Abstract
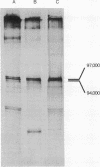
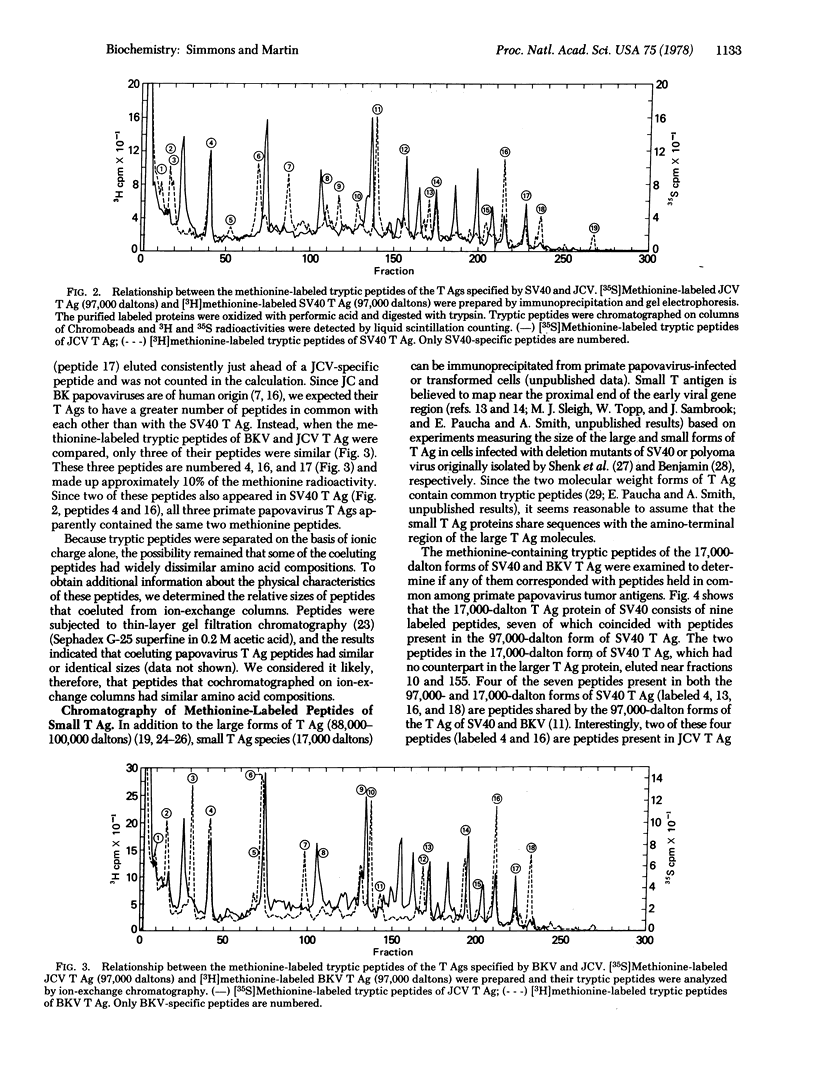
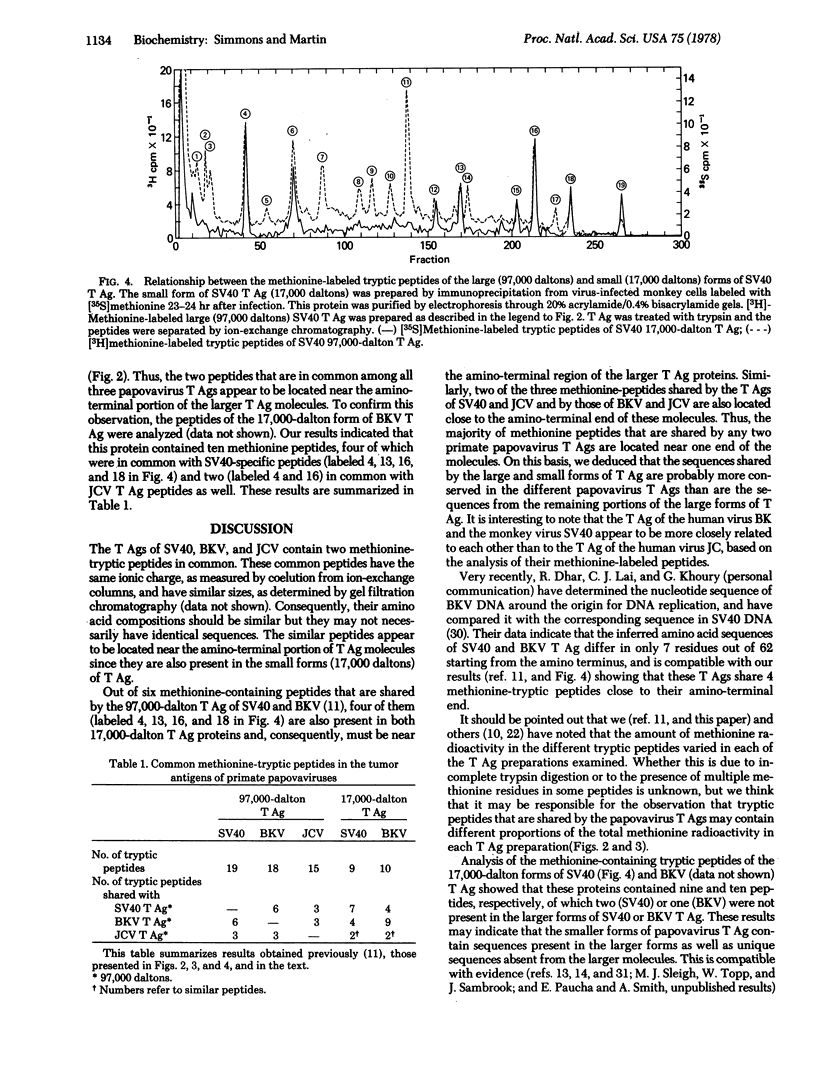
The tumor antigens directed by human papovaviruses BK and JC and the monkey papovavirus simian virus 40 have two methionine-containing tryptic peptides in common. These peptides are constituents of the small forms of papovavirus tumor antigen (17,000 daltons) which are present in lytically infected and transformed cells and which are believed to share some amino acid sequences with the amino-terminal portion of the larger tumor antigen species (97,000 daltons). In addition to the two peptides, which are present in all three papovavirus tumor antigens, the larger forms of the tumor antigens specified by simian virus 40 and BK virus share four other methionine-containing tryptic peptides, two of which are also present in the smaller (17,000 daltons) species of antigen. The occurrence of common peptides at the amino-terminal portion of tumor antigens of primate papovaviruses suggests that these conserved regions may play a fundamental role in the function of these proteins and in the propagation of these viruses in nature. The tryptic peptides of the small forms of papovavirus tumor antigen were examined and compared to those present in the large species. Out of a total of nine and ten methionine-containing peptides in the 17,000-dalton tumor antigens of simian virus 40 and BK virus, seven and nine peptides, respectively, are constituents of the corresponding larger (97,000 daltons) forms of the antigen.
Keywords: simian virus 40, BK virus, JC virus, immunoprecipitation, peptide analysis
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad-Zadeh C., Allet B., Greenblatt J., Weil R. Two forms of simian-virus-40-specific T-antigen in abortive and lytic infection. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1097–1101. doi: 10.1073/pnas.73.4.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. Host range mutants of polyoma virus. Proc Natl Acad Sci U S A. 1970 Sep;67(1):394–399. doi: 10.1073/pnas.67.1.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll R. B., Smith A. E. Monomer molecular weight of T antigen from simian virus 40-infected and transformed cells. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2254–2258. doi: 10.1073/pnas.73.7.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. V., Cole C. N., Smith A. E., Paucha E., Tegtmeyer P., Rundell K., Berg P. Organization and expression of early genes of simian virus 40. Proc Natl Acad Sci U S A. 1978 Jan;75(1):117–121. doi: 10.1073/pnas.75.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fey G., Hirt B. Fingerprints of polyoma virus proteins and mouse histones. Cold Spring Harb Symp Quant Biol. 1975;39(Pt 1):235–241. doi: 10.1101/sqb.1974.039.01.030. [DOI] [PubMed] [Google Scholar]
- Gardner S. D., Field A. M., Coleman D. V., Hulme B. New human papovavirus (B.K.) isolated from urine after renal transplantation. Lancet. 1971 Jun 19;1(7712):1253–1257. doi: 10.1016/s0140-6736(71)91776-4. [DOI] [PubMed] [Google Scholar]
- Howley P. M., Khoury G., Takemoto K. K., Martin M. A. Polynucleotide sequences common to the genomes of simian virus 40 and the human papovaviruses JC and BK. Virology. 1976 Aug;73(1):303–307. doi: 10.1016/0042-6822(76)90084-2. [DOI] [PubMed] [Google Scholar]
- Ito Y., Spurr N., Dulbecco R. Characterization of polyoma virus T antigen. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1259–1263. doi: 10.1073/pnas.74.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S., Kronvall G. The use of protein A-containing Staphylococcus aureus as a solid phase anti-IgG reagent in radioimmunoassays as exemplified in the quantitation of alpha-fetoprotein in normal human adult serum. Eur J Immunol. 1974 Jan;4(1):29–33. doi: 10.1002/eji.1830040108. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Lewis A. M., Jr, Rowe W. P. Studies of nondefective Adenovirus 2-Simian virus 40 hybrid viruses. 8. Association of Simian virus 40 transplantation antigen with a specific region of the early viral genome. J Virol. 1973 Oct;12(4):836–840. doi: 10.1128/jvi.12.4.836-840.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K., Hunter T., Walter G., Linke H. Evidence for simian virus 40 (SV40) coding of SV40 T-antigen and the SV40-specific proteins in HeLa cells infected with nondefective adenovirus type 2-SV40 hybrid viruses. J Virol. 1977 Oct;24(1):151–169. doi: 10.1128/jvi.24.1.151-169.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer H. R., Allen R. C. Useful buffer and gel systems for polyacrylamide gel electrophoresis. Z Klin Chem Klin Biochem. 1972 May;10(5):220–225. doi: 10.1515/cclm.1972.10.5.220. [DOI] [PubMed] [Google Scholar]
- Newell N., Lai C. J., Khoury G., Kelly T. J., Jr Electron microscope study of the base sequence homology between simian virus 40 and human papovavirus BK. J Virol. 1978 Jan;25(1):193–201. doi: 10.1128/jvi.25.1.193-201.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn J. E., Robertson S. M., Padgett B. L., Walker D. L., Weisblum B. Comparison of JC and BK human papovaviruses with simian virus 40: DNA homology studies. J Virol. 1976 Aug;19(2):675–684. doi: 10.1128/jvi.19.2.675-684.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett B. L., Hunt J. M., Walker D. L. Specificity of the Tumor-specific transplantation antigen induced by JC virus, a human polyomavirus. Intervirology. 1977;8(3):182–185. doi: 10.1159/000148893. [DOI] [PubMed] [Google Scholar]
- Padgett B. L., Walker D. L., ZuRhein G. M., Eckroade R. J., Dessel B. H. Cultivation of papova-like virus from human brain with progressive multifocal leucoencephalopathy. Lancet. 1971 Jun 19;1(7712):1257–1260. doi: 10.1016/s0140-6736(71)91777-6. [DOI] [PubMed] [Google Scholar]
- Prives C., Gilboa E., Revel M., Winocour E. Cell-free translation of simian virus 40 early messenger RNA coding for viral T-antigen. Proc Natl Acad Sci U S A. 1977 Feb;74(2):457–461. doi: 10.1073/pnas.74.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radola B. J. Thin-layer gel filtration of proteins. I. Method. J Chromatogr. 1968 Nov 5;38(1):61–77. doi: 10.1016/0021-9673(68)85009-5. [DOI] [PubMed] [Google Scholar]
- Rundell K., Tegtmeyer P., Wright P. J., Di Mayorca G. Identification of the human papovavirus T antigen and comparison with the simian virus 40 protein a. Virology. 1977 Oct 1;82(1):206–213. doi: 10.1016/0042-6822(77)90043-5. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah K. V., Ozer H. L., Ghazey H. N., Kelly T. J., Jr Common structural antigen of papovaviruses of the simian virus 40-polyoma subgroup. J Virol. 1977 Jan;21(1):179–186. doi: 10.1128/jvi.21.1.179-186.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Carbon J., Berg P. Construction and analysis of viable deletion mutants of simian virus 40. J Virol. 1976 May;18(2):664–671. doi: 10.1128/jvi.18.2.664-671.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. T., Takemoto K. K., Martin M. A. Relationship between the methionine tryptic peptides of simian virus 40 and BK virus tumor antigens. J Virol. 1977 Oct;24(1):319–325. doi: 10.1128/jvi.24.1.319-325.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M., O'Neill C., Berryman S., Waxman V. Anchorage and growth regulation in normal and virus-transformed cells. Int J Cancer. 1968 Sep 15;3(5):683–693. doi: 10.1002/ijc.2910030517. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Martin M. A. Transformation of hamster kidney cells by BK papovavirus DNA. J Virol. 1975 Jan;17(1):247–253. doi: 10.1128/jvi.17.1.247-253.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeyer P., Schwartz M., Collins J. K., Rundell K. Regulation of tumor antigen synthesis by simain virus 40 gene A. J Virol. 1975 Jul;16(1):168–178. doi: 10.1128/jvi.16.1.168-178.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimmappaya B., Weissman S. M. The early region of SV40 DNA may have more than one gene. Cell. 1977 Aug;11(4):837–843. doi: 10.1016/0092-8674(77)90295-1. [DOI] [PubMed] [Google Scholar]
- Vogt V. M., Eisenman R., Diggelmann H. Generation of avian myeloblastosis virus structural proteins by proteolytic cleavage of a precursor polypeptide. J Mol Biol. 1975 Aug 15;96(3):471–493. doi: 10.1016/0022-2836(75)90174-6. [DOI] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]