Abstract
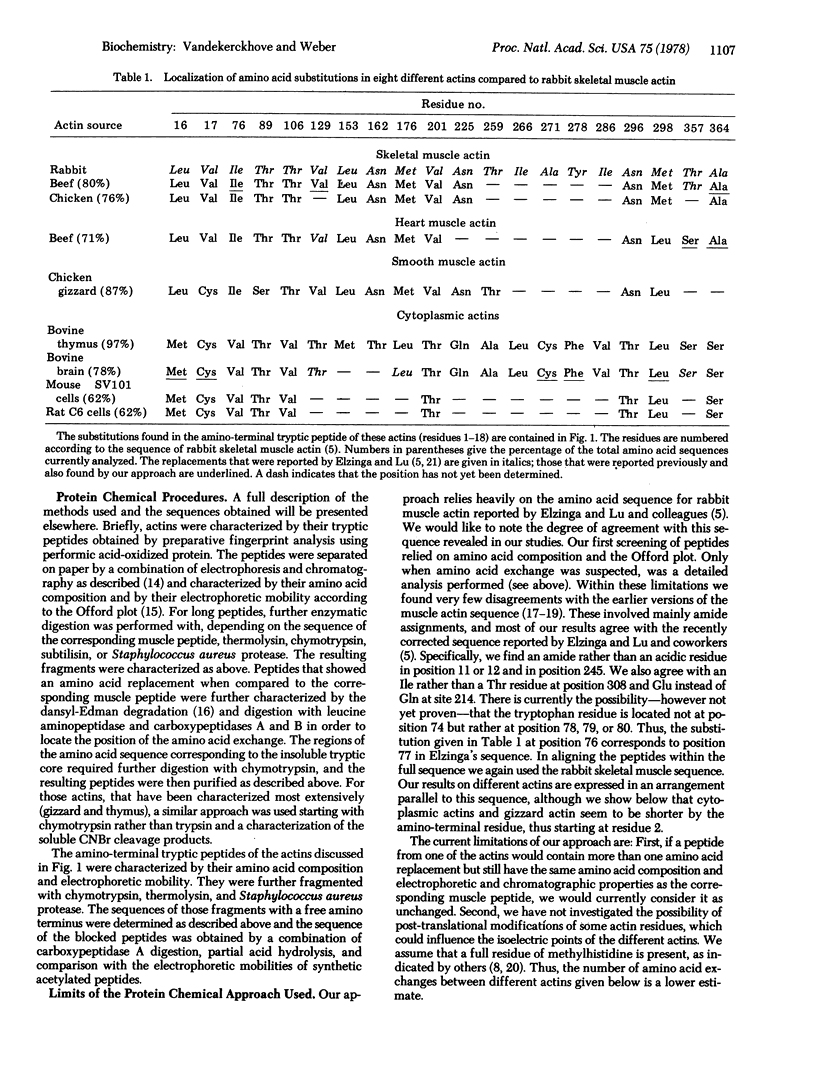
Muscle and cytoplasmic actins from several species have been compared by extensive fingerprint analysis and by partial amino acid sequence determination with the known amino acid sequence of rabbit muscle actin. Although complete sequences have not been established, the following characteristics are apparent. (a) Cytoplasmic actins are the products of two different genes. The difference seen in isoelectric focusing studies is probably determined only by the nature of the three amino-terminal acidic residues. (b) Mammalian cytoplasmic actins are exceedingly similar and perhaps identical. (c) Cytoplasmic actins may differ by at least 25 amino acid replacement from rabbit muscle actin. These replacements have been identified for calf thymus actin; however, other cytoplasmic actins show the same replacements. (d) The replacements always involve—except for the first five residues—neutral amino acid residues. (e) The replacements are not randomly distributed. Residues 18-75 are constant whereas residues 2-18 and 259-298 show many substitutions. (f) The main component of smooth muscle actin from chicken gizzard shows the charge characteristics found at the amino terminus of the less acidic cytoplasmic actin species. In the rest of the polypeptide chain, gizzard actin resembles skeletal muscle actin, although two substitutions of the cytoplasmic type have been identified. (g) Heart muscle actin is very similar to skeletal muscle actin. Only two amino acid replacements have been found; they are of the cytoplasmic type. (h) Skeletal muscle actins from chicken and beef have not shown a replacement.
Keywords: smooth muscle, brain, thymus, protein sequence, isoelectric focusing
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bray D., Thomas C. Unpolymerized actin in fibroblasts and brain. J Mol Biol. 1976 Aug 25;105(4):527–544. doi: 10.1016/0022-2836(76)90233-3. [DOI] [PubMed] [Google Scholar]
- Bridgen J. The amino acid sequence around four cysteine residues in trout actin. Biochem J. 1971 Jul;123(4):591–600. doi: 10.1042/bj1230591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Hartley B. S. Chemical studies on methionyl-tRNA synthetase from Escherichia coli. J Mol Biol. 1970 Sep 14;52(2):165–178. doi: 10.1016/0022-2836(70)90023-9. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Elzinga M. The primary structure of actin from rabbit skeletal muscle. Completion and analysis of the amino acid sequence. J Biol Chem. 1975 Aug 10;250(15):5915–5920. [PubMed] [Google Scholar]
- Elzinga M., Collins J. H., Kuehl W. M., Adelstein R. S. Complete amino-acid sequence of actin of rabbit skeletal muscle. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2687–2691. doi: 10.1073/pnas.70.9.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga M., Maron B. J., Adelstein R. S. Human heart and platelet actins are products of different genes. Science. 1976 Jan 9;191(4222):94–95. doi: 10.1126/science.1246600. [DOI] [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Gruenstein E., Rich A. Non-identity of muscle and non-muscle actins. Biochem Biophys Res Commun. 1975 May 19;64(2):472–477. doi: 10.1016/0006-291x(75)90345-9. [DOI] [PubMed] [Google Scholar]
- Izant J. G., Lazarides E. Invariance and heterogeneity in the major structural and regulatory proteins of chick muscle cells revealed by two-dimensional gel electrophoresis. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1450–1454. doi: 10.1073/pnas.74.4.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarides E., Lindberg U. Actin is the naturally occurring inhibitor of deoxyribonuclease I. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4742–4746. doi: 10.1073/pnas.71.12.4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Pollack R., Osborn M., Weber K. Patterns of organization of actin and myosin in normal and transformed cultured cells. Proc Natl Acad Sci U S A. 1975 Mar;72(3):994–998. doi: 10.1073/pnas.72.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Weihing R. R. Actin and myosin and cell movement. CRC Crit Rev Biochem. 1974 Jan;2(1):1–65. doi: 10.3109/10409237409105443. [DOI] [PubMed] [Google Scholar]
- Rubenstein P. A., Spudich J. A. Actin microheterogeneity in chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Jan;74(1):120–123. doi: 10.1073/pnas.74.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Vandekerckhove J. S., Gielen J. G., Van Montagu M. C. Sequence of the A-protein of coliphage MS2. II. Isolation and sequence determination of chymotryptic peptides. J Biol Chem. 1977 Nov 10;252(21):7761–7772. [PubMed] [Google Scholar]
- Weber K., Koch R., Herzog W., Vandekerckhove J. The isolation of tubulin and actin from mouse 3T3 cells transformed by simian virus 40 (SV3T3 cells), an established cell line growing in culture. Eur J Biochem. 1977 Aug 15;78(1):27–32. doi: 10.1111/j.1432-1033.1977.tb11710.x. [DOI] [PubMed] [Google Scholar]
- Whalen R. G., Butler-Browne G. S., Gros F. Protein synthesis and actin heterogeneity in calf muscle cells in culture. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2018–2022. doi: 10.1073/pnas.73.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]


