Abstract
The localization of DNA sequences coding for ribosomal RNA was studied by hybridization of purified ribosomal RNA to DNA from chromatin fragments prepared by limited digestion of Physarum nuclei with staphylococcal nuclease. The 32P-labeled 19S and 26S RNA hybridized to DNA from nucleosome monomers, dimers, trimers, and higher oligomers, separated by sucrose gradient centrifugation, although the level of hybridization to DNA from nucleosome fractions was less than the level of hybridization to undigested nuclear DNA. The distribution of 19S and 26S rDNA sequences in the nucleosome fractions differed from the distribution of bulk DNA in that the rDNA sequences were recovered primarily in two fractions containing monomer-sized DNA lengths (140-160 base pairs). The percentage of DNA hybridizing to 19S plus 26S RNA was greater in peak A, the more slowly sedimenting monomer peak, than in any other chromatin fraction at all stages of digestion. Peak A and monomer particles differed in protein content and distribution. The presence of ribosomal cistrons in an altered nucleosome configuration may be related to changes in functional states of rDNA chromatin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., FAULKNER R., MIRSKY A. E. ACETYLATION AND METHYLATION OF HISTONES AND THEIR POSSIBLE ROLE IN THE REGULATION OF RNA SYNTHESIS. Proc Natl Acad Sci U S A. 1964 May;51:786–794. doi: 10.1073/pnas.51.5.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axel R., Cedar H., Felsenfield G. The structure of the globin genes in chromatin. Biochemistry. 1975 Jun 3;14(11):2489–2495. doi: 10.1021/bi00682a031. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun R., Evans T. E. Replication of nuclear satellite and mitochondrial DNA in the mitotic cycle of Physarum. Biochim Biophys Acta. 1969 Jun 17;182(2):511–522. doi: 10.1016/0005-2787(69)90203-2. [DOI] [PubMed] [Google Scholar]
- Casey J., Davidson N. Rates of formation and thermal stabilities of RNA:DNA and DNA:DNA duplexes at high concentrations of formamide. Nucleic Acids Res. 1977;4(5):1539–1552. doi: 10.1093/nar/4.5.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foe V. E., Wilkinson L. E., Laird C. D. Comparative organization of active transcription units in Oncopeltus fasciatus. Cell. 1976 Sep;9(1):131–146. doi: 10.1016/0092-8674(76)90059-3. [DOI] [PubMed] [Google Scholar]
- Hall L., Braun R. The organisation of genes for transfer RNA and ribosomal RNA in amoebae and plasmodia of Physarum polycephalum. Eur J Biochem. 1977 Jun 1;76(1):165–174. doi: 10.1111/j.1432-1033.1977.tb11582.x. [DOI] [PubMed] [Google Scholar]
- Hall L., Turnock G. Synthesis of ribosomal RNA during the mitotic cycle on the slime mould Physarum polycephalum. Eur J Biochem. 1976 Mar 1;62(3):471–477. doi: 10.1111/j.1432-1033.1976.tb10180.x. [DOI] [PubMed] [Google Scholar]
- Johnson E. M., Littau V. C., Allfrey V. G., Bradbury E. M., Matthews H. R. The subunit structure of chromatin from Physarum polycephalum. Nucleic Acids Res. 1976 Dec;3(12):3313–3329. doi: 10.1093/nar/3.12.3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis D. J., Gorovsky M. A. Subunit structure of rDNA-containing chromatin. Biochemistry. 1976 Feb 24;15(4):750–755. doi: 10.1021/bi00649a005. [DOI] [PubMed] [Google Scholar]
- Mohberg J., Rusch H. P. Isolation and DNA content of nuclei of Physarum polycephalum. Exp Cell Res. 1971 Jun;66(2):305–316. doi: 10.1016/0014-4827(71)90682-3. [DOI] [PubMed] [Google Scholar]
- Molgaard H. V., Matthews H. R., Bradbury E. M. Organisation of genes for ribosomal RNA in Physarum polycephalum. Eur J Biochem. 1976 Sep 15;68(2):541–549. doi: 10.1111/j.1432-1033.1976.tb10842.x. [DOI] [PubMed] [Google Scholar]
- Reeves R. Analysis and reconstruction of Xenopus ribosomal chromatin nucleosomes. Eur J Biochem. 1977 May 16;75(2):545–560. doi: 10.1111/j.1432-1033.1977.tb11555.x. [DOI] [PubMed] [Google Scholar]
- Reeves R., Jones A. Genomic transcriptional activity and the structure of chromatin. Nature. 1976 Apr 8;260(5551):495–500. doi: 10.1038/260495a0. [DOI] [PubMed] [Google Scholar]
- Reeves R. Ribosomal genes of Xenopus laevis: evidence of nucleosomes in transcriptionally active chromatin. Science. 1976 Oct 29;194(4264):529–532. doi: 10.1126/science.973136. [DOI] [PubMed] [Google Scholar]
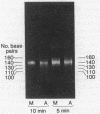
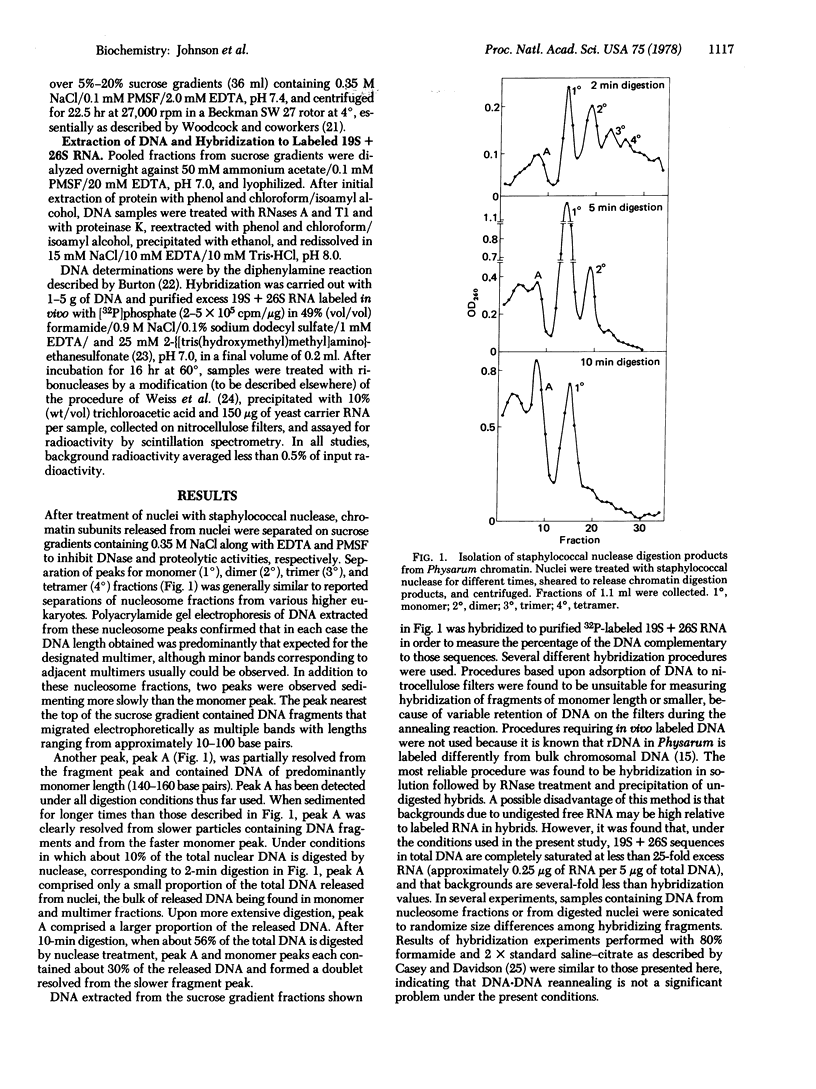
- Scheer U., Trendelenburg M. F., Krohne G., Franke W. W. Lengths and patterns of transcriptional units in the amplified nucleoli of oocytes of Xenopus laevis. Chromosoma. 1977 Mar 16;60(2):147–167. doi: 10.1007/BF00288462. [DOI] [PubMed] [Google Scholar]
- Staron K., Jerzmanowski A., Tyniec B., Urbanska A., Toczko K. Nucleoprotein chromatin subunit from Physarum polycephalum. Biochim Biophys Acta. 1977 Mar 2;475(1):131–138. doi: 10.1016/0005-2787(77)90347-1. [DOI] [PubMed] [Google Scholar]
- Tien Kuo M., Sahasrabuddhe C. G., Saunders G. F. Presence of messenger specifying sequences in the DNA of chromatin subunits. Proc Natl Acad Sci U S A. 1976 May;73(5):1572–1575. doi: 10.1073/pnas.73.5.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Repeated structure of chromatin in metaphase nuclei of Physarum. FEBS Lett. 1976 Apr 15;64(1):190–192. doi: 10.1016/0014-5793(76)80280-3. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Braun R. Structure of ribosomal DNA in Physarum polycephalum. J Mol Biol. 1976 Sep 25;106(3):567–587. doi: 10.1016/0022-2836(76)90252-7. [DOI] [PubMed] [Google Scholar]
- Wall R., Darnell J. E. Presence of cell and virus specific sequences in the same molecules of nuclear RNA from virus transformed cells. Nat New Biol. 1971 Jul 21;232(29):73–76. doi: 10.1038/newbio232073a0. [DOI] [PubMed] [Google Scholar]
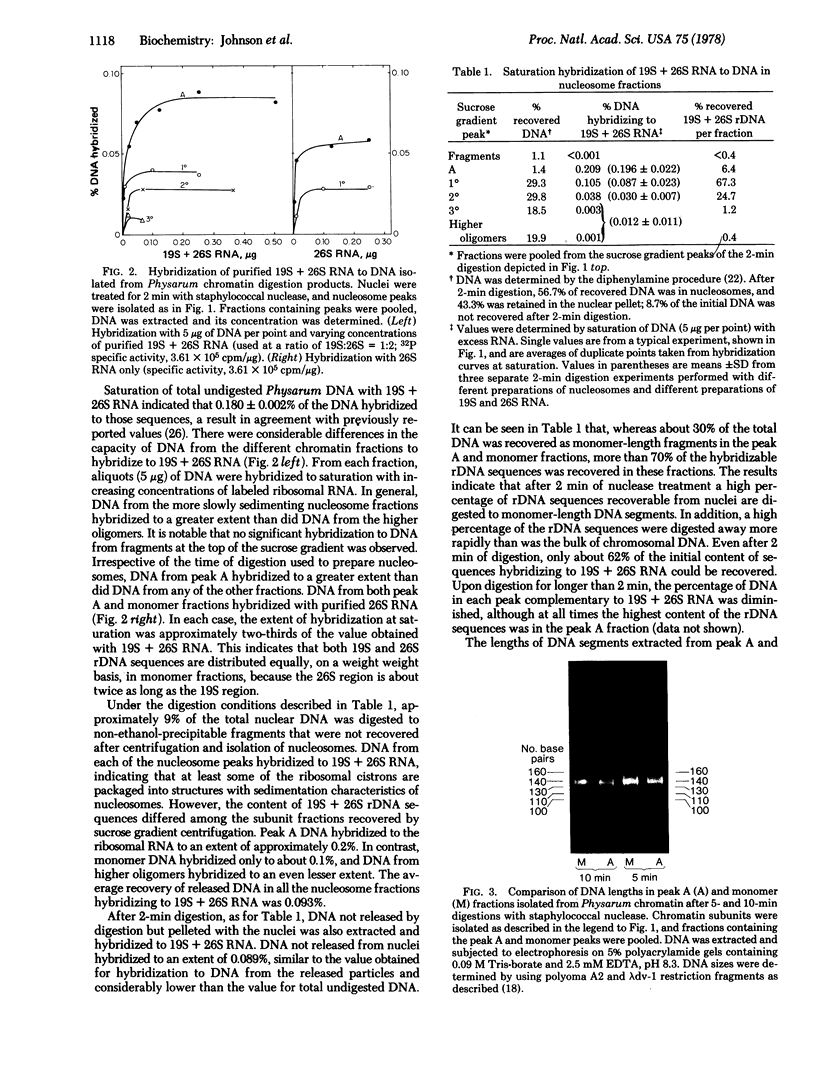
- Weintraub H., Worcel A., Alberts B. A model for chromatin based upon two symmetrically paired half-nucleosomes. Cell. 1976 Nov;9(3):409–417. doi: 10.1016/0092-8674(76)90085-4. [DOI] [PubMed] [Google Scholar]
- Weiss M. J., Sweet R. W., Gulati S. C., Harter D. H. Nucleic acid sequence relationships among "slow" viruses of sheep. Virology. 1976 Jun;71(2):395–401. doi: 10.1016/0042-6822(76)90367-6. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Frado L. L., Hatch C. L., Ricciardiello L. Fine structure of active ribosomal genes. Chromosoma. 1976 Oct 12;58(1):33–39. doi: 10.1007/BF00293438. [DOI] [PubMed] [Google Scholar]
- Woodcock C. L., Sweetman H. E., Frado L. L. Structural repeating units in chromatin. II. Their isolation and partial characterization. Exp Cell Res. 1976 Jan;97:111–119. doi: 10.1016/0014-4827(76)90660-1. [DOI] [PubMed] [Google Scholar]