Abstract
Objective:
To determine the cumulative rate and characteristics of recurrent thromboembolic events after acute ischemic stroke in patients with cancer.
Methods:
We retrospectively identified consecutive adult patients with active systemic cancer diagnosed with acute ischemic stroke at a tertiary-care cancer center from 2005 through 2009. Two neurologists independently reviewed all electronic records to ascertain the composite outcome of recurrent ischemic stroke, myocardial infarction, systemic embolism, TIA, or venous thromboembolism. Kaplan-Meier statistics were used to determine cumulative outcome rates. In exploratory analyses, Cox proportional hazard analysis was used to evaluate potential independent associations between a priori selected clinical factors and recurrent thromboembolic events.
Results:
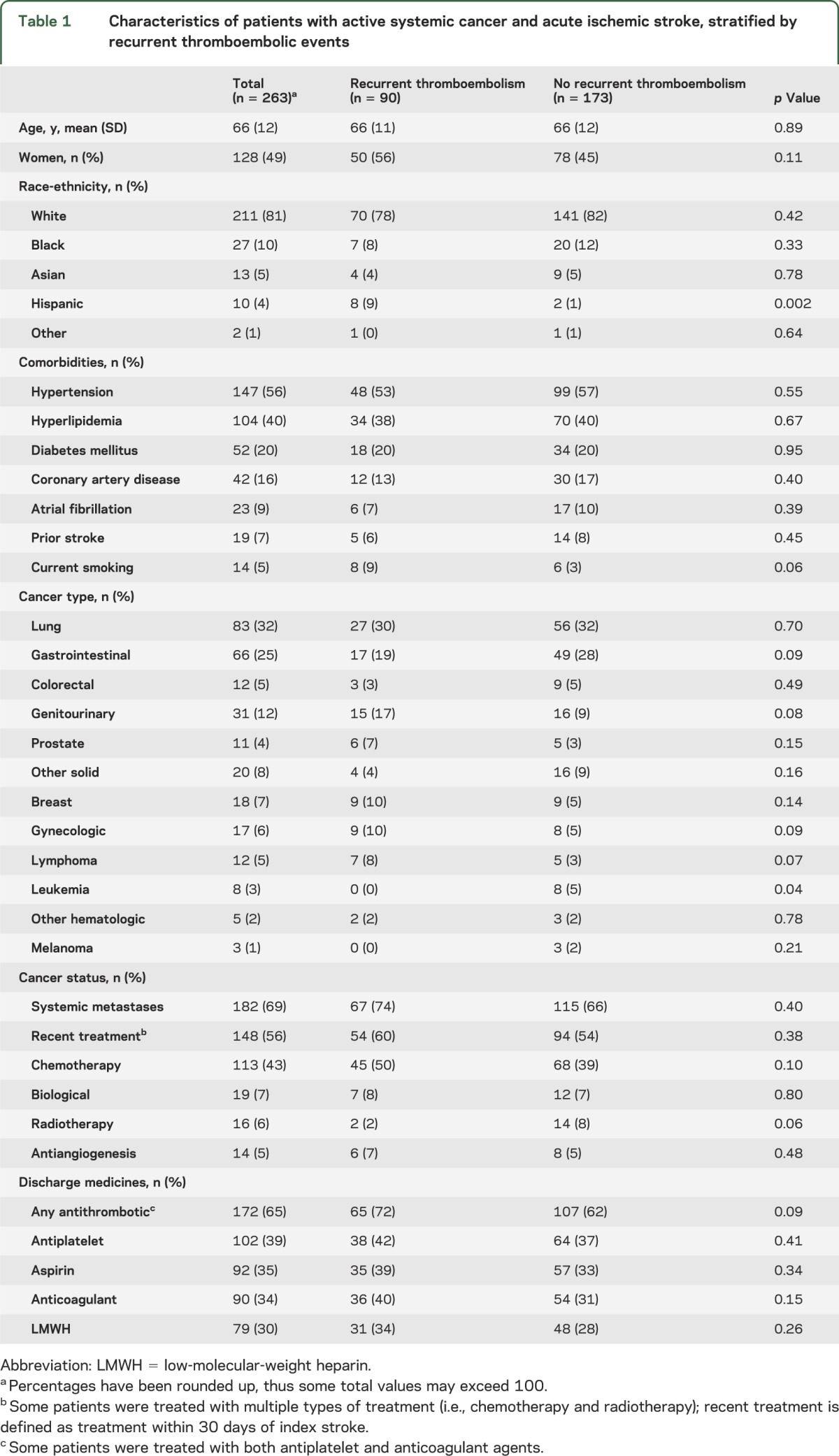
Among 263 study patients, complete follow-up until death was available in 230 (87%). Most patients had an adenocarcinoma as their underlying cancer (60%) and had systemic metastases (69%). Despite a median survival of 84 days (interquartile range 24–419 days), 90 patients (34%; 95% confidence interval 28%–40%) had 117 recurrent thromboembolic events, consisting of 57 cases of venous thromboembolism, 36 recurrent ischemic strokes, 13 myocardial infarctions, 10 cases of systemic embolism, and one TIA. Kaplan-Meier rates of recurrent thromboembolism were 21%, 31%, and 37% at 1, 3, and 6 months, respectively; cumulative rates of recurrent ischemic stroke were 7%, 13%, and 16%. Adenocarcinoma histology (hazard ratio 1.65, 95% confidence interval 1.02–2.68) was independently associated with recurrent thromboembolism.
Conclusions:
Patients with acute ischemic stroke in the setting of active cancer (especially adenocarcinoma) face a substantial short-term risk of recurrent ischemic stroke and other types of thromboembolism.
Cancer often causes a hypercoagulable state. This is due to various intrinsic and extrinsic mechanisms, including the secretion of procoagulant substances by tumor cells, endothelial dysfunction caused by the release of inflammatory cytokines from native immune cells, and complications of cancer therapy.1–4 As a result, patients with cancer frequently have arterial and venous thromboembolic events, including ischemic stroke, which is the second most common brain lesion in patients with cancer and occasionally serves as its initial presentation.5–8
Patients with cancer who develop ischemic strokes often have metastatic disease and may be at high risk for other thromboembolic events.7–10 Their cause of stroke frequently differs from the general population and is commonly attributed to unconventional mechanisms related to hypercoagulability, such as nonbacterial thrombotic endocarditis (NBTE).5,8,10,11 Transcranial Doppler ultrasound frequently demonstrates high rates of microemboli in patients with cancer and stroke, suggesting that these patients are at high risk of recurrent stroke.12 However, to the authors' knowledge, only one study has previously reported the risk of recurrent ischemic stroke in patients with active cancer, and this study is limited by a small and outdated cohort.13 In addition, no large-scale study has systematically evaluated the incidence or characteristics of recurrent thromboembolic events in this study population.
We therefore conducted a retrospective cohort study to delineate the rate, type, and potential predictors of recurrent thromboembolism in patients with cancer and acute ischemic stroke. Our a priori study hypotheses were that recurrent thromboembolic events (including recurrent stroke) are very common in patients with active cancer and stroke, and that NBTE is associated with an increased risk of recurrence because it is a direct manifestation of cancer-mediated hypercoagulability.
METHODS
Study design.
This was a retrospective cohort study of patients with active systemic cancer diagnosed with acute ischemic stroke in inpatient and emergency department settings at the Memorial Sloan-Kettering Cancer Center (MSKCC). Patients initially diagnosed with stroke at an outside hospital and subsequently transferred to MSKCC for further inpatient care were included in the study, while patients initially diagnosed with stroke at other centers and subsequently evaluated by MSKCC physicians solely as an outpatient were excluded because these patients often had incomplete diagnostic evaluations. MSKCC is an urban, tertiary-care hospital specializing in the care of patients with confirmed or suspected cancer. Although it is not a primary stroke center, patients treated at MSKCC often receive all their medical care there and are closely followed for adverse events; all patient records, including those from outside facilities, are comprehensively collected in electronic format. Furthermore, MSKCC patients diagnosed with stroke at other facilities are often transferred to MSKCC for completion of their stroke evaluation.
Standard protocol approvals, registrations, and patient consents.
The MSKCC Institutional Review Board approved this study; the need for informed consent was waived because of minimal risk to patients.
Study subjects.
We identified consecutive adult patients (age 18 years or older) with active systemic cancer who were diagnosed with an acute ischemic stroke at MSKCC from January 1, 2005 through December 31, 2009. Active systemic cancer was defined, as in previous studies, as a diagnosis of or treatment for any systemic cancer besides local basal cell or squamous cell carcinoma of the skin within the previous 6 months, or known recurrent or metastatic disease10,12,14; patients with primary CNS tumors were excluded. Acute ischemic stroke was defined as any new neurologic deficit with corresponding MRI evidence of acute ischemia and no clinical or radiologic indication of a noncerebrovascular etiology. Specific MRI characteristics and protocols used to diagnose stroke are defined in appendix e-1 on the Neurology® Web site at Neurology.org. Patients diagnosed with stroke solely on the basis of CT were excluded given the nonspecific nature of CT lesions in patients with cancer and the resultant possibility of diagnostic error. We screened for patients by searching MSKCC's administrative databases for ICD-9-CM codes for ischemic stroke (codes 433.01, 433.11, 433.21, 433.31, 433.81, 433.91, 434.01, 434.11, and 434.91), and by reviewing the Department of Neurology's comprehensive clinical database for any patients diagnosed with “stroke,” “ischemic stroke,” or “transient ischemic attack.” All charts identified in this manner were then reviewed by a board-certified neurologist to determine eligibility.
Measurements.
A reviewing neurologist certified in calculating the modified Rankin Scale (mRS) score15 collected data about patients' demographics, vascular risk factors, use of antithrombotic agents, type of cancer, presence of metastases, cancer treatments, d-dimer levels, vascular imaging, echocardiography, mRS score at discharge, and hospital disposition. In line with guidelines for retrospective cohort studies, data were collected on standardized abstraction forms that underwent several modifications before formal use based on mock abstractions and investigator meetings.16,17 In addition, all abstracted variables were defined in advance in a data dictionary for use by the study neurologists during chart review.
Two board-certified neurologists determined by consensus the index stroke's specific mechanism, if known; the index stroke subtype according to the modified TOAST (Trial of Org 10172 in Acute Stroke Treatment Study) criteria18; and whether the stroke was due to a known (termed conventional in prior reports in this area) mechanism such as large-artery atherosclerosis, small-vessel occlusion, or atrial fibrillation.10,12 Specific details regarding the methods used by reviewing neurologists to determine stroke mechanisms are provided in appendix e-1.
Outcomes.
Comprehensive medical records, including all inpatient and outpatient encounters, were reviewed from the initial hospitalization through July 31, 2012 to ascertain outcomes. The primary outcome was a recurrent thromboembolic event defined as a composite of any recurrent ischemic stroke, TIA, myocardial infarction, systemic embolism, deep vein thrombosis, or pulmonary embolism. Secondary outcomes were recurrent ischemic stroke, intracranial hemorrhage, symptomatic intracranial hemorrhage, major bleeding, and death. All outcomes are defined in detail in appendix e-1.
Statistical analysis.
Descriptive statistics were used to characterize patients' baseline characteristics and crude outcome rates. Kaplan-Meier survival statistics were used to calculate cumulative outcome rates. Patients were censored at the time of an outcome of interest, last available follow-up visit, or death. Multivariable Cox proportional hazard analysis was used to explore potential predictors of recurrent thromboembolism. Based on the results of previous studies and anticipated sample and effect sizes, the following clinical variables plus age and sex were selected a priori: hypertension, adenocarcinoma histology, known systemic metastases, unconventional stroke mechanism, chemotherapy within 30 days, suspected or confirmed NBTE, and lung cancer.11,12,19–23 Stepwise reverse selection was used to remove variables whose independent association with recurrent thromboembolism was not significant at a threshold of p < 0.20. In the final model, the threshold of statistical significance was p < 0.05.
In an exploratory manner, we used the final model above to perform 2 additional Cox proportional hazards analyses of the association between the type of antithrombotic therapy (e.g., therapeutic-dose anticoagulation vs antiplatelet agents) and the hazard of (1) recurrent thromboembolism and (2) death. Patients were assigned to the anticoagulation group if at the time of hospital discharge they were receiving any therapeutic-dose anticoagulant including warfarin, low-molecular-weight heparin or another heparin derivative, or any factor Xa or direct thrombin inhibitor. Conversely, patients were assigned to the antiplatelet group if they were treated with aspirin, clopidogrel, or dipyridamole and no therapeutic-dose anticoagulation.
Additional exploratory analyses were performed to analyze the effect of recurrent thromboembolism on survival; these methods are described in appendix e-1. All statistical analyses were performed using Stata MP version 12 (StataCorp, College Station, TX).
RESULTS
Description of the cohort.
The final cohort comprised 263 patients and complete follow-up until death was available in 230 (87%). The median number of follow-up encounters was 5 (interquartile range [IQR] 1–22) and the median number of encounters per month was 1.1 (IQR 0.2–2.0). Among patients who survived their index stroke hospitalization, the median number of follow-up encounters was 7 (IQR 1–24) and the median number of encounters per month was 1.2 (IQR 0.4–2.1). Mean age was 66 years (SD 12) and 49% were women. Vascular risk factors were common (table 1). Most patients had solid tumors, particularly of the lung (32%) and gastrointestinal tract (25%). Adenocarcinoma histology was frequent (60%). Cancer was widespread in most patients, with 69% having known metastases. Median time from diagnosis of underlying cancer to index stroke was 9.7 months (IQR 2.3–30.8 months). Stroke was the initial manifestation of patients' underlying cancer in only one patient; however, 32 patients (12%) were diagnosed with their index stroke within 1 month of cancer diagnosis. d-Dimer was measured in 36 patients within 72 hours of their index stroke, and was elevated in 18 (50%; 95% confidence interval [CI] 33%–67%); the median level was 2.32 μg/mL (IQR 1.01–7.69 μg/mL).
Table 1.
Characteristics of patients with active systemic cancer and acute ischemic stroke, stratified by recurrent thromboembolic events
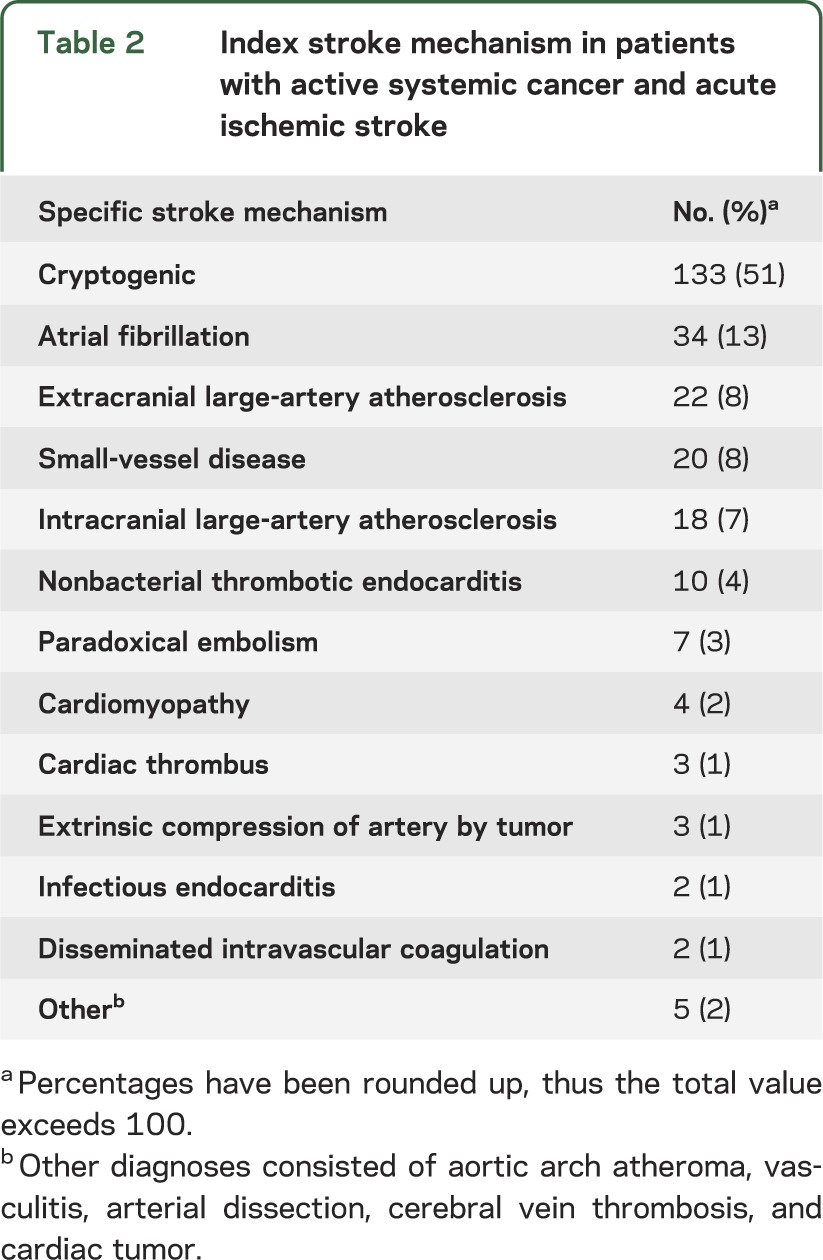
The mechanism of the index stroke could not be determined in 51% of patients (table 2) despite generally thorough diagnostic evaluations, with most patients undergoing echocardiography (82% transthoracic, 14% transesophageal, and 84% either), cranial and neck vessel imaging (76%), and inpatient cardiac rhythm analysis (100%). The rate of echocardiography and vessel imaging was significantly higher in patients who lived past 30 days from their index stroke than in those who died within 30 days (echocardiography, 90% vs 68%, p < 0.001; vessel imaging, 85% vs 55%, p < 0.001). Stroke mechanisms according to TOAST criteria comprised 22% cardioembolism, 15% large-artery atherosclerosis, 8% small-vessel occlusion, 5% other determined causes, and 51% undetermined. Confirmed strokes from cancer-related hypercoagulable mechanisms such as NBTE (n = 10) and disseminated intravascular coagulation (n = 2) were rare (5%), but many patients with cryptogenic stroke were suspected to have NBTE (76 patients, 57% of cryptogenic group) because their underlying cancer was advanced or had adenocarcinoma histology and their strokes had cardioembolic radiographic appearances (i.e., wedge-shaped or cortical infarctions in multiple vascular territories).24,25 The median mRS score at hospital discharge was 3 (IQR 2–5), and most patients were discharged home (56%). In-hospital mortality was 13%.
Table 2.
Index stroke mechanism in patients with active systemic cancer and acute ischemic stroke
Rate of outcomes.
Despite a median survival of 84 days (IQR 24–419 days), 117 cases of recurrent thromboembolism occurred in 90 patients (34%), consisting of 40 cases of deep vein thrombosis, 36 recurrent ischemic strokes, 17 cases of pulmonary embolism, 13 myocardial infarctions, 10 cases of systemic embolism, and one TIA. Most (76%) of these events were diagnosed in the emergency room or inpatient setting at MSKCC. In addition, most (n = 65, 56%) were diagnosed at a clinical encounter after hospital discharge for index stroke. Adenocarcinoma histology was more common in patients with recurrent thromboembolism than in those without recurrent thromboembolism (68% vs 56%, p = 0.07), as was adenocarcinoma histology with known systemic metastases (57% vs 43%, p = 0.04). Cumulative rates of recurrent thromboembolism were 21% (95% CI 17%–27%), 31% (95% CI 25%–38%), and 37% (95% CI 30%–44%) at 1, 3, and 6 months, respectively (figure 1). The corresponding cumulative rates of recurrent ischemic stroke were 7% (95% CI 4%–11%), 13% (95% CI 9%–19%), and 16% (95% CI 11%–22%) (figure 2). Given concern that these high rates could be attributable to bias from an especially sick cohort treated at a tertiary-care cancer hospital, we performed a post hoc Kaplan-Meier analysis of recurrent ischemic stroke limited to patients without known systemic metastases; the rates of recurrent ischemic stroke in these patients were lower but still high: 4% (95% CI 1%–11%) at 1 month, 7% (95% CI 3%–16%) at 3 months, and 11% (95% CI 5%–21%) at 6 months. Crude rates of bleeding outcomes were low: any intracranial hemorrhage in 8 patients (3%), symptomatic intracranial hemorrhage in 7 patients (3%), and major bleeding in 11 patients (4%).
Figure 1. Cumulative rate of recurrent thromboembolism in patients with cancer and acute ischemic stroke.
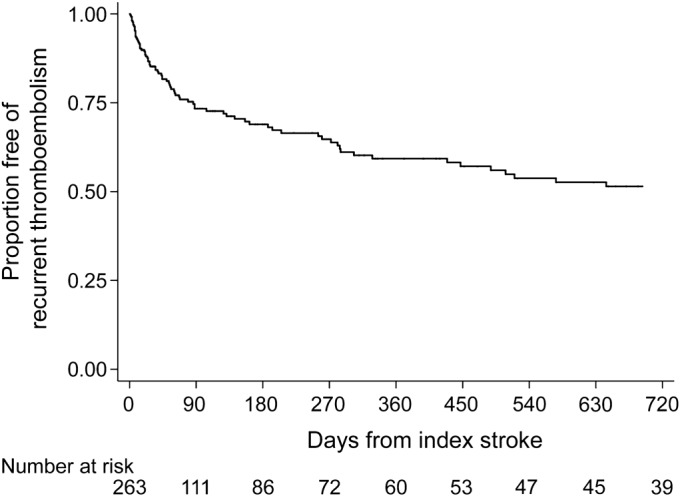
Kaplan-Meier plot of survival free of recurrent thromboembolic events defined as a composite of any recurrent ischemic stroke, TIA, myocardial infarction, systemic embolism, deep vein thrombosis, or pulmonary embolism.
Figure 2. Cumulative rate of recurrent ischemic stroke in patients with cancer and acute ischemic stroke.
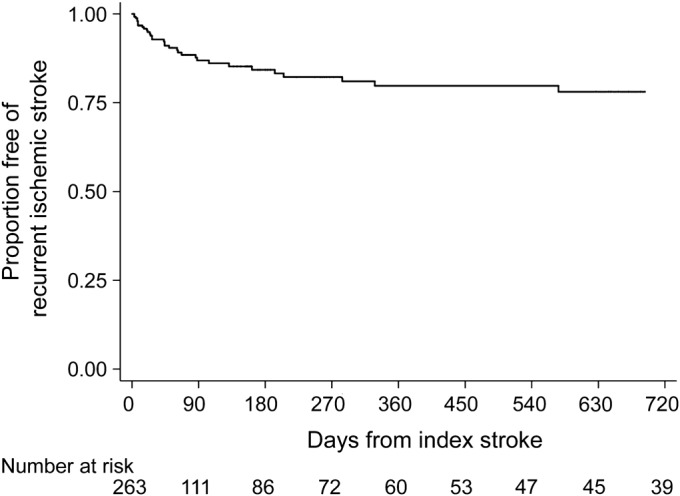
Kaplan-Meier plot of survival free of recurrent ischemic stroke.
Predictors of outcomes.
In multivariable analysis, only adenocarcinoma histology was independently associated with recurrent thromboembolism (hazard ratio [HR] 1.65, 95% CI 1.02–2.68), although there were notable but statistically nonsignificant associations with suspected or confirmed NBTE (HR 1.53, 95% CI 0.96–2.44) and recent chemotherapy (HR 1.33, 95% CI 0.87–2.03) (figure 3). In an exploratory analysis comparing rates of recurrent thromboembolism in those receiving antiplatelet vs anticoagulant therapy, there was no significant difference between groups (HR 1.19, 95% CI 0.72–1.97). Similarly, type of antithrombotic therapy was not associated with death (HR 1.03, 95% CI 0.75–1.42).
Figure 3. Cumulative rate of recurrent thromboembolism stratified by adenocarcinoma histology in patients with cancer and stroke.
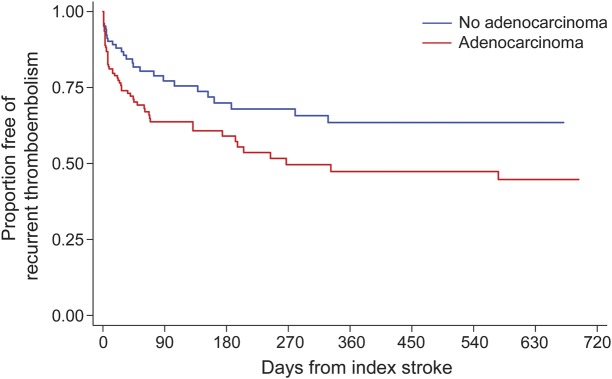
Kaplan-Meier plot of survival free of recurrent thromboembolic events, stratified by adenocarcinoma histology. Recurrent thromboembolic events were defined as a composite of recurrent ischemic stroke, TIA, myocardial infarction, systemic artery thrombosis, deep vein thrombosis, or pulmonary embolism.
In Cox proportional hazard analysis, recurrent thromboembolism was associated with higher mortality (HR 2.12, 95% CI 1.61–2.80; figure e-1), including in analyses restricted to patients with adenocarcinomas (HR 2.22, 95% CI 1.58–3.12).
DISCUSSION
In this large, retrospective cohort study, we found that patients with acute ischemic stroke in the setting of active systemic cancer face very high rates of recurrent thromboembolism despite a median survival of only 84 days. Recurrent thromboembolism in these patients consisted of both arterial and venous events, including recurrent ischemic stroke, which accounted for 31% of all thromboembolic events. In addition, recurrent thromboembolism appeared to negatively affect survival, increasing the hazard of death approximately 2-fold. Adenocarcinoma histology was the only clinical variable independently associated with recurrent thromboembolism in our cohort, although there were also notable but statistically nonsignificant associations with suspected or confirmed NBTE and recent chemotherapy.
The mechanism of stroke in cancer patients is often undetermined, although we and other investigators suspect that many patients with cryptogenic strokes have NBTE as their underlying stroke mechanism.23 NBTE arises from cancer-mediated hypercoagulability and consists of sterile, small (<5 mm) platelet-fibrin vegetations on normal cardiac valves.26 Because these lesions are small and prone to embolization, they are rarely detected by echocardiography, including transesophageal studies.27 A large autopsy study demonstrated that NBTE is the leading cause of symptomatic ischemic stroke in patients with cancer, typically occurring in those with disseminated cancer, particularly from adenocarcinoma.5 Strokes from NBTE generally result in diffuse, small- to moderate-size embolic cerebral infarctions,25,28 which was the radiographic infarct pattern seen in most patients with cryptogenic stroke in our cohort. However, the infarct pattern seen in these patients could also have arisen from undetected atrial fibrillation or other conventional cardiac sources. Furthermore, some of these patients could have had thrombus formation directly within the cerebral arteries, but this is unlikely because a prior transcranial Doppler study in patients with cancer and acute ischemic stroke demonstrated embolic signals in 58% of those with cryptogenic stroke.12
The cumulative rate of recurrent ischemic stroke in patients with cancer appears to be considerably higher than in the general stroke population. The 3- and 6-month cumulative rates of recurrent ischemic stroke in our cohort were approximately 3 times the overall rates seen in a recent large study of patients with ischemic stroke, thereby highlighting the unique susceptibility of patients with cancer to recurrent ischemic stroke.29 A prior study of 33 patients with active cancer and ischemic stroke at a tertiary-care center from 1988 to 1992 reported a 6% recurrent stroke rate, but data from this study are limited by the small and outdated cohort and likely poor diagnostic ascertainment—there is no mention of methods or degree of patient follow-up.13 Therefore, while previous studies have shown that venous thromboembolism is more common after stroke in general,11,30,31 to our knowledge, this is the first study to demonstrate that arterial thromboembolic events also frequently occur after ischemic stroke in patients with active cancer.
Antithrombotic therapy type did not seem to affect outcome rates in our study, but because this was a nonrandomized study, these results may be attributable to indication bias, in that patients with a higher likelihood of recurrent thromboembolism may have been preferentially treated with anticoagulation (mostly with low-molecular-weight heparins because these agents are superior to warfarin for cancer-mediated venous thromboembolism).14 Only randomized trials, such as one that we are currently conducting at MSKCC (clinicaltrials.gov identifier NCT01763606), comparing anticoagulation to antiplatelet therapy will be able to determine the optimal antithrombotic strategy for these patients.
Adenocarcinoma histology independently predicted recurrent thromboembolism in our cohort and has been shown previously to be associated with transcranial Doppler microemboli in patients with cancer and ischemic stroke.12 Patients with adenocarcinoma may be more prone to developing cancer-mediated hypercoagulability or “Trousseau syndrome,” which is historically notable because both Trousseau and the patient in whom he first described this disorder had visceral adenocarcinomas.3,4
This study has several important limitations. First, it was a single-center study conducted at an urban, tertiary-care cancer hospital, and its results may not be applicable to other settings. Second, although MSKCC closely follows its patients and vigorously pursues patients' medical records from outside facilities, data were collected retrospectively and outcomes were determined based on clinical diagnoses; therefore, some outcomes of interest may have been missed and our recurrent thromboembolism rates may be underestimated. For instance, there were few TIA outcomes in our study, which may be attributable to underdiagnosis, or alternatively may reflect our prespecified diagnostic ascertainment strategy that prioritized specificity over sensitivity. In addition, MSKCC is not a certified primary stroke center, so recurrent stroke rates may be especially prone to underestimation, because some patients with recurrent stroke may have been preferentially transported to and diagnosed at primary stroke centers. Third, diagnostic evaluations were not standardized; 24% of patients did not have vessel imaging and 16% did not have echocardiography, which may partly account for the high rate of cryptogenic stroke. Fourth, patient follow-up in our study was not performed in a prospective, systematic manner, so some patients may have been lost to follow-up, which could have introduced misclassification bias leading to an error in cumulative outcome rates. However, this is unlikely given that 87% of our patients had follow-up until death, and it would not have affected our high crude outcome rates. Fifth, based on the width of the CIs, our exploratory multivariable analysis may have been underpowered to detect some clinically meaningful associations. Sixth, we did not collect long-term functional data and thus could not determine the impact of recurrent thromboembolism on long-term functional status.
Patients with cancer and acute ischemic stroke face a very high risk of stroke recurrence and other forms of thromboembolism, even though typical life expectancies are short. Adenocarcinoma histology may increase the risk of recurrent thromboembolism even further. Our findings should be confirmed in a prospective, community-based cohort. If confirmed, these clinical observations may be helpful in motivating further research into the fundamental pathophysiologic mechanisms underlying ischemic stroke in patients with cancer, and guiding attempts to improve preventive therapy for this disabling complication of an already serious disease.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Robert J. Young for his assistance in revising the manuscript through substantive editing. Dr. Young is affiliated with the Department of Radiology at the Memorial Sloan-Kettering Cancer Center in New York, NY.
GLOSSARY
- CI
confidence interval
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, ninth revision, Clinical Modification
- IQR
interquartile range
- mRS
modified Rankin Scale
- MSKCC
Memorial Sloan-Kettering Cancer Center
- NBTE
nonbacterial thrombotic endocarditis
- TOAST
Trial of Org 10172 in Acute Stroke Treatment Study
Footnotes
Editorial, page 15
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Babak B. Navi conceived and designed the study, analyzed and interpreted the data, and drafted and revised the manuscript for intellectual content. Samuel Singer, Alexander E. Merkler, and Natalie T. Cheng designed the study, analyzed and interpreted the data, and revised the article critically for important intellectual content. Jacqueline B. Stone analyzed and interpreted the data and revised the article critically for important intellectual content. Hooman Kamel and Costantino Iadecola designed the study, analyzed and interpreted the data, and revised the article critically for important intellectual content. Mitchell S.V. Elkind analyzed and interpreted the data and revised the article critically for important intellectual content. Lisa M. DeAngelis designed the study, analyzed and interpreted the data, and revised the article critically for important intellectual content.
STUDY FUNDING
Supported by an NIH KL2 grant administered to Dr. Navi through the Cornell Clinical and Translational Science Center (CTSC).
DISCLOSURE
B. Navi is supported by NIH grant KL2 TR000458-06 administered through the Weill Cornell CTSC. S. Singer, A. Merkler, N. Cheng, J. Stone, H. Kamel, and C. Iadecola report no disclosures relevant to the manuscript. M. Elkind is an editor for Neurology®. L. DeAngelis receives research support through NIH grant KL2 (for mentorship of Babak Navi). Go to Neurology.org for full disclosures.
REFERENCES
- 1.Bick RL. Cancer-associated thrombosis. N Engl J Med 2003;349:109–111 [DOI] [PubMed] [Google Scholar]
- 2.Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol 2005;6:401–410 [DOI] [PubMed] [Google Scholar]
- 3.Sack GH, Jr, Levin J, Bell WR. Trousseau's syndrome and other manifestations of chronic disseminated coagulopathy in patients with neoplasms: clinical, pathophysiologic, and therapeutic features. Medicine 1977;56:1–37 [PubMed] [Google Scholar]
- 4.Dammacco F, Vacca A, Procaccio P, Ria R, Marech I, Racanelli V. Cancer-related coagulopathy (Trousseau's syndrome): review of the literature and experience of a single center of internal medicine. Clin Exp Med 2013;13:85–97 [DOI] [PubMed] [Google Scholar]
- 5.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine 1985;64:16–35 [DOI] [PubMed] [Google Scholar]
- 6.Taccone FS, Jeangette SM, Blecic SA. First-ever stroke as initial presentation of systemic cancer. J Stroke Cerebrovasc Dis 2008;17:169–174 [DOI] [PubMed] [Google Scholar]
- 7.Cestari DM, Weine DM, Panageas KS, Segal AZ, DeAngelis LM. Stroke in patients with cancer: incidence and etiology. Neurology 2004;62:2025–2030 [DOI] [PubMed] [Google Scholar]
- 8.Rogers LR. Cerebrovascular complications in patients with cancer. Semin Neurol 2010;30:311–319 [DOI] [PubMed] [Google Scholar]
- 9.Zhang YY, Chan DK, Cordato D, Shen Q, Sheng AZ. Stroke risk factor, pattern and outcome in patients with cancer. Acta Neurol Scand 2006;114:378–383 [DOI] [PubMed] [Google Scholar]
- 10.Kim SG, Hong JM, Kim HY, et al. Ischemic stroke in cancer patients with and without conventional mechanisms: a multicenter study in Korea. Stroke 2010;41:798–801 [DOI] [PubMed] [Google Scholar]
- 11.Schwarzbach CJ, Schaefer A, Ebert A, et al. Stroke and cancer: the importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke 2012;43:3029–3034 [DOI] [PubMed] [Google Scholar]
- 12.Seok JM, Kim SG, Kim JW, et al. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol 2010;68:213–219 [DOI] [PubMed] [Google Scholar]
- 13.Chaturvedi S, Ansell J, Recht L. Should cerebral ischemic events in cancer patients be considered a manifestation of hypercoagulability? Stroke 1994;25:1215–1218 [DOI] [PubMed] [Google Scholar]
- 14.Lee AY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med 2003;349:146–153 [DOI] [PubMed] [Google Scholar]
- 15.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19:604–607 [DOI] [PubMed] [Google Scholar]
- 16.Lowenstein SR. Medical record reviews in emergency medicine: the blessing and the curse. Ann Emerg Med 2005;45:452–455 [DOI] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. BMJ 2007;335:806–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adams HP, Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41 [DOI] [PubMed] [Google Scholar]
- 19.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002;360:1903–1913 [DOI] [PubMed] [Google Scholar]
- 20.Zoller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow-up study from Sweden. Eur J Cancer 2012;48:1875–1883 [DOI] [PubMed] [Google Scholar]
- 21.Li SH, Chen WH, Tang Y, et al. Incidence of ischemic stroke post-chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg 2006;108:150–156 [DOI] [PubMed] [Google Scholar]
- 22.Chen PC, Muo CH, Lee YT, Yu YH, Sung FC. Lung cancer and incidence of stroke: a population-based cohort study. Stroke 2011;42:3034–3039 [DOI] [PubMed] [Google Scholar]
- 23.Bal S, Menon B, Demchuk A. Relationship of endocarditis, disseminated intravascular coagulation, and embolic signals in cancer with stroke. Ann Neurol 2012;71:146. [DOI] [PubMed] [Google Scholar]
- 24.Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Arch Neurol 2003;60:1730–1734 [DOI] [PubMed] [Google Scholar]
- 25.Singhal AB, Topcuoglu MA, Buonanno FS. Acute ischemic stroke patterns in infective and nonbacterial thrombotic endocarditis: a diffusion-weighted magnetic resonance imaging study. Stroke 2002;33:1267–1273 [DOI] [PubMed] [Google Scholar]
- 26.el-Shami K, Griffiths E, Streiff M. Nonbacterial thrombotic endocarditis in cancer patients: pathogenesis, diagnosis, and treatment. Oncologist 2007;12:518–523 [DOI] [PubMed] [Google Scholar]
- 27.Dutta T, Karas MG, Segal AZ, Kizer JR. Yield of transesophageal echocardiography for nonbacterial thrombotic endocarditis and other cardiac sources of embolism in cancer patients with cerebral ischemia. Am J Cardiol 2006;97:894–898 [DOI] [PubMed] [Google Scholar]
- 28.Rogers LR, Cho ES, Kempin S, Posner JB. Cerebral infarction from non-bacterial thrombotic endocarditis: clinical and pathological study including the effects of anticoagulation. Am J Med 1987;83:746–756 [DOI] [PubMed] [Google Scholar]
- 29.Navi BB, Kamel H, Sidney S, Klingman JG, Nguyen-Huynh MN, Johnston SC. Validation of the Stroke Prognostic Instrument-II in a large, modern, community-based cohort of ischemic stroke survivors. Stroke 2011;42:3392–3396 [DOI] [PubMed] [Google Scholar]
- 30.Zhang YY, Cordato D, Shen Q, Sheng AZ, Hung WT, Chan DK. Risk factor, pattern, etiology and outcome in ischemic stroke patients with cancer: a nested case-control study. Cerebrovasc Dis 2007;23:181–187 [DOI] [PubMed] [Google Scholar]
- 31.Oberndorfer S, Nussgruber V, Berger O, Lahrmann H, Grisold W. Stroke in cancer patients: a risk factor analysis. J Neurooncol 2009;94:227. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


