drug abuse, mother to the newborn and occupational exposure. Hepatitis B infection is an international health concern because 2 billion subjects are infected with this virus,[2,3] and 350 million subjects suffer from chronic hepatitis B infection among whom 75% are Asian.[4,5] About 45%, 43% and 12% of people live in hyper-endemic (prevalence ≥ 8%), miso-endemic (prevalence: 2-7%) and hypo-endemic (prevalence < 2%) regions, respectively.[6,7] According to World Health Organization and Center for Disease Control reports, Iran is among the countries with intermediate prevalence regarding chronic hepatitis B infection,[6,7] According to the estimate of meta-analyses prevalence of chronic hepatitis B infection in Iran is 2.7%.[8] The incidence of this disease in the US was 14 in 100,000 individuals in 1980 with reduced to 3 in 100,000 in 1989.[9] In Iran an important risk factor for this disease is IV drug abuse.[10] There is no cure for acute or chronic infections and present treatments are very costly.[11] Now vaccination id the most efficacious and economical means for prevention of this disease.[11] Hepatitis B vaccination for newborns and persons in high-risk conditions such as health care workers according to the national vaccination program was started in 1993. In this program vaccination is performed by intramuscular injection of 0.5 cc recombinant deoxyribonucleic acid vaccine in between 0, 1, and 6 months after the first injection. From 2006, vaccination was performed for under 18 year old adolescents as well.[12] The vaccine was produced in 1980 and from 1990 it was used in children vaccination in many countries.[12]
There are different reports on the immunizing rate of this vaccine in Iran. Studies in Iran and other parts of the world have reported an immunizing rate of 71-95% for this vaccine.[13,14,15,16,17,18,19,20,21,22,23,24,25,26, 27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75] Studies have shown that various factors may affect the efficacy of hepatitis B vaccine including vaccine type, times of vaccination,[76] genetic background,[77] age,[78] weight, smoking, and alcohol consumption.[79]
Considering different studies on the efficacy of hepatitis B vaccination, in order to validate the results of these studies for identification of the national load of hepatitis B infection and better control of this disease from educational, research and health care aspects and for measurement of the effect of vaccine, a meta-analysis was performed on the studies published from 1997 to 2010.
MATERIALS AND METHODS
This was a meta-analysis on the efficacy of hepatitis B vaccine which was performed by a systematic review of the existing evidence.
Search strategy
In this study, International databases, including Information Sciences Institute, PubMed, scopus and google scholar and Iranian databases, including Iranmedex, Scientific Information Data base, and Magiran were searched, and studies about the efficacy of hepatitis B vaccination which were published from 1997 to 2010 were evaluated. Following keywords or a combination of them, were used for search: Efficacy, hepatitis B, immunity, vaccination, HBV, virus, response, immunogenicity. For those articles which full text was not available in databases we contacted the author via E-mail to receive fulltext articles.
Inclusion criteria
At first a list of titles and abstracts of all articles in the aforementioned databases was prepared and related articles entered the study independently. All studies which had assessed the efficacy of hepatitis B vaccine in Iranian population at least 1 month after the last (third) dose of the vaccine entered the study. The selected studies were performed cross-sectional between 1997 and 2010. All observational studies (case-control and cohort), clinical trials on two different kinds of vaccines, trials with historical controls, and before-after studies were excluded from the study. In some studies from which several articles had been published, only one article with the largest sample size was selected.
Outcome assessment
The study outcome was the efficacy of hepatitis B vaccine which is assessed by the measurement of the titer of the antibody against the hepatitis B surface antigen by enzyme-linked immunosorbent assay method. Subjects are divided into three groups according to the antibody titer (IU/L): (1) Antibody titer > 100 IU/L, (2) antibody titer between 10 and 100 IU/L, and (3) antibody titer < 10 IU/L, which were considered as positive response (immune), weak positive response (immune), and no response (not immune), respectively. So in this meta-analysis antibody titer > 10 IU/L was considered as the desirable response of the main outcome.
Data extraction
According to inclusion and exclusion criteria, a list of accepted articles was prepared, then a checklist (including name of the author, year of publication, study place, sample size, the amount of positive or desirable response to vaccine, gender, age group, characteristics of study population, the time of antibody titer measurement, and type of the study) was designed for final assessment of the articles.
Quality of the studies
The quality of the study regarding the study method and reportingmethod was evaluated. For evaluation a standard checklist i.e. strengthening the reporting of observational studies in epidemiology (STROBE) for cross-sectional studies was used. It consisted of 6 stions and 22 titles.[80] In this study 64 articles were accepted among which 2 studies were published as abstracts. The advantages and disadvantages of the studies were assessed by the checklist and in the report of these studies about 70% of the titles of STROBE checklist were addressed.
The description of the studies
Totally 121 articles were found by a search in national and international databases. Six articles were excluded because they were repeated, and 9 were excluded due to low quality. Among the remainder 106 articles, 36 ones were excluded due to inconsistency with the criteria of the current study, and 6 other studies were excluded due to unavailability of the fulltext article or required data. From 64 accepted articles, 14 articles had been performed on children under 10 years old,[51,52,53,54,55,56,57,58,59,60,61,62,63,64] 38 articles on adults (more than 18 years old),[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] and 12 on individuals with a specific disease (thalassemia, acquired immunodeficiency syndrome [AIDS], hemodialysis)[64,65,66,67,68,69,70,71,72,73,74,75] [Figure 1].
Figure 1.

Follow diagram of systematic review and searches for efficacy of hepatitis B vaccine in I.R. Iran
Characteristics of the population in different studies
From 64 accepted articles, 14 articles had been performed on children under 10-year-old,[51,52,53,54,55,56,57,58,59,60,61,62,63,64] 38 articles on adults (more than 18-year-old),[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] and 12 on individuals with a specific disease (thalassemia, AIDS, hemodialysis).[64,65,66,67,68,69,70,71,72,73,74,75] 39 studies have assessed the efficacy of the vaccine on both genders (separately and in combination).[14,15,18,19,20,21,22,25,27,29,30,34,35,36,39,40,43,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,67,68,70,71,73,75]
Statistical analysis
Due to the variability of the studied subjects and accepted articles for assessment of hepatitis B vaccine efficacy, studies were divided into some groups and analysis was done in each group separately. The efficacy of hepatitis B vaccine and sample size was extracted from each study, so for measurement of variance in each study, binominaldistribution was used. In order to assess the consistency of the studies Cochran's Q test and I2 index were used. Galbraith plot was used for graphic presentation. For calculation of the pooled effect measure inverse variance was used for fixed effect and Dersimonian-Lirdmethod was used for random effect. For presentation of the meta-analysis results Forest plot was used. In this plot the size of the square shows sample size in each study and lines in both sides show the 95% confidence interval (CI) for the efficacy of vaccine in each study. In order to detect publication bias, Funnel plot, and Egger's regression were used. Data analysis was performed by R 2.15.1 software.
RESULTS
Due to the variability of subjects in different studies, we could not merge the results and analyses them totally, so studies were divided into three groups and analysis was done in each group, separately.
The efficacy of hepatitis B vaccine in the general population
There 52 studies conducted on the general population between 1997 and 2010. These studies included all age groups (from 8 months to 55 years). Sample size was between 36 and 600 subjects. The lowest efficacy (antibody titer > 10 IU/L) was observed in Zanjan (48%), and the highest was seen in Lorestan and Babol (100%). Heterogeneity test (P < 0.005), I2 index (I2 = 96.65%), and Galbraith plot showed a high inconsistency among the studies [Figure 2]. According to the meta-regression model, publication year, age group and administration method were factors responsible for inconsistency. So studies on two age groups, children (under 10 years old) and adults (more than 18-year-old), were analysed separately and in combination. Meta-analysis estimate of the efficacy of hepatitis B vaccine in 95% CI was 82.82% (95% CI: 75.8-89.8%), 87.87% (95% CI: 85.6-90%), and 86.37% (95% CI: 83.9-88.7%) in children, adults, and general population, respectively. In sensitivity analysis for evaluation of the effect of each study on the final result by excluding the studies one by one, the efficacy of hepatitis B vaccine was constant with little fluctuation. In another word, meta-analysis model enjoyed a high stability and considering this low fluctuation, the highest effect was observed with the study of Saffar et al. in 1993[32] and Rafizade et al. in 2003[64] which changed the efficacy between 0.0065 and 0.008. For assessment of publication bias, Funnel plot was used. This plot shows the distribution of all 52 studies in horizontal axis (the efficacy of vaccine), and vertical (accuracy of each study) and the points have formed an asymmetrical pattern. Egger's regression quantitatively confirmed the publication bias (P < 0.005).
Figure 2.

Galbraith plot of the efficacy of hepatitis B vaccine in the general population
According to the results of meta-regression model, meta-analysis estimate of the efficacy of hepatitis B vaccine in general population was 94.9% (95% CI: 93-97%) after inter-dermal injection and 85% (95% CI: 82.7-88.1%) after intramuscular injection.
The efficacy of hepatitis B vaccine in specific diseases
In the period between 1997 and 2010, we found 5 studies on hemodialysis patients[65,66,70,72,73] and 4 patients on thalassemia patients,[16,32,67,74] and 3 studies on patients with AIDS.[68,69,71] Sample size in these studies was between 37 and 215. The lowest efficacy (29.1%) for hepatitis B vaccine was observed in AIDS patients in Kermanshah,[71] and the highest was 92% and among thalassemia patients in Sari.[33] Due to inconsistency between studies I2 = 94.3, P < 0.001), the meta-analysis estimate of efficacy by random model was 59.6% (95% CI: 48-71%) [Figure 3].
Figure 3.
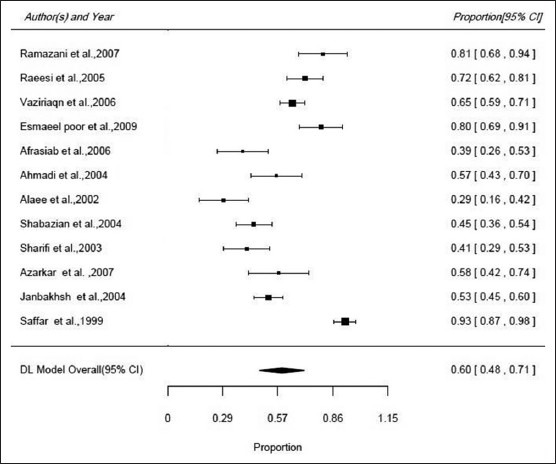
Forest plot of the efficacy of hepatitis B vaccine in specific diseases
According to the results of Egger's regression (P = 0.6) and Funnel plot, there wasn't significant publication bias. Sensitivity analysis showed that by excluding the studies one by one, the efficacy of hepatitis B vaccine was constant with little fluctuation (range of the estimate of efficacy: 57.7-62.3%). Therefore meta-analysis model enjoyed a high stability.
The efficacy of hepatitis B vaccine in the general population regarding gender
Totally 32 studies had assessed the efficacy of hepatitis B vaccine in two genders. The meta-analysis estimate by random model in 95% of confidence interval was calculated using Forest plot. Odds ratio (OR) of efficacy of hepatitis B vaccine (female to male) was 1.88 (95% CI: 1.42-2.48) [Figure 4]. According to the results of Egger's regression (P = 0.36) and Funnel plot, there wasn't significant publication bias [Figure 5]. Sensitivity analysis showed that by excluding the studies one by one (range of the estimate of OR: 1.59-1.93) showed that the study conducted by Sarkari et al.[22] by female to male ratio of 1.59 increased the final estimate of OR by 0.29. Due to inconsistency between studies I2 = 96.65, P < 0.001), the meta-analysis method showed that publication year and age were the factors responsible for heterogeneity. Therefore meta-analysis estimated the OR of efficacy of hepatitis B vaccine to be 1.2 (95% CI: 0.97-1.42) in children and 2.43 (95% CI: 1.65-3.59) in adults [Figure 4].
Figure 4.
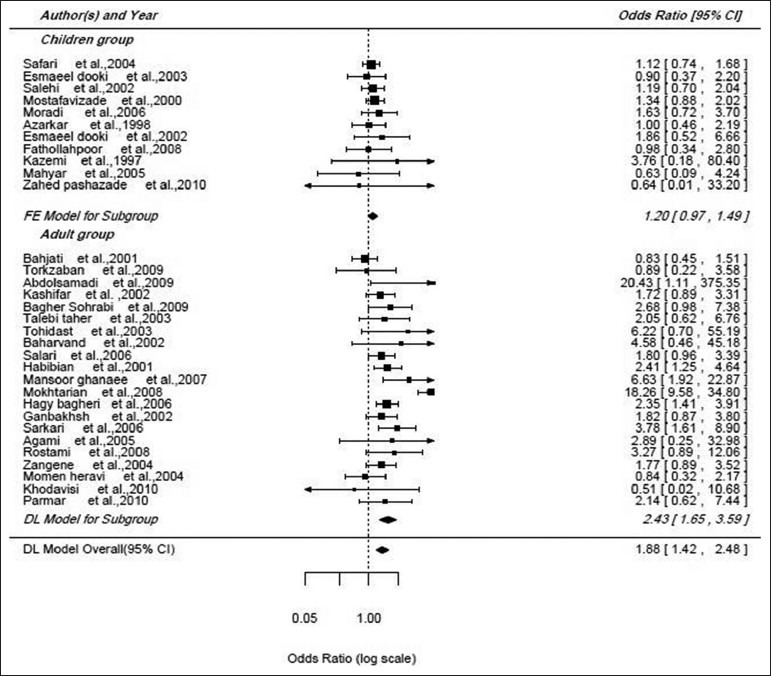
Forest plot of the efficacy of hepatitis B vaccine in the general population regarding Age
Figure 5.
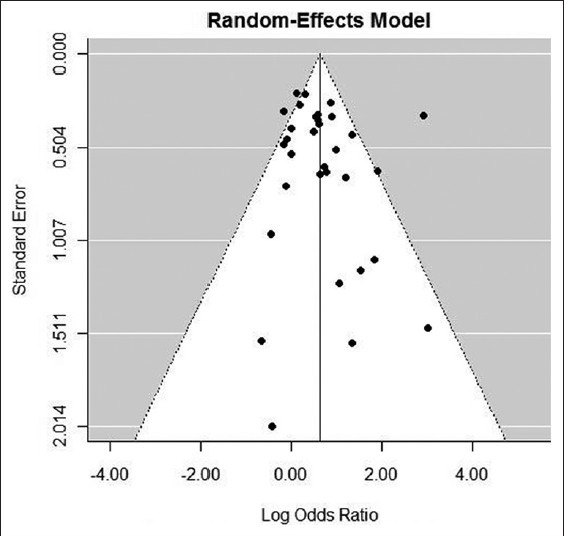
Funnel plot of the efficacy of hepatitis B vaccine in the general population regarding gender
The efficacy of hepatitis B vaccine in specific diseases regarding gender
We found 7 studies that have evaluated the efficacy of hepatitis B vaccine in people suffering from specific diseases regarding gender.[67,68,69,70,71,73,75] According to Forest plot and meta-analysis estimate by random model in 95% confidence interval, the female to male OR for the efficacy of vaccine was 0.86 (95% CI: 1.9-0.39). According to the results of Egger's regression (P = 0.72) and Funnel plot, there wasn't significant publication bias. Sensitivity analysis showed that the efficacy of hepatitis B vaccine was constant with little fluctuation (range of the estimate of OR: 0.73-1.1). Heterogeneity test (I2 = 63.9, P = 0.1) and Funnel plot showed that studies have a moderate heterogeneity.
DISCUSSION
This was a comprehensive systematic review and meta-analysis on cross-sectional studies conducted to assess the efficacy of hepatitis B vaccine during the period between 1997 and 2010. Due to the variability in subjects participated in the studies, meta-analysis was performed on two groups: General population and those suffering from specific diseases. The meta-analysis estimate of the efficacy of hepatitis B vaccine in different studies was 86.3% (95% CI: 83.9-88.7%) in general population and 59.6% (95% CI: 47.9-71.2%) in the population suffering from a specific disease. In other words, suffering from a specific disease such as thalassemia, chronic renal failure, or human immunodeficiency virus infection lowers the efficacy of hepatitis B vaccine. It can be explained by these two reasons: (1) Individuals in general population are physically stronger than those suffering from an important disease and (2) general population consists of people in different ages, but those with specific diseases were mostly older than 30 years.
The results of meta-regression model showed that meta-regression coefficient was significant for publication year, age and route of administration which shows a significant change in the efficacy of hepatitis B vaccine in different age groups and routes of administration. The comparison between studies on adults (older than 18-year-old) and children (younger than 10 years old) shows that efficacy in adults is somewhat more than children, but the difference is not significant. In the study conducted by Fisman et al. in 2002 who assessed the responsiveness to recombinant hepatitis B vaccine, the responsiveness was increased to 30 years old and then decreased.[81] Meta-analysis showed that efficacy is higher by administration of vaccine via interdermal router than intramuscular which was consistent with the study of Fabrizi et al.[82] but was inconsistent with the study of Afzali et al.[83] In the accepted studies, the lowest OR for the efficacy of vaccine was observed in the study of Khodavisi et al.[15] in Hamedan, and the highest was seen in Abdolsamadi et al. study[48] in Tehran, although the meta-anlysis considering gender showed that the female to male ratio for the efficacy of the vaccine was 1.87 (95% CI: 1.42-2.48); it means that the probability of efficacy in females is 0.87 more than males and the efficacy of vaccine is lower in males which was confirmed in children and adults. In patients suffering from a specific disease, the efficacy was higher among males than females, so female gender is an attenuating factor for efficacy of hepatitis B vaccine. Totally, there is controversy about the effect of gender on the efficacy of hepatitis B vaccine. Some researches mention that responsiveness to vaccine is higher among females, which is not approved in other studies. Ferraz et al. have found a higher titer of antibody in females after vaccination.[84]
Mansori et al. showed that males had a better responsiveness than females.[85] Holenger believes that geneder is not an effective factor on responsiveness.[86] This study showed that the efficacy of hepatitis B vaccine is lower in males and those with specific diseases which is consistent with textbooks.
CONCLUSION
Prevention is an important issue in public health. This study showed that the efficacy of hepatitis B vaccine in general population is more than 80%. Therefore booster dose is not required in the general population, but it may be required in subjects with a specific disease.
ACKNOWLEDGMENTS
We are thankful to professor Mohammad Hasan Lotfi and Amir Hooshang Mehrparvar for his teaching and guidance.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Zuckerman JN, Zuckerman AJ. The epidemiology of hepatitis B. Clin Liver Dis. 1999;3:179–87. [Google Scholar]
- 2.Daniels D, Grytdal S, Wasley A. Centers for Disease Control and Prevention (CDC). Surveillance for acute viral hepatitis-United States, 2007. MMWR Surveill Summ. 2009;58:1–27. [PubMed] [Google Scholar]
- 3.Alter MJ. Epidemiology and prevention of hepatitis B. Semin Liver Dis. 2003;23:39–46. doi: 10.1055/s-2003-37583. [DOI] [PubMed] [Google Scholar]
- 4.Kao JH, Chen PJ, Lai MY, Chen DS. Occult hepatitis B virus infection and clinical outcomes of patients with chronic hepatitis C. J Clin Microbiol. 2002;40:4068–71. doi: 10.1128/JCM.40.11.4068-4071.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andrade AF, Oliveira-Silva M, Silva SG, Motta IJ, Bonvicino CR. Seroprevalence of hepatitis B and C virus markers among blood donors in Rio de Janeiro, Brazil, 1998-2005. Mem Inst Oswaldo Cruz. 2006;101:673–6. doi: 10.1590/s0074-02762006000600016. [DOI] [PubMed] [Google Scholar]
- 6.Mast EE, Margolis HS, Fiore AE, Brink EW, Goldstein ST, Wang SA, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: Recommendations of the advisory committee on immunization practices (ACIP) part 1: Immunization of infants, children, and adolescents. MMWR Recomm Rep. 2005;54:1–31. [PubMed] [Google Scholar]
- 7.Geneva: WHO; 2001. World Health Organization. Introduction of hepatitis B vaccine into childhood immunization services. (unpublished document WHO/V&B/01.31 available on request from Department of Vaccines and Biologicals, World Health Organization, 1211 Geneva 27, Switzerland. [Google Scholar]
- 8.Poorolajal J, Majdzadeh R. Prevalence of chronic hepatitis B infection in Iran: A review article. J Res Med Sci. 2009;14:249–58. [PMC free article] [PubMed] [Google Scholar]
- 9.Goldstein ST, Alter MJ, Williams IT, Moyer LA, Judson FN, Mottram K, et al. Incidence and risk factors for acute hepatitis B in the United States, 1982-1998: Implications for vaccination programs. J Infect Dis. 2002;185:713–9. doi: 10.1086/339192. [DOI] [PubMed] [Google Scholar]
- 10.Alizade M, Hooshang A, Alavian SM, JAfari Kh, Yazdi N. Prevalence HIV-Ab and HCV-Ab and HbsAg in addicted prisoners of central jail of Hamadan. J Res Med Sci. 2002;7:311–3. [Google Scholar]
- 11.Bonanni P, Pesavento G, Boccalini S, Bechini A. Perspectives of public health: Present and foreseen impact of vaccination on the epidemiology of hepatitis B. J Hepatol. 2003;39(Suppl 1):S224–9. doi: 10.1016/s0168-8278(03)00315-5. [DOI] [PubMed] [Google Scholar]
- 12.The Office of Deputy Ministry of Health. Ministry of Health and Medical Education. 5th ed. Tehran (Iran): Seda Publishing; 1998. Vaccination and Immunity Guideline. [Google Scholar]
- 13.Parmar Z, Khadivi R, Sadeghi B, Rahimi M. Immunization following hepatitis B mass vaccination in the 18 years old student in chaharmahalvabakhtyari province in Iran. J Shahrekord Univ Med Sci. 2009;13:35–41. [Google Scholar]
- 14.Kazemi H, Yadegarinia D, Rasheki H. Evaluation of hepatitis B antibody and factors related to hepatitis B vaccination in Tehran Hospital staffs, 2010. J Dent Sch Shahid Beheshti Univ Med Sci. 2010;35:114–8. [Google Scholar]
- 15.Khodavisi M, Mohamadi N, Omidi A, Amini R. Determination of Anti-HBS titre hepatitis B in student of nursing and midwifery faculty in Hamadan. J Hamadan Nurs Midwifery Fac. 2010;18:45–9. [Google Scholar]
- 16.Azarkeivan A, Nasiritoosi M, Hajibeigi B, Afradi H, Eslami M, Ghazizadeh Sh. Evaluation of immune response to hepatitis B vaccination and effects of booster dose in chronic transfusion patients. J Iran Univ Med Sci. 2009;16:32–9. [Google Scholar]
- 17.Alavian SM, Akbari H, Ahmadzadasl M, Kazem M, Davodi A. Vaccination status of dentists participating in the forty-second international congress of dentists against hepatitis B and Infection control practices. J Islam Dent Assoc Iran. 2007;7:48–56. [Google Scholar]
- 18.Moemenheravi M, Sharifa, Mousavi Gh. Evaluation of serum anti-HBs in health personnel vaccinated in Shahidbeheshti hospital of Kashan. J Feiz. 2006;10:11–4. [Google Scholar]
- 19.Zangeneh M, Poopak B, Khavari M, Valikhani M, Parsania M. Evaluation of immunogenicity of hepatitis B vaccination in health workers. J Islam Azad Univ Med Sci-Tehran Med Branch. 2004;14:13–22. [Google Scholar]
- 20.Rostami H, Farmani S, Mirzaee A, Ghorbanzade R. Evaluation of immune response to hepatitis B in workers at risk. J Urmia Nurs Midwifery Fac. 2008;6:178–82. [Google Scholar]
- 21.Ajami A, Abediyan F. Immunogenicity of hepatitis B vaccine in medical sciences students-2004. J Mazandaran Univ Med Sci. 2006;16:72–7. [Google Scholar]
- 22.Sarkari B, Zargar MA, Mohammadi R, Asgarian SH. Prevalence of hepatitis B antibodies in health-care workers in Yasuj hospitals, Armaghane-danesh bimonthly. J Yasuj Univ Med Sci. 2006;11:97–106. [Google Scholar]
- 23.Khaki M, Ghavamian M. The evaluation of recombinant HBS. Ag vaccine immunity in vaccinated medical group and hospital personnel in Borujerd-2004. J Arak Univ Med Sci. 2005;8:1–6. [Google Scholar]
- 24.Izadpanah AM, Mashreghy Moghadam HR, Ziaee M, Foadaldini M, Ebadian FS. Anti HBs level in nursing staff of Birjand university of medical sciences. J Birjand Univ Med Sci. 2008;15:80–6. [Google Scholar]
- 25.Janbakhsh A, Hatami H, Sayyad B, Eini P, Hashemian A. The rate of immune response to hepatitis B vaccination in health centers staff in Kermanshah, 2002. J Kermanshah Univ Med Sci. 2003;7:48–53. [Google Scholar]
- 26.Davodzade M, Rajabipoor F, Shafizade F, Ghorbanivaghee A. Evaluation anti-HBs level in vaccinated Interns in Lorestan University of medical sciences. J Lorestan Univ Med Sci. 2004;23:57–60. [Google Scholar]
- 27.Hajybagheri K, Kanani Sh, Moradi Gh, Yousefinejad V. Response to hepatitis B vaccine in medical staff of hospitals in Kurdistan-2006. Iran J Infect Dis Trop Med. 2008;13:53–7. [Google Scholar]
- 28.Aminzade Z, Shabani Z, Gachkar L, Sayyadi A. Frequency of positive HBs Ag in pregnant women of Zanjan. J Rafsenjan Univ Med Sci. 2004;3:126–32. [Google Scholar]
- 29.Mokhtarian K, Yazdanparast M, Tarshizi R, Moghani M. Evaluation levels of antibodies against hepatitis B in staffs of Hajar Hospital, 2007-2008. J Shahrekord Univ Med Sci. 2009;11:35–9. [Google Scholar]
- 30.Rajbar M, Keramat F, Keshavarz F. Immunogenicity of hepatitis B vaccination in staff of HamedanSina Hospital (2001) Sci J Hamdan Univ Med Sci. 2002;7:55–8. [Google Scholar]
- 31.Ayatollahi J, Sharifi MR, Sabzi F, Zare AR. Blood level Anti-HBs due to HB vaccine in health care personnel of ShahidSadoughi Hospital – Yazd. Iran J Obstet Gynecol Infertility. 2004;7:48–51. [Google Scholar]
- 32.Saffar M, Nikkhah M, Anvari M. Measure antibody persistence of hepatitis B virus in Thalassemia recipients of health personnel 6 years after vaccination in Sari. J Mazandaran Univ Med Sci. 2001;11:60–5. [Google Scholar]
- 33.Baba Mahmoodi F. Evaluation of hepatitis B antibody (HBS) levels in nursing staff of GaemshahrRazi hospital and it's variation with duration of immunity post HB vaccination. J Mazandaran Univ Med Sci. 2000;10:48–53. [Google Scholar]
- 34.Mansour Ghanati F, Fallah MS, Jokar F, Jafarshad R, Aramy M, Ale-Ismail A, et al. Safety of students vaccinated against hepatitis B in Guilan University of Medical Sciences. Iran J Infect Dis Trop Med. 2008;13:47–51. [Google Scholar]
- 35.Habibiyan R. Efficacy of complete hepatitis B-vaccination in health care workers. J Shahrekord Univ Med Sci. 2003;5:42–7. [Google Scholar]
- 36.Salari M, Alavian SM, Tadrisi D, Karimi A, Sadeghian H, Asadzandi M, et al. Safety of hepatitis B immunization in health care workers. Kowsar Med J. 2006;11:343–52. [Google Scholar]
- 37.Mohamadjafari R, Saadati N, Vaziri Sh, Sooranian A. Frequency of positive HBs Ag in pregnant women referred to health centers in Ahvaz City. J Iranian Inst Health Sci Res. 2004;3:237–43. [Google Scholar]
- 38.Asefzade M, Sharifi M, Aliaee A. Prevalence of HBsAg carriers and antiHBsAg in health care workers of Boali-Sina teaching hospital in Qazvin. J Qazvin Univ Med Sci. 2004;32:41–6. [Google Scholar]
- 39.Baharvand M, Iramloo Z. Survey of anti-HBs titers in vaccinated dental students of ShahidBeheshti University of Medical Sciences in 2002. J Dent Sch Shahid Beheshti Univ Med Sci. 2003;21:653–60. [Google Scholar]
- 40.Tohidast Z, Farahani Z, Salmani Gh. Titer of HBs Ab in the faculty members, residents and student of dental college after three rounds of vaccination in Tehran university of medical sciences (2002-2004) J Islam Dent Assoc Iran. 2005;17:98–103. [Google Scholar]
- 41.Keshavarz M, Ahoee M, Babakhani A, Haratipoor H. Efficacy of hepatitis B vaccine in medical students in Shahrood-2004. Med J Mashad Univ Med Sci. 2007;3:53–9. [Google Scholar]
- 42.Talebi Taher M, Akbari M, Rezaee M, Ashaerii N, Omrani Z, Ghaderian H, et al. Determination of anti-HBS titre mean induced by hepatitis B vaccine among health care workers in Firoozgar hospital in Tehran. Razi j Med Sci. 2004;11:789–95. [Google Scholar]
- 43.Sohrabi MB, Sarafha J, Zolfaghari P, Eskandari Z. HBs Ab level in clinical personnel of Immam Hossein Hospital of Shahrood. J Blood. 2009;6:65–4. [Google Scholar]
- 44.Savadkoohi R, Hoseinian MA. Level of blood anti-HBs in the hospital staff of Amirkola. J Babol Univ Med Sci. 2003;5:38–42. [Google Scholar]
- 45.Hagybagheri K, Rahimi A, Mansoorifar Sh. Evaluation of serum anti-HBs and related factors in staff vaccinated of Tohid Hospital in Sanandaj. J Kordestan Univ Med Sci. 2001;5:27–9. [Google Scholar]
- 46.Kashifar M, Hasanjaniroshan MR. Response to hepatitis B vaccination in hospital personnel Yahyanejad-2002. J Babol Univ Med Sci. 2004;6:39–42. [Google Scholar]
- 47.Taghavi N, Eilami O, Nabavi M, Azargashb E, Yadegarynia D, Alizadeh AM. Immunogenicity of recombinant hepatitis B vaccine in health care worker of Boo-Ali Hospital in Tehran, Iran, 2002-2004. Iran J Clin Infect Dis. 2006;1:67–70. [Google Scholar]
- 48.Abdolsamadi HR, BakianianVaziri P, Abdollahzadeh Sh, Mani Kashani KH, Vahedi M. Immune response to hepatitis B vaccine among dental students. Iran J Public Health. 2009;38:113–8. [Google Scholar]
- 49.Torkzaban P, Abdolsamadi H, Vaziri P. Efficacy rate of hepatitis B vaccination in vaccinated dentists in Hamadan city. J Dent Res. 2009;6:62–6. [Google Scholar]
- 50.Behjati M, Mirhoseini NA, Ayatollahi J. Titer of HBs Ab in 8 year old children vaccinated in Yazd city schools (2001) J Shaheed Sadoughi Univ Med Sci. 2002;10:3–7. [Google Scholar]
- 51.Zahedpashay Y, Ahmadpourkacho M, Pour Nasrollah M, Bijani A, Saadat H, Mazloumi A. Immune response in preterm infants to hepatitis B vaccine. J Babol Univ Med Sci. 2010;13:34–9. [Google Scholar]
- 52.Mahyar A. Survey of antibody titers against recombinant hepatitis B vaccination in 6 years old children. J Guilan Univ Med Sci. 2007;16:39–43. [Google Scholar]
- 53.Kazemi SA, Azizi N. Evaluation of the effects of hepatitis B vaccination in children after 3 dose vaccine in Zanjan. J Zanjan Univ Med Sci. 1993;22:5–10. [Google Scholar]
- 54.Fathollahporr A, Hemmatpoor S, Ghaderi E. Protective effect of hepatitis B vaccine in vaccinated children 12-24 months in the city of Sanandaj. Iran J Infect Dis Trop Med. 2008;13:65–70. [Google Scholar]
- 55.Esmaeeldooki M, Seyedkala F. Evaluation of serum anti-HBs children have been vaccinated against hepatitis B in children admitted to Mirkala Hospital in Babol. Feyz J. 2002;24:45–8. [Google Scholar]
- 56.Azarkar Z. Efficacy of hepatitis B vaccine in children from 12-16 months in Mashad Health Centers. J Qazvin Univ Med Sci. 2004;29:38–41. [Google Scholar]
- 57.Moradi A, Khodabakhshi B, Ghaemi E, Mansoorian A, Sarikhani A, Saeedi M. The rate of response to hepatitis B vaccination in children less than one year of Gorgan. J Gorgan Univ Med Sci. 2008;10:50–3. [Google Scholar]
- 58.Mostafavizade K, Salehi H, Imani R, Haghayegh MR. Antibody levels following vaccination against hepatitis B virus surface antigen after a period of 5-6 years old children new to school in Sharekord. Res Med Sci. 2000;6:194–6. [Google Scholar]
- 59.Salehi M, Saneimoghadam E, Khosravi S. Evaluation of immune response of hepatitis B vaccination in Zahedaninfants. Zahedan J Res Med Sci. 2002;4:155–8. [Google Scholar]
- 60.Esmail Duki MR, Zahed Pasha Y, Mahdavi A, Haji Ahmadi M. Comparison of the effect of hepatitis B vaccination in normal and low birth weight neonates. J Babol Univ Med Sci. 2003;5:13–8. [Google Scholar]
- 61.Rafiee M, Majidi J, Hashemi F, Hoseinpoor S, Nejati N, Mostafa gharabaghi M, et al. Comparison of hepatitis B surface antigen antibody response in premature and received infants after vaccination with hepatitis B vaccine in hospital's children center Alzahra. Med J Tabriz Univ Med Sci Health Serv. 2006;28:55–8. [Google Scholar]
- 62.Safari M, Yazdanpanah B. Decline of hepatitis B antibody level in vaccinated 5-7 year-old children. J Zahedan Med Res. 2010;12:24–8. [Google Scholar]
- 63.Saffar MJ, Rezaee MS. Persistence of hepatitis B vaccine safety ten years after public vaccination in infancy. J Semnan Univ Med Sci. 2003;5:63–71. [Google Scholar]
- 64.Rafizade B, Kazemi A, Amirmoghadami HR, Moosavinasab N. Evaluation of serum anti-HBs in 5-6 years old children vaccinated in Zanjan-2003. J Zanjan Univ Med Sci Health Serv. 2004;47:49–55. [Google Scholar]
- 65.Ramazani A, Aghakhani A, Eslamifar A, Banifazl M, Gachkar L, Taeb J, et al. Long-term safety of hepatitis B vaccine in hemodialytic patient. Iran J Clin Infect Dis. 2009;13:21–4. [Google Scholar]
- 66.Raeissi N, Habibian R, Zandian K. Determination of HBs Ab stability after HBV vaccination in multitransfused patients, Hajar Hospital, Shahrekord, 2003. J Blood. 2006;3:259–63. [Google Scholar]
- 67.Varesvazirian M, Vahidi A, Shamsadini S, Shamsadini A. Evaluation blood level Anti-HBs due to HB vaccine in thalassemia children in Kerman, 2007. J Kerman Univ Med Sci. 2007;6:309–15. [Google Scholar]
- 68.Esmaeilpoor N, Mirzaei N, Chaman R, Rasoulinegad M, Haji abdolbaghi M, Raham M, et al. Investigating HIV/AIDS patient's immune response to hepatitis B vaccination. J Shahrood Univ Med Sci. 2010;5:1–4. [Google Scholar]
- 69.Afrasiabian SH, Hajibageri K, Yousefinejad V, Esmaeeli Nasab NE, Sayfi SH. Response to hepatitis B vaccine in HIV-Infected patients, Armaghane-danesh bimonthly. J Yasuj Univ Med Sci. 2008;12:11–8. [Google Scholar]
- 70.Ahmadi F, Maziar S, Ramazani A, Pezeshki M, Khatami M, Mahdavi M, et al. Evaluation immune hepatitis B and factors in response to vaccination in hemodialysis patients (2004) Iran J Clin Infect Dis. 2004;9:38–41. [Google Scholar]
- 71.Alaee K, Mansouri D, Alaee A. The rate of response to hepatitis B vaccination in in HIV positive patient referred to HIV/AIDS advice and care centers in Kermanshah. J Babol Univ Med Sci. 2004;6:46–51. [Google Scholar]
- 72.Shahbazian H, Dinparast R. Evaluation of the immune response and efficacy of intradermal injection of hepatitis B vaccine in hemodialysis patient. Sci Med J Ahwaz Jundishapur Univ Med Sci. 2006;5:499–504. [Google Scholar]
- 73.Sharifi M, Talebitaher M, Sadeghi M. Antibody response to hepatitis B vaccination in hemodialytic patient. J Qazvin Univ Med Sci. 2003;25:47–52. [Google Scholar]
- 74.Azarkar Z, Sharifizade Gh. The effectiveness of vaccination against hepatitis B in children with thalassemia major-Khorasan (2007) Ofogh-e-Danesh. 2008;14:44–7. [Google Scholar]
- 75.Janbakhsh A, Vaziri S, Sayad B, Afsharian M, Rezaei M, et al. Immune response to standard dose of hepatitis B vaccine in HIV positive clients of Kermanshah behavioral diseases counseling center. Hepat Mon. 2006;6:71–4. [Google Scholar]
- 76.Koike Y, Yoo YC, Mitobe M, Oka T, Okuma K, Tono-oka S, et al. Enhancing activity of mycobacterial cell-derived adjuvants on immunogenicity of recombinant human hepatitis B virus vaccine. Vaccine. 1998;16:1982–9. doi: 10.1016/s0264-410x(98)00084-x. [DOI] [PubMed] [Google Scholar]
- 77.Jafarzadeh A, Khoshnoodi J, Ghorbani SH, Hazrati S, Mazaheri B, Shokri F. Differential immunogenicity of recombinant hepatitis B vaccine n Iranian neonates: Influence of ethnicity and environmental factors. Iran J Immunol. 2004;2:98–103. [Google Scholar]
- 78.Koff RS. Hepatitis vaccine. In: Schiff ER, Sorrell MF, Maddery WC, editors. Disease of the Liver. 8th ed. New York: Lippincott Williams and Wilkins; 1999. [Google Scholar]
- 79.Alimonos K, Nafziger AN, Murray J, Bertino JS., Jr Prediction of response to hepatitis B vaccine in health care workers: Whose titers of antibody to hepatitis B surface antigen should be determined after a three-dose series, and what are the implications in terms of cost-effectiveness? Clin Infect Dis. 1998;26:566–71. doi: 10.1086/514575. [DOI] [PubMed] [Google Scholar]
- 80.STROBE Statement — Checklist of items that should be included in reports of cross-sectional studies. [Last accessed date Dec 2010]. Available from: http://www.strobe-statement.org/
- 81.Fisman DN, Agrawal D, Leder K. The effect of age on immunologic response to recombinant hepatitis B vaccine: A meta-analysis. Clin Infect Dis. 2002;35:1368–75. doi: 10.1086/344271. [DOI] [PubMed] [Google Scholar]
- 82.Fabrizi F, Martin P, Dixit V, Bunnapradist S, Dulai G. Meta-analysis: The effect of age on immunological response to hepatitis B vaccine in end-stage renal disease. Aliment Pharmacol Ther. 2004;20:1053–62. doi: 10.1111/j.1365-2036.2004.02264.x. [DOI] [PubMed] [Google Scholar]
- 83.Afzali H, Vali Gh, Khalifehsoltani A. Comparative effect of intradermal and intramuscular injections of hepatitis B vaccine. Arch Iran Med. 2003;6:9–12. [Google Scholar]
- 84.Ferraz ML, Silva AE, Kemp VL, Cruz CN, Guimarães Rx. Evaluation of the immunological response to hepatitis B vaccine in health care professionals. Rev Assoc Med Bras. 1992;38:5–8. [PubMed] [Google Scholar]
- 85.Mansori D. Survey of incidence and affect of hepatitis B vaccine in students. J Babol Med Univ Sci. 1993;2:39–42. [Google Scholar]
- 86.Park K. 3rd ed. Tehran: Samat; 2002. Text Book of Preventive and Social Medicine Translated by ShojaiTehrani H; pp. 113–21. [Google Scholar]


