Abstract
Objective:
To identify a possible functional imaging biomarker sensitive to the earliest neural changes in premanifest Huntington disease (preHD), allowing early therapeutic approaches aimed at preventing or delaying clinical onset.
Methods:
Sixteen preHD and 18 healthy participants were submitted to anatomical acquisitions and functional MRI (fMRI) acquisitions during the execution of the exogenous covert orienting of attention task. Due to strong a priori hypothesis, all fMRI correlation analyses were restricted to the following: (1) the frontal oculomotor cortex identified by the means of a prosaccadic task, comprising frontal eye fields and supplementary frontal eye fields; and (2) the data collected during inhibition of return, a phenomenon occurring during the executed task. In preHD, multiple regression analysis was performed between fMRI data and the probability to develop the disease in the next 5 years (p5HD). Moreover, mean blood oxygen level–dependent (BOLD) signal changes in the frontal oculomotor cortex and striatal volumes were linearly correlated with p5HD.
Results:
In preHD, multiple regression analysis showed that clusters of activity strongly correlated with p5HD in the right frontal oculomotor cortex. Importantly, mean BOLD signal changes of this region correlated with p5HD (r2 = 0.52). Among the considered striatal volumes, a modest correlation (r2 = 0.29) was observed in the right putamen and p5HD.
Conclusion:
fMRI activations in the right-frontal oculomotor cortex during inhibition of return can be considered a possible functional imaging biomarker in preHD.
Huntington disease (HD) is an adult-onset inherited disorder characterized by progressive motor abnormalities and dementia. Studies focused on the detection of reliable measures of disease progression in premanifest HD (preHD) demonstrated that structural neuroimaging by means of striatal measure1–5 and functional neuroimaging (fMRI)6–13 are sensitive in detecting the earliest neural changes.
Impaired anatomical14 and functional connectivity15 between caudates and the frontal cortex, in particular the frontal eye fields (FEFs), were identified in preHD. FEFs are involved in saccades and exogenous covert orienting of spatial attention (COVAT).16 Accordingly, in patients with HD, an abnormal time course of inhibition of return (IOR)17,18 was observed during the execution of COVAT.
During this task,19 visual attention is directed, by reflex, toward a spatial location using a cue stimulus. When a second stimulus (target) is presented with an interval >250 ms (stimulus onset asynchrony [SOA]), a facilitation is observed when the target is presented in the opposite location respective to the cue (invalid trial), while an inhibition is elicited when the target is presented in the same location as the cue (valid trial). The phenomenon of inhibition is defined as IOR.20
We investigated whether the blood oxygen level–dependent (BOLD) signal changes in the frontal oculomotor cortex, comprising FEFs and supplementary FEFs, during IOR were correlated with the probability to develop disease onset in the next 5 years (p5HD).21 Our aim was to identify a possible fMRI biomarker that could represent an outcome measure in clinical trials for preHD individuals, aimed at preventing or delaying clinical onset.
METHODS
Standard protocol approvals, registrations, and patient consents.
PreHD participants were selected among those who decided to undergo the program for predictive genetic testing, according to the protocol approved by our institutional review board, in agreement with the international guidelines for genetic testing in HD (International Huntington Association and World Federation of Neurology Research Group on Huntington's Chorea, 1994). The study protocol was approved by the local ethics committee and informed consent was obtained from each subject in accordance with the Helsinki Declaration.
Participants.
Inclusion criteria for preHD were as follows: 1) molecular diagnosis for HD with CAG triplet expansion ≥39; 2) absence of neurologic diseases; 3) scores on Unified Huntington Disease Rating Scale: motor assessment = 0 and diagnostic confidence level = 0,22 as judged by a neurologist experienced in HD (table 1). For preHD individuals, we calculated p5HD.21 None of the preHD individuals presented motor or cognitive symptoms consistent with possible HD clinical onset. One preHD individual was not included in the study for claustrophobia, and 1 control subject for alcohol abuse before the MRI execution. Sixteen preHD participants (mean age 31 ± 7.9 years; 8 male) and 18 healthy participants (mean age 29 ± 5.4 years; 9 male) participated in this study. The 2 groups did not differ with respect to age or years of education. All preHD participants had low p5HD (median p5HD = 0.04), indicating that our sample was far from the clinical onset. Except for one control, all the participants were right-handed as determined by means of Edinburgh Inventory Scale.23
Table 1.
Demographic and clinical data for the preHD and control groups
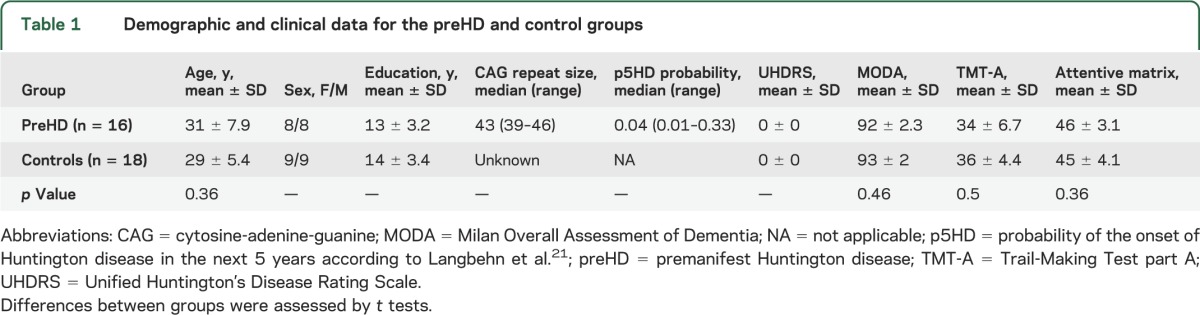
All subjects underwent a neuropsychological battery including screening tests for global cognitive abilities (Milan Overall Dementia Assessment and Raven's Progressive Matrices) and tests for the assessment of attention and executive functions (Forward and Backward Digit Span, Dual Task, Stroop-Color-Word Interference Task, Phonemic Fluency, Ruff Figural Fluency Test, Trail-Making Test, and Attention Matrices). A brief description of tests administrated is provided in appendix e-1 on the Neurology® Web site at Neurology.org. All tests were used in their Italian standardized version whenever available. The obtained scores were in the normal range for all subjects and t tests corrected for multiple comparisons showed no difference between groups (table 1).
Volumetric and functional MRI data acquisition.
We used a 1.5T Siemens Magnetom Avanto magnetic resonance scanner (Erlangen, Germany). The protocol included 3D magnetization-prepared rapid acquisition gradient echo sequence (repetition time [TR] = 1,640, echo time [TE] = 2.12 ms, inversion time = 552 ms, voxel size: 1 × 1 × 1 mm, 256 × 256 matrix, 160 slices, slice thickness = 1 mm, gap = 0.5 mm) and T2*-weighted images, obtained using echoplanar imaging in axial orientation (TR = 2,000 ms, TE = 52 ms, voxel size = 4 × 4 mm, 64 × 64 matrix, 21 slices, slice thickness = 5 mm, no gap).
fMRI tasks.
During a single fMRI session, participants were presented with 3 event-related tasks: COVAT, prosaccadic task (PST), and antisaccadic task. We investigated BOLD signal changes during the execution of COVAT, and identified the frontal oculomotor cortex by means of PST. Therefore, we present methods and results for COVAT extensively, but briefly for PST. Reaction times (RT) and eye movements (IRIS infrared eye-tracking system; sample at 64 Hz) were collected during the scanning session.
COVAT.
Participants were presented with 3 horizontally arranged boxes, with a fixation cross in the central box. The cue stimulus was presented in one of the 2 lateral boxes; then a second stimulus (target) was presented either in the same box (valid trial) or in the opposite box (invalid trial). Participants were instructed to maintain central fixation and to respond to the target as quickly as possible, considering that target and cue locations were independently presented (appendix e-2, figure e-1).
Based on previous studies, we used 250-ms SOAs in the hypothesis of IOR with accelerated time course17 and 900-ms SOA in the hypothesis of IOR with delayed time course.18
PST.
We used the same structure of COVAT (overlap paradigm),24 but without the occurrence of the cue. Participants were instructed to execute a saccadic eye movement to the target as soon as it appeared and then to return to fixate the central cross.24
Data analyses.
Two preHD and 3 healthy participants were excluded due to the excessive head movements (>2 mm) during the fMRI session,25 detected after the application of the motion correction algorithm during the preprocessing step of the fMRI data.
COVAT data analyses were performed in 14 preHD and 15 healthy participants, and PST data analyses in 15 preHD and 16 healthy participants.
Behavioral data analyses.
COVAT.
Mean RT at 250 ms SOA and at 900 ms SOA were calculated for each participant and entered in a repeated-measures analysis of variance (ANOVA) with group (preHD, controls) as between-subjects factor and trial (valid, invalid) and SOA (250 ms, 900 ms) as within-subject factors. On the basis of the strong a priori hypothesis that IOR effect could emerge at different SOA in preHD and in control group, planned comparisons were also implemented. This allowed us to identify the SOA (250 ms or 900 ms) that elicited the IOR effect. The trials at the identified SOA were used to implement regression analyses for fMRI data (see following). Regression analyses were also performed between RT and p5HD.
PST.
Mean saccadic latencies were entered in a repeated-measures ANOVA with group (preHD, controls) as between-subjects factor and SOA (250 ms, 900 ms) as within-subject factor.
Volumetric MRI data analyses.
Left and right caudate and putamen volumes were measured on the T1-weighted magnetization-prepared rapid gradient echo images. An initial segmentation and labelling were performed automatically by means of a model-based segmentation tool FIRST (FSL). The resulting segmentations of the striatal structures were subsequently refined, section by section, on the 3 planes, by an experienced operator (M.L.M.). The posterior limit of the segmented caudate nucleus corresponded to the posterior margin of the body at the level of the posterior third segment of the cella media of the lateral ventricle. The tail of the caudate nucleus was not included due to its difficult identification. The volume of each structure was extracted and corrected for intracranial volume.26 A repeated-measures ANOVA with group (preHD, controls) as between-subjects factor and structure (caudate, putamen) and side (right, left) as within-subjects factors were used to test differences between groups. Moreover, for the preHD group, correlations were calculated between the volumes of caudates–putamina and p5HD. To exclude the possible influence of age on striatal volumes, we calculated the correlation between each striatal structure and age in the control group.
fMRI data analyses.
fMRI data were analyzed in the framework of the general linear model using SPM8 (Wellcome Trust Centre for Neuroimaging; http://www.fil.ion.ucl.ac.uk) and Marsbar software (http://marsbar.sourceforge.net/) running on Matlab 7.12 (MathWorks, Natick, MA).
Single subject analyses.
The images preprocessing included realignment to the first volume, normalization to Montreal Neurological Institute template, and smoothing (full width at half maximum isotropic Gaussian kernel = 8 mm). For each task, 4 experimental conditions (COVAT: valid and invalid trials at SOA = 250 ms and at SOA = 900 ms; PST: left and right trials at SOA = 250 ms and at SOA = 900 ms) were modeled with time and dispersion derivatives in the design matrixes. Contrast images of interest of each single experimental condition were produced.
Group analyses.
Random effect analyses were implemented in order to account for intersubjects variability and to generalize inferences from which populations were drawn.27
Contrast images from single-subjects analyses were used to implement 2 × 2 × 2 ANOVAs for COVAT and PST with group (preHD, controls) as between-subjects factor and trial (COVAT: valid, invalid; PST: left, right) and SOA (COVAT and PST: 250 ms, 900 ms) as within-subjects factors.
For COVAT, multisubject statistical maps were produced for (1) the main effect of the task for each group and (2) the between-groups comparison. Inferences were drawn from cluster-level significance scores.28 In order to form clusters, a voxel-level threshold of p < 0.001 uncorrected was applied and subsequently a threshold of p < 0.05 family-wise error (FWE)–corrected was applied at cluster level.
When significant results in the between-groups statistical comparison were detected, we proceeded extracting the BOLD signal % changes in the region involved at the single-subject level. These values were then entered in a nonparametric analysis (Mann-Whitney U tests) to strengthen the between-groups comparison.
For PST, multisubject statistical map of the main effect of the task was produced (voxel-level threshold = p < 0.05 FWE-corrected; cluster-level threshold = p < 0.05 FWE-corrected). From this map, masks of the right and left frontal oculomotor cortex, comprising FEFs and supplementary FEFs, were created. These masks were applied to the regression analyses.
Regression analyses.
In the preHD group, multiple regression analyses, restricted to the previous identified masks (implicit masking: p = 0.001), were conducted between p5HD and the contrast images obtained for the conditions for which the strongest behavioral effect of IOR was observed (SOA = 900 ms; valid and invalid condition).
Then, in the detected correlation clusters, mean BOLD signal % changes were extracted at single-subject level in 6-mm spherical regions of interest (ROIs) centered in the signal peak of each cluster. Pearson product-moment correlation coefficients were calculated between these values and p5HD. In order to reinforce these results, mean BOLD signal % changes of the right and left frontal oculomotor cortex were extracted in each single subject and entered in a correlation analysis with p5HD. Analyses were also conducted in the control group using the corresponding age at the time of scanning.
RESULTS
Behavioral results.
COVAT.
We detected no effect for group factor.
The planned comparisons showed a significant difference between invalid and valid trials (IOR effect) at 900-ms SOA but not at 250-ms SOA in preHD (F1,25 = 18.03; p = 0.0003) and in controls (F1,25 = 15.09; p = 0.0007), thus indicating that the IOR effect emerged in both groups at the same SOA (900 ms) (see also appendix e-3). No significant correlations were found between behavioral data (means of the reaction times) and p5HD. Detailed information is reported in appendix e-3.
PST.
No effect was detected for group factor and no interaction between factors was observed.
Volumetric MRI results.
ANOVA showed significant differences between groups (F1,27 = 6.95; p =0 0.014): preHD participants had smaller striatal volumes than healthy participants.
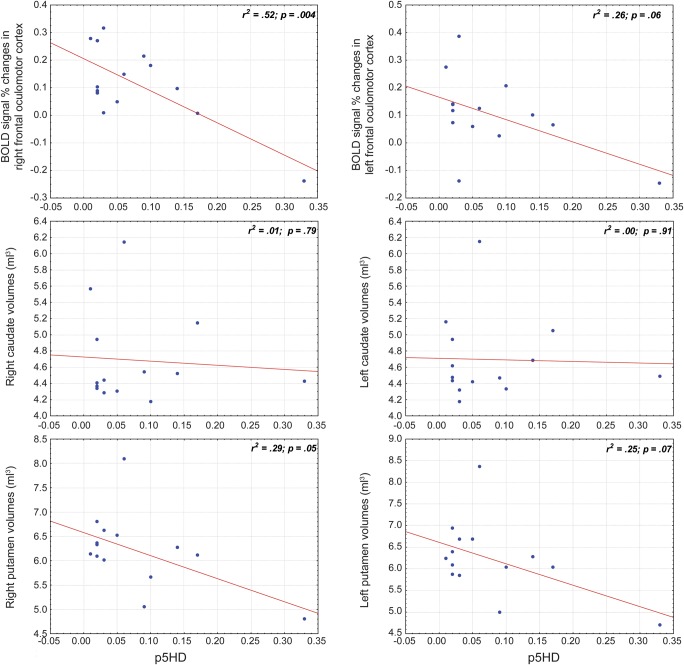
In the preHD group, a significant correlation emerged between p5HD and right putamen volume (r2 = 0.29; p = 0.05), while no correlation emerged for the remaining striatal structures. ANOVA analyses using the mean volumes of caudates and putamina showed similar results. A tendency toward significance was observed between the mean putamen volumes and p5HD (r2 = 0.27; p = 0.06); no significant correlations were found between the mean caudate volumes and p5HD (r2 = 0.0034; p = 0.8439). In healthy participants, no correlation was observed between age and the striatal volumes (table 2, figure 1).
Table 2.
Mean volumes of the striatal structures for both groups
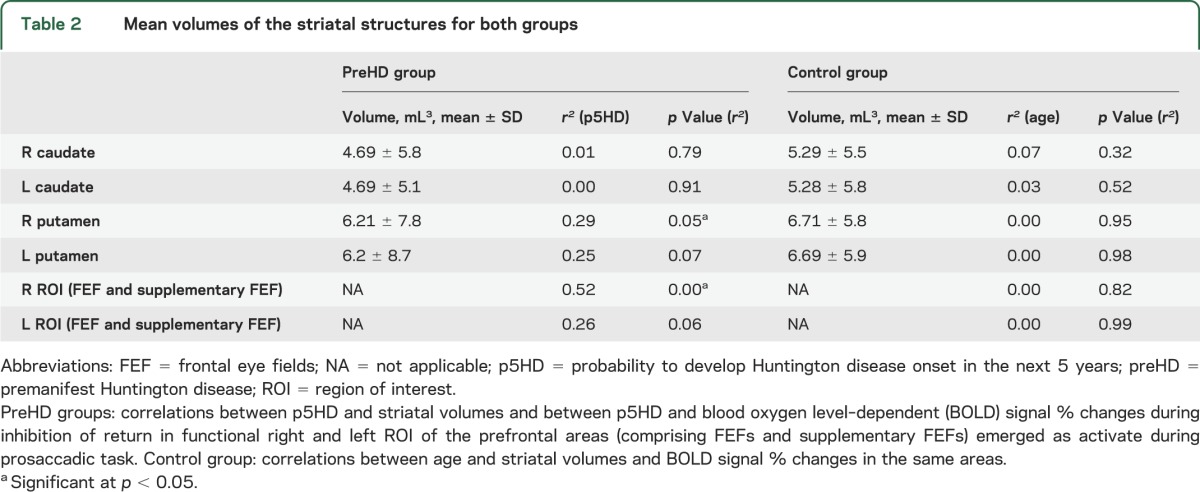
Figure 1. Correlations between MRI data and p5HD.
Premanifest Huntington disease participants: regression analyses and relative r2 between probability to develop Huntington disease onset in the next 5 years (p5HD) and functional MRI data (blood oxygen level–dependent signal % changes in left and right regions of interest) and between p5HD and anatomical data (left and right caudate and putamen).
fMRI results.
Full factorial analyses.
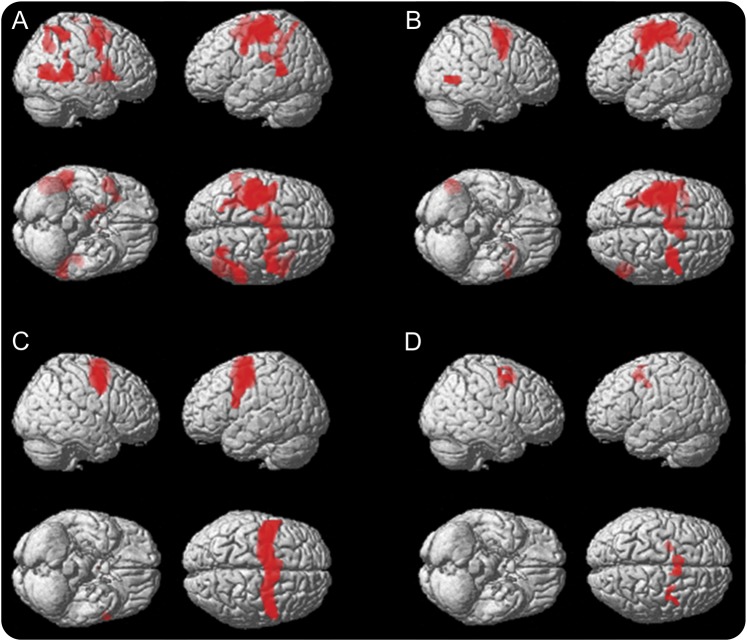
The random effect analysis of COVAT fMRI data revealed a similar network of the main effect of the task in each group (figure 2, A and B). The between-groups comparison revealed that preHD participants showed increased BOLD signal in the right middle temporal gyrus (peak voxel in 56, −36, 6, 278 voxels). Nonparametric analysis of the mean BOLD signal % changes in this cluster confirmed differences between the 2 groups (U(25) = −3.47, p = 0.000). The multisubject statistical map for the main effect of PST showed a widespread network of activity comprising, among others, the bilateral frontal oculomotor cortex. The left and right masks built from these frontal areas of this map comprised the supposed bilateral FEFs and supplementary FEFs (figure 2C).
Figure 2. fMRI results during the execution of visuospatial attention task.
(A) Main effect of exogenous covert orienting of spatial attention (COVAT) in premanifest Huntington disease (preHD) participants (voxel-level threshold = p < 0.05 family-wise error [FWE]–corrected, cluster-level threshold = p < 0.05 FWE-corrected). (B) Main effect of COVAT in healthy participants (voxel-level threshold = p < 0.05 FWE-corrected, cluster-level threshold = p < 0.05 FWE-corrected). (C) Masks built of the frontal oculomotor cortex emerged in the multisubjects statistical map of the main effect of the prosaccadic task (p < 0.05 FWE-corrected) and comprising the supposed bilateral frontal eye fields (FEF) and supplemental FEF. (D) Clusters emerged in the multiple regression analysis restricted to the regions of interest (p < 0.001 uncorrected) conducted in preHD participants between probability to develop Huntington disease onset in the next 5 years and the contrast images obtained for the conditions for which the strongest behavioral effect of inhibition of return was observed (i.e., stimulus onset asynchrony = 900 ms; valid and invalid condition).
Regression analyses.
In preHD, the multiple regression analysis showed several clusters in right supplementary FEF and FEF where BOLD signal % changes correlated with p5HD (table 3; figure 2D). The corresponding analysis in the control group, using age instead of p5HD, did not show any significant result.
Table 3.
PreHD participants: Clusters of correlation between blood oxygen level–dependent signal % changes during inhibition of return (valid and invalid trial) and p5HD
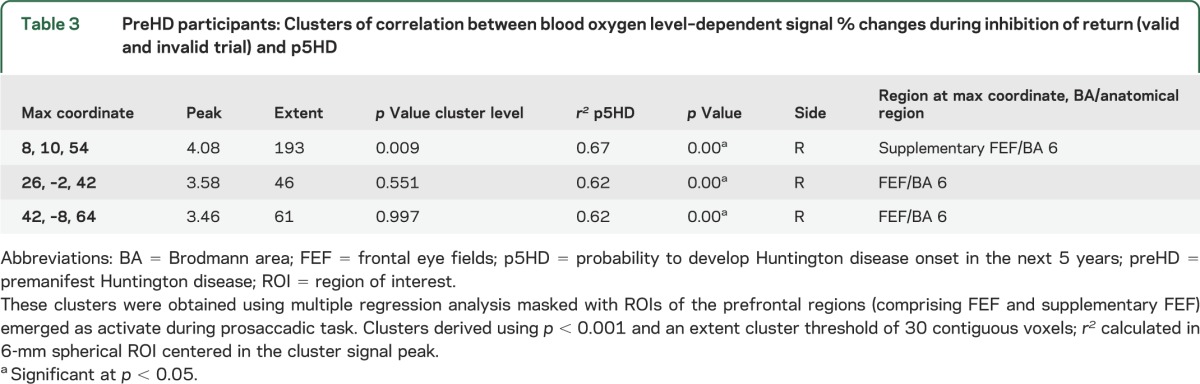
Correlation indices calculated in the 6-mm spherical ROIs showed very strong results, yielding r2 = 0.67 and r2 = 0.62, respectively, in right supplementary FEF and FEF (table 3).
In the preHD group, the mean BOLD signal % changes calculated for the whole right frontal oculomotor cortex was correlated with p5HD (r2 = 0.52, p = 0.004) (table 2). A tendency toward significance was also observed between the whole left frontal oculomotor cortex (r2 = 0.26, p = 0.064) and p5HD (table 2). The corresponding analysis in the control group did not show any significant result.
DISCUSSION
Our results showed that the mean BOLD signal % changes of several clusters identified in the right frontal oculomotor cortex, comprising FEFs and supplementary FEFs, were strongly correlated with p5HD (yielding r2 = 0.67) in a group of preHD participants during IOR phenomenon.
The correlation was still very strong (r2 = 0.52) considering the whole right frontal oculomotor cortex. This indicates that our results were not spurious, but the outcome of a regional brain activity strictly correlates with the supposed subclinical disease progression.
The correlation scores are robust for several reasons: (1) the experiment was conceived starting from strong a priori hypothesis of the involvement of FEFs in HD,12,15 of the abnormal time course of IOR in HD,17,18 and of saccade and attention alterations in preHD; (2) preHD participants had very low p5HD, indicating that probably they were far from onset; (3) an independent localizer task (PST) was used29 to identify the frontal oculomotor cortex; (4) to estimate the supposed subclinical disease progression, we used an accurate approach (p5HD).7 P5HD, in fact, is the only predictive model based on a very large collection of patients with HD and at-risk individuals.21 The comparison with other prediction models has demonstrated the consistency of this model, and its utility in research perspective.30 Our results support the hypothesis that functional neuroimaging studies are sensitive in detecting the earliest neural changes in far-from-clinical-onset preHD individuals.6,7
It is widely accepted that FEFs are involved in visual spatial attention and eye movements. During the execution of COVAT, patients with HD presented abnormal IOR time course. These results were explained as reduced inhibitory activity of the basal ganglia, which accelerated IOR,17 and a slowing disengagement processes, which delayed IOR.18 Although IOR is still under debate, it has been conceptualized as an inhibitory activity over the superior colliculus, determined cortically by structures involved in both ocular movement and attention, such as FEFs.16 Considering the role of FEFs in eye movements, it is expected that patients with HD present saccades alterations as earliest clinical signs.31 Further supporting this view, in individuals with preHD, the variability of the voluntary-guided saccadic latencies correlated with the disease severity31 and with fiber tracking streamlines connecting FEFs with the caudate body.14
We confirmed previous findings of smaller striatal volumes in preHD participants in comparison to controls,3,4 therefore supporting the hypothesis that selective brain degeneration begins decades before diagnosable HD. We did not demonstrate significant correlations between the single striatal volumes and p5HD except for the right putamen (r2 = 0.29). Probably this finding could be related to the small sample size, rather than a different involvement of the striatal structures.
Although behavioral performance did not differ between preHD participants and controls, preHD participants showed increased task-related activity in the right middle temporal gyrus in comparison to healthy participants. This result confirms that brain activity is increased in preHD individuals during different fMRI tasks,8,9 suggesting that enhanced cortical activation might be a physiologic response to compensate striatal or localized cortical dysfunction.7,32
Methodologic limitations of our study are the small sample size and the estimated age at onset based on CAG repeat length, which is an estimate itself with its own variability. A further intrinsic limitation could be represented by a biased selection of the HD at-risk population used for the development of Langbehn model. This population, in fact, represents people requiring predictive genetic test and not the entire world HD at-risk population. In addition, it is well-known that the CAG repeat length is not the only determinant of the HD onset, particularly in individuals with small expansion size, and possible contributions of both genetic and environmental factors also should be taken into consideration.21
The functional imaging findings we identified are likely to be considered a possible imaging biomarker tracking disease progression since the very early stage of the disease. Although imaging biomarkers are powerful for the detection of the earliest events in HD neurodegeneration, they are expensive and time-consuming. Longitudinal studies with larger samples are needed to test the validity of this index, and to develop easier clinical procedures to be used as possible outcome measures in future therapeutic trials.
Supplementary Material
ACKNOWLEDGMENT
This article is dedicated to the memory of Mario Savoiardo, MD, a neuroradiologist known worldwide for his numerous collaborations, his vast knowledge and experience in MRI, and his keen scientific contributions, who died on January 30, 2014. He was admired for his peerless professional talent and capacities, the endless time dedicated to studying and discussing the most difficult clinical cases, his numerous interests in art, music, and photography. We will miss his brilliant intelligence and judgment, but, most of all, his warm-heartedness and friendship.
GLOSSARY
- ANOVA
analysis of variance
- BOLD
blood oxygen level–dependent
- COVAT
covert orienting of spatial attention
- FEF
frontal eye field
- fMRI
functional MRI
- FWE
family-wise error
- HD
Huntington disease
- IOR
inhibition of return
- p5HD
probability to develop Huntington disease onset in the next 5 years
- preHD
premanifest Huntington disease
- PST
prosaccadic task
- ROI
region of interest
- RT
reaction times
- SOA
stimulus onset asynchrony
- TE
echo time
- TR
repetition time
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
S. Ferraro: study concept and design, analysis and interpretation of data, acquisition of data, statistical analysis, study supervision. Dr. Nanetti: study concept and design, drafting the manuscript for content. Dr. Piacentini: analysis and interpretation of data, acquisition of data. Dr. Mandelli: analysis and interpretation of data, acquisition of data, drafting the manuscript for content. N. Bertolino: analysis and interpretation of data, acquisition of data. Dr. Ghielmetti: analysis and interpretation of data, acquisition of data. F. Epifani: analysis and interpretation of data, acquisition of data. A. Nigri: analysis and interpretation of data, acquisition of data. Dr. Taroni: study concept and design, revising the manuscript for content. Dr. Bruzzone: study concept and design, revising the manuscript for content. Dr. Di Donato: revising the manuscript for content. Dr. Savoiardo: revising the manuscript for content. Dr. Mariotti: study concept and design, revising the manuscript for content. Dr. Grisoli: study concept and design, drafting and revising the manuscript for content, study supervision or coordination.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Aylward EH, Codori AM, Barta PE, Pearlson GD, Harris GJ, Brandt J. Basal ganglia volume and proximity to onset in presymptomatic Huntington disease. Arch Neurol 1996;53:1293–1296 [DOI] [PubMed] [Google Scholar]
- 2.Aylward EH, Codori AM, Rosenblatt A, et al. Rate of caudate atrophy in presymptomatic and symptomatic stages of Huntington's disease. Mov Disord 2000;15:552–560 [DOI] [PubMed] [Google Scholar]
- 3.Aylward EH, Sparks BF, Field KM, et al. Onset and rate of striatal atrophy in preclinical Huntington disease. Neurology 2004;63:66–72 [DOI] [PubMed] [Google Scholar]
- 4.Paulsen JS, Zimbelman JL, Hinton SC, et al. fMRI biomarker of early neuronal dysfunction in presymptomatic Huntington's Disease. AJNR Am J Neuroradiol 2004;25:1715–1721 [PMC free article] [PubMed] [Google Scholar]
- 5.Paulsen JS, Langbehn DR, Stout JC, et al. Detection of Huntington's disease decades before diagnosis: the predict-HD study. J Neurol Neurosurg Psychiatry 2008;79:874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulsen JS. Functional imaging in Huntington's disease. Exp Neurol 2009;216:272–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weir DW, Sturrock A, Leavitt BR. Development of biomarkers for Huntington's disease. Lancet Neurol 2011;10:573–590 [DOI] [PubMed] [Google Scholar]
- 8.Wolf RC, Vasic N, Schonfeldt-Lecuona C, Landwehrmeyer GB, Ecker D. Dorsolateral prefrontal cortex dysfunction in presymptomatic Huntington's disease: evidence from event-related fMRI. Brain 2007;130:2845–2857 [DOI] [PubMed] [Google Scholar]
- 9.Wolf RC, Grön G, Sambataro F, et al. Brain activation and functional connectivity in premanifest Huntington's disease during states of intrinsic and phasic alertness. Hum Brain Mapp 2012;33:2161–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zimbelman JL, Paulsen JS, Mikos A, Reynolds NC, Hoffmann RG, Rao SM. fMRI detection of early neural dysfunction in preclinical Huntington's disease. J Int Neuropsychol Soc 2007;13:758–769 [DOI] [PubMed] [Google Scholar]
- 11.Saft C, Schuttke A, Beste C, Andrich J, Heindel W, Pfleiderer B. fMRI reveals altered auditory processing in manifest and premanifest Huntington's Disease. Neuropsychologia 2008;46:1279–1289 [DOI] [PubMed] [Google Scholar]
- 12.Kloppel S, Draganski B, Siebner HR, Tabrizi SJ, Weiller C, Frackowiak RS. Functional compensation of motor function in pre-symptomatic Huntington's disease. Brain 2009;132:1624–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Novak MJ, Warren JD, Henley SM, Draganski B, Frackowiak RS, Tabrizi SJ. Altered brain mechanisms of emotion processing in pre-manifest Huntington's disease. Brain 2012;135:1165–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kloppel S, Draganski B, Golding CV, et al. White matter connections reflect changes in voluntary-guided saccades in pre-symptomatic Huntington's disease. Brain 2008;131:196–204 [DOI] [PubMed] [Google Scholar]
- 15.Unschuld PG, Joel SE, Liu X, et al. Impaired cortico-striatal functional connectivity in prodromal Huntington's disease. Neurosci Lett 2012;514:204–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbetta M, Akbudak E, Conturo TE, et al. A common network of functional areas for attention and eye movements. Neuron 1998;21:761–773 [DOI] [PubMed] [Google Scholar]
- 17.Fielding J, Georgiou-Karistianis N, Bradshaw J, Millist L, Churchyard A, White O. Accelerated time-course of inhibition of return in Huntington's disease. Behav Brain Res 2006;166:211–219 [DOI] [PubMed] [Google Scholar]
- 18.Couette M, Bachoud-Levi AC, Brugieres P, Sieroff E, Bartolomeo P. Orienting of spatial attention in Huntington's disease. Neuropsychologia 2008;46:1391–1400 [DOI] [PubMed] [Google Scholar]
- 19.Posner MI, Walker JA, Friedrich FJ, Rafal RD. Effects of parietal injury on covert orienting of attention. J Neurosci 1984;4:1863–1874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lepsien J, Pollmann S. Covert reorienting and inhibition of return: an event-related fMRI study. J Cogn Neurosci 2002;14:127–144 [DOI] [PubMed] [Google Scholar]
- 21.Langbehn DR, Brinkman RR, Falush D, Paulsen JS, Hayden MR; International Huntington's Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington's disease based on CAG length. Clin Genet 2004;65:267–277 [DOI] [PubMed] [Google Scholar]
- 22.Huntington Study Group. Unified Huntington's Disease Rating Scale: reliability and consistency. Huntington study group. Mov Disord 1996;11:136–142 [DOI] [PubMed] [Google Scholar]
- 23.Oldfield RC. The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 1971;9:97–113 [DOI] [PubMed] [Google Scholar]
- 24.Domagalik A, Beldzik E, Fafrowicz M, Oginska H, Marek T. Neural networks related to pro-saccades and anti-saccades revealed by independent component analysis. Neuroimage 2012;62:1325–1333 [DOI] [PubMed] [Google Scholar]
- 25.Breiter HC, Aharon I, Kahneman D, Dale A, Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron 2001;30:619–639 [DOI] [PubMed] [Google Scholar]
- 26.Mandelli ML, Savoiardo M, Minati L, et al. Decreased diffusivity in the caudate nucleus of presymptomatic Huntington disease gene carriers: which explanation? AJNR Am J Neuroradiol 2010;31:706–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friston KJ, Holmes AP, Worsley KJ. How many subjects constitute a study? Neuroimage 1999;10:1–5 [DOI] [PubMed] [Google Scholar]
- 28.Friston KJ, Holmes A, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: Levels of inference and power. Neuroimage 1996;4:223–235 [DOI] [PubMed] [Google Scholar]
- 29.Vul E, Harris C, Winkielman P, Pashler H. Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspect Psychol Sci 2009;4:274–290 [DOI] [PubMed] [Google Scholar]
- 30.Langbehn DR, Hayden MR, Paulsen JS; PREDICT-HD Investigators of the Huntington Study Group. CAG-repeat length and the age of onset in Huntington disease (HD): a review and validation study of statistical approaches. Am J Med Genet B Neuropsychiatr Genet 2010;153B:397–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Golding CV, Danchaivijitr C, Hodgson TL, Tabrizi SJ, Kennard C. Identification of an oculomotor biomarker of preclinical Huntington disease. Neurology 2006;67:485–487 [DOI] [PubMed] [Google Scholar]
- 32.Rosas HD, Hevelone ND, Zaleta AK, Greve DN, Salat DH, Fischl B. Regional cortical thinning in preclinical Huntington disease and its relationship to cognition. Neurology 2005;65:745–747 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.