Abstract
To determine whether an angiotensin-converting enzyme inhibitor (lisinopril) or calcium channel blocker (amlodipine) is superior to a diuretic (chlorthalidone) in reducing cardiovascular disease incidence in gender subgroups, we carried out a prespecified subgroup analysis of 15,638 female and 17,719 male participants in the Antihypertensive and Lipid-Lowering to Prevent Heart Attack Trial (ALLHAT). Total follow-up (active treatment + passive surveillance using national administrative databases to ascertain deaths and hospitalizations) was 8 to 13 years. The primary outcome was fatal coronary heart disease or nonfatal myocardial infarction. Secondary outcomes included all-cause mortality, stroke, combined cardiovascular disease (coronary heart disease death, nonfatal myocardial infarction, stroke, angina, coronary revascularization, heart failure, or peripheral vascular disease), and end-stage renal disease. In-trial rates of heart failure, stroke, and combined cardiovascular disease were significantly higher for lisinopril compared to chlorthalidone, and rates of heart failure were significantly higher for amlodipine compared to chlorthalidone in both men and women. There were no significant treatment gender interactions. These findings did not persist through the extension period with the exception of the heart failure result for amlodipine versus chlorthalidone, which did not differ significantly by gender. For both women and men, rates were not lower in the amlodipine or lisinopril groups than in the chlorthalidone group for either the primary coronary heart disease outcome or any other cardiovascular disease outcome, and chlorthalidone-based treatment resulted in the lowest risk of heart failure. Neither lisinopril nor amlodipine is superior to chlorthalidone for initial treatment of hypertension in either women or men.
Keywords: hypertension, gender, diuretic, calcium channel blocker, ACE inhibitor
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality among women in developed countries, and elevated blood pressure (BP) is a leading contributor to this phenomenon.1, 2
While the benefits of lowering elevated BP in reducing CVD morbidity and mortality in the population as a whole have been well-established, until recently, data documenting the specific benefits of antihypertensive treatment on reducing the CV complications of hypertension in women were not available.3 Furthermore, prior to the Antihypertensive and Lipid-Lowering to Prevent Heart Attack Trial (ALLHAT), CVD outcome data comparing the effects of non-diuretic-based antihypertensive therapy (i.e., angiotensin-converting enzyme [ACE] inhibitors or calcium channel blockers [CCBs]) to diuretic therapy in women were absent. This is particularly significant, because hypertension is highly prevalent, difficult to control, and associated with a high incidence of CVD complications in older women, the population that was enrolled in ALLHAT.4–6 Data from the Framingham Heart Study showed an age-related decline in BP control rates that was more pronounced in women than in men.6 Among the oldest participants with hypertension, only 23% of women (vs. 38% of men) were controlled to BP <140/90 mmHg. A cross-sectional analysis of data from the 2005 National Ambulatory Medical Care Survey and the National Hospital Ambulatory Medical Care Survey also showed that among patients with hypertension, women were less likely than men to meet BP control targets.7 The gender disparity was particularly large in the elderly, where the odds ratio of having controlled BP in women compared to men was 0.62 (95% CI, 0.45–0.85). It is not clear from these studies whether the gender disparity in BP control rates in elderly patients was related to true treatment resistance among women due to biological factors or to incompletely understood characteristics of the health care delivery system.
ALLHAT was a randomized, double-blind, NHLBI-sponsored trial that recruited 42,418 participants and compared an ACE inhibitor (lisinopril), an alpha blocker (doxazosin), and a calcium channel blocker (amlodipine), each with a diuretic (chlorthalidone).8, 9 ALLHAT reported that chlorthalidone was not surpassed in preventing CHD compared to doxazosin, lisinopril, or amlodipine; was more effective than these agents in preventing heart failure (HF); and was more effective than doxazosin or lisinopril in preventing stroke and a composite of CVD outcomes.10 Results for the doxazosin arm, which was terminated early, have been reported in several papers, and are not included here.11–17
The ALLHAT cohort included 47% women with a mean age of 67 years at enrollment and pre-specified subgroups in the trial included women and men.8, 9 Thus, ALLHAT offers an excellent opportunity to compare the effects on CVD outcomes of representatives of the newer classes of antihypertensive agents versus those of the diuretic chlorthalidone in a large population of older, higher risk women. In addition, we tested whether 5 years of in-trial randomized antihypertensive treatment resulted in persistence or de novo development of mortality and morbidity differences over extended follow-up of 8–13 years.18–21 The primary outcome for the long-term (in-trial plus post-trial) analyses was CVD mortality. This report details the results of the in-trial and post-trial analyses by gender. In particular, we now examine in detail the interactions of gender and treatment in determining CVD outcomes with emphasis on treatment effects in women.
Methods
The rationale and design of ALLHAT have been presented elsewhere7 and more extensive details are in the online supplement.8 Briefly, participants were women and men age ≥55 years with hypertension and at least one additional risk factor for CHD events.8, 9 All participants gave written informed consent, and all centers obtained institutional review board approval. The study adhered to the principles of the Declaration of Helsinki and Title 45, U.S. Code of Federal Regulations, Part 46, Protection of Human Subjects.
Participants (n = 33,357) were assigned by a computer-generated randomization schedule to chlorthalidone, amlodipine, or lisinopril in a ratio of 1.7:1:1, respectively. Goal BP in each randomized group was <140/90 mmHg achieved by titrating the assigned study drug (step 1) and adding open-label agents (step 2 or 3) when necessary. Method of BP measurement is detailed elsewhere.22
The primary outcome was a composite of fatal CHD or nonfatal MI.8–10 Four major pre-specified secondary outcomes were: 1) all-cause mortality; 2) fatal and nonfatal stroke; 3) combined CHD (the primary outcome, coronary revascularization, hospitalized angina); and 4) combined CVD (combined CHD, stroke, other treated angina, HF [fatal, hospitalized, or treated non-hospitalized], and peripheral arterial disease). Individual components of the combined outcomes also were examined. Other pre-specified secondary outcomes included cancer and end-stage renal disease (ESRD) (dialysis, renal transplant or death). For the in-trial plus post-trial periods, the primary outcome was CV mortality and secondary outcomes were mortality, stroke, CHD, HF, CVD, and ESRD. For details on the use of national databases, see online supplement and Cushman et al.18 For the post-trial period, data are not available on medications, serum chemistries, or blood pressure levels.
Statistical Analyses
Baseline characteristics and intermediate outcomes were compared across treatments within each gender subgroup using analysis of variance for continuous covariates and contingency table analyses for categorical data. Data were analyzed according to participants’ randomized treatment assignments regardless of their subsequent medications (i.e., intention-to-treat analysis). Six-year cumulative event rates for the in-trial data and 10-year event rates for the post-trial data were calculated using the Kaplan-Meier procedure. Cox proportional hazards models were used to obtain hazard ratios (HRs) and 95% confidence intervals (CIs) for time-to-event outcomes and included the participant’s entire trial experience for both the in-trial and the combined in-trial plus post-trial periods. Heterogeneity of treatment effects across gender subgroups was examined by testing for treatment x gender interaction with the proportional hazards model using P<.05. Given the many multivariate, subgroup, and interaction analyses performed, statistical significance at the .05 level should be interpreted with caution. Stata version 11 (Stata Corp, College Station, Tex) was used for all analyses. Further details are provided in the major ALLHAT outcomes paper,10 the ALLHAT extension paper,18 and the ALLHAT extension protocol (www.allhat.org).
Results
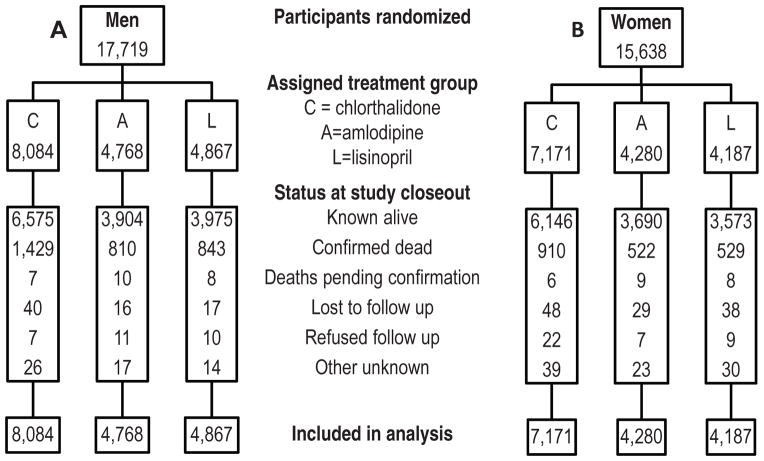
A consort diagram (Figure 1) shows the number of participants who entered the trial and their status at the end of the in-trial period (March, 2002) by gender. There were 17,719 men and 15,638 women randomized into ALLHAT.
Figure 1.
Consort diagrams for men (A) and women (B)
Baseline characteristics
Women accounted for 47% of the ALLHAT population (Table 1). They were slightly older (by ½ year), were more likely to be Black (41% vs. 30%) and Hispanic (19% vs. 13%), and had lower educational attainment. While there was little difference by gender in percentages of those 55–64, there were fewer women than men among those aged 65–80 years old (49% vs. 53%), whereas there were more women among those aged 80 years and older (8.4% vs. 4.8%). The percent on treatment at entry were similar in men and women (~90%). Women compared with men had slightly higher systolic BP (147.0 mmHg vs. 145.6 mmHg), higher HDL and LDL-cholesterol (52 mg/dL vs. 42 mg/dL and 141mg/dL vs. 131 mg/dL, respectively), higher BMI (30.6 vs. 29.1), slightly lower estimated GFR (76.6 ml/min/1.73m2vs. 78.7 ml/min/1.73m2), and were more likely to be participants in the lipid-lowering trial (25.5% vs. 23.6%) due to the ALLHAT-LLT eligibility criteria. Women were more likely to have diabetes (43.9% vs. 39.5%), less likely to have a history of CHD (19.3% vs 30.9%) or other form of atherosclerotic CVD (48.2% vs. 54.5%), including MI, stroke, or coronary revascularization, and fewer were smokers (19.4% vs 24.1%). The prevalence of atrial fibrillation at baseline was low and about half that in men (0.7% vs. 1.3%). There were no significant differences across the 3 drug treatment groups in baseline BP or other baseline characteristics within either gender subgroup, except for: 1) history of MI or stroke in women, which was lower in the lisinopril group (16.6%) than in the chlorthalidone and amlodipine groups (18.5% and 18.8%, respectively); 2) history of CHD in men, lower in the amlodipine (29.7%) versus chlorthalidone group (31.5%); and 3) estimated GFR in men, which was lower in chlorthalidone group (78.3) versus amlodipine group (79.1).
Table 1.
Baseline characteristics of ALLHAT participants by gender*
| Characteristic | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Chlorthalidone | Amlodipine | Lisinopril | All Male | Chlorthalidone | Amlodipine | Lisinopril | All Female | |
| Number randomized | 8,084 | 4,768 | 4,867 | 17,719 | 7,171 | 4,280 | 4,187 | 15,638 |
| Age, years, mean (sd) | 66.69(7.2) | 66.66(7.3) | 66.76(7.3) | 66.7(7.3) | 67.13(8.2) | 67.14(8.2) | 67.0(8.2) | 67.1(8.2) |
| 55–64 | 3,422(42.3) | 2,006(42.1) | 2,023(41.6) | 7,451(42.1) | 3,049(42.5) | 1,838(42.9) | 1,846(44.1) | 6,733(43.1) |
| 65–80 | 4,269(52.8) | 2,538(53.2) | 2,605(53.5) | 9,412(53.1) | 3,515(49.0) | 2,080(48.6) | 1,994(47.6) | 7,589(48.5) |
| 80+ | 393(4.9) | 224(4.7) | 239(4.9) | 856(4.8) | 607(8.5) | 362(8.5) | 347(8.3) | 1,316(8.4) |
| Ethnicity† | ||||||||
| Black | 2,434(30.1) | 1,464(30.7) | 1,464(30.1) | 5,362(30.3) | 2,935(40.9) | 1,749(40.9) | 1,746(41.7) | 6,430(41.1) |
| Non-Black | 5,650(69.9) | 3,304(69.3) | 3,403(69.9) | 12,357(69.7) | 4,236(59.1) | 2,531(59.1) | 2,441(58.3) | 9,208(58.9) |
| White, non-Hispanic | 4,413(54.6) | 2,629(55.1) | 2,654(54.5) | 9,696(54.7) | 2,789(38.9) | 1,676(39.2) | 1,608(38.4) | 6,073(38.8) |
| Black, non-Hispanic | 2,243(27.8) | 1,323(27.8) | 1,348(27.7) | 4,914(27.7) | 2,628(36.7) | 1,588(37.1) | 1,572(37.5) | 5,788(37.0) |
| White Hispanic | 854(10.6) | 478(10.0) | 521(10.7) | 1,853(10.5) | 1,058(14.8) | 630(14.7) | 615(14.7) | 2,303(14.7) |
| Black Hispanic | 191(2.4) | 141(3.0) | 116(2.4) | 448(2.5) | 307(4.3) | 161(3.8) | 174(4.2) | 642(4.1) |
| Other | 383(4.7) | 197(4.1) | 228(4.7) | 808(4.6) | 389(5.4) | 225(5.3) | 218(5.2) | 832(5.3) |
| Education, yrs, mean (sd) | 11.5(4.1) | 11.5(4.0) | 11.4(4.1) | 11.48(4.1) | 10.4(3.9) | 10.4(3.8) | 10.4(4.0) | 10.37(3.9) |
| Receiving antihypertensive treatment, n(%) | 7,249(90.0) | 4,261(89.4) | 4,355(89.5) | 15,865(90.0) | 6,505(90.7) | 3,910(91.4) | 3,809(91.0) | 14,224(91.0) |
| Blood pressure, mmHg, mean (sd) | ||||||||
| SBP | 145.5(16.0) | 145.8(15.7) | 145.6(15.7) | 145.6(15.8) | 147.1(15.4) | 146.7(15.6) | 147.3(15.3) | 147.0(15.4) |
| DBP | 84.3(10.1) | 84.2(10.2) | 84.4(10.0) | 84.3(10.1) | 83.8(10.0) | 83.6(10.1) | 83.8(10.0) | 83.7(10.0) |
| Treated at baseline | ||||||||
| SBP | 144.3(15.9) | 144.7(15.7) | 144.4(15.6) | 144.5(15.8) | 146.1(15.3) | 145.6(15.5) | 146.4(15.3) | 146.0(15.4) |
| DBP | 83.6(10.0) | 83.5(10.1) | 83.8(10.0) | 83.6(10.0) | 83.2(9.9) | 83.1(10.0) | 83.3(9.9) | 83.2(9.9) |
| Untreated at baseline | ||||||||
| SBP | 155.5(12.0) | 155.3(12.5) | 155.8(12.5) | 155.5(12.3) | 156.7(12.12) | 158.2(11.4) | 156.9(12.2) | 157.1(12.0) |
| DBP | 90.0(8.9) | 90.1(9.4) | 89.3(8.9) | 89.8(9.1) | 89.0(9.3) | 89.2(9.4) | 88.8(9.7) | 89.0(9.4) |
| Baseline lipid profile, mg/dL,-mean (sd) | ||||||||
| Cholesterol | ||||||||
| Total | 206.5(40.5) | 207.1(40.2) | 206.8(39.5) | 206.7(40.1) | 227.3(44.5) | 227.1(45.7) | 226.0(43.1) | 226.9(44.5) |
| HDL | 42.4(12.9) | 42.8(12.7) | 42.2(12.5) | 42.4(12.7) | 51.9(15.3) | 52.1(15.3) | 51.8(15.0) | 51.9(15.2) |
| LDL | 130.9(34.9) | 131.1(34.5) | 131.7(34.3) | 131.2(34.6) | 141.6(39.1) | 140.6(39.4) | 140.9(38.4) | 141.2(39.0) |
| Fasting triglycerides | 174.4(137.1) | 172.2(135.2) | 173.0(140.7) | 173.4(137.6) | 170.7(120.9) | 173.1(131.7) | 172.5(137.9) | 171.8(128.6) |
| Total Glucose mg/dL | 123.2(58.6) | 122.4(56.9) | 122.0(55.5) | 122.6(57.3) | 127.2(64.6) | 127.1(64.4) | 126.1(63.1) | 126.9(64.2) |
| Fasting Glucose mg/dL | 121.8(55.8) | 121.0(53.4) | 120.8(52.2) | 121.3(54.2) | 125.6(61.2) | 125.4(60.6) | 125.1(60.0) | 125.4(60.7) |
| Diabetes classification | ||||||||
| Diabetes‡ | 3,006(39.1) | 1,835(40.6) | 1,797(39.0) | 6,638(39.5) | 2,988(44.4) | 1,762(43.6) | 1,713(43.5) | 6,463(43.9) |
| Impaired fasting glucose§ | 389(5.1) | 233(5.2) | 249(5.4) | 871(5.2) | 239(3.6) | 131(3.2) | 158(4.0) | 528(3.6) |
| Normoglycemic|| | 4,289(55.8) | 2,447(54.2) | 2,557(55.6) | 9,293(55.3) | 3,502(52.0) | 2,147(53.1) | 2,070(52.5) | 7,719(52.5) |
| Lipid trial participants** | 1,931(23.9) | 1,116(23.4) | 1,127(23.2) | 4,174(23.6) | 1,824(25.4) | 1,124(26.3) | 1,040(24.8) | 3,988(25.5) |
| History of CHD | 2,523(31.5) | 1,402(29.7) | 1,496(31.0) | 5,421(30.9) | 1,420(19.9) | 800(18.8) | 774(18.6) | 2,994(19.3) |
| Cigarette smoker | 1,933(23.9) | 1,153(24.2) | 1,187(24.4) | 4,273(24.1) | 1,409(19.7) | 827(19.3) | 794(19.0) | 3,030(19.4) |
| Atherosclerotic CVD | 4,407(54.5) | 2,568(53.9) | 2,687(55.2) | 9,662(54.5) | 3,493(48.7) | 2,046(47.8) | 1,997(47.7) | 7,536(48.2) |
| History of MI or stroke | 2,258(27.9) | 1,293(27.1) | 1,364(28.0) | 4,915(27.7) | 1,323(18.5) | 805(18.8) | 694(16.6) | 2,822(18.1) |
| History of coronary revascularization | 1,442(17.8) | 820(17.2) | 900(18.5) | 3,162(17.9) | 544(7.6) | 286(6.7) | 318(7.6) | 1,148(7.3) |
| Other Atherosclerotic CVD†† | 1,841(22.8) | 1,085(22.8) | 1,128(23.2) | 4,054(22.9) | 1,763(25.0) | 1,060(25.0) | 1,024(24.5) | 3,847(24.6) |
| ST-T wave‡‡ | 807(10.1) | 464(9.8) | 460(9.6) | 1,731(9.9) | 765(10.8) | 444(10.5) | 480(11.6) | 1,689(10.9) |
| LVH by Electrocardiogram | 1,355(16.8) | 818(17.2) | 788(16.2) | 2,961(16.7) | 1,112(15.5) | 715(16.7) | 686(16.4) | 2,513(16.1) |
| LVH by Echocardiogram | 331(4.1) | 181(3.8) | 191(4.0) | 703(4.0) | 364(5.1) | 230(5.4) | 221(5.1) | 805(5.2) |
| Estimated GFR (ml/min/1.73 m2), mean (sd) | 78.3(19.1) | 79.1(19.4) | 78.7(19.8) | 78.7(19.4) | 76.6(20.2) | 77.0(20.0) | 76.5(19.9) | 76.6(20.1) |
| Body mass index, mean (sd) | 29.1(5.2) | 29.1(5.2) | 29.1(5.2) | 29.1(5.2) | 30.5(7.0) | 30.7(7.3) | 30.6(7.0) | 30.6(7.1) |
| Atrial Fibrillation/Flutter, n(%) | 98(1.4) | 57(1.4) | 54(1.3) | 209(1.3) | 39(0.7) | 31(0.9) | 23(0.7) | 93(0.7) |
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular diseases; DBP, diastolic blood pressure; GFR, glomerular filtration rat ; LVH, left ventricular hypertrophy ; sd, standard deviation; yrs, years ; SBP, systolic blood pressure.
All results are presented as percentages of the number of participants randomized to the treatment groups unless otherwise indicated.
Ethnicity is by self-report
Diabetes = History of diabetes at baseline or fasting glucose ≥ 126 mg/dL;
Impaired fasting glucose = No history and baseline fasting glucose is 110 to 125 mg/dL inclusive;
Normoglycemic = Not classified as impaired fasting glucose, no history of DM and at least one fasting glucose or non-fasting glucose is < 110 mg/dL.
Participants randomized to the ALLHAT Lipid Trial, an open label substudy of Pravastatin versus Usual Care in those participants with elevated cholesterol
Other atherosclerotic CVD is any of history of angina pectoris; history of intermittent claudication, gangrene, or ischemic ulcers; history of transient ischemic attack; coronary, peripheral vascular, or carotid stenosis ≥ 50% documented by angiography or Doppler studies; ischemic heart disease documented by reversible or fixed ischemia on stress thallium or dipyridamole thallium.
ST-T wave is any major ST segment depression or T-wave inversion on any electrocardiogram in the past two years. ST depression ≥ 1 mm for ≥ 1 minute on exercise testing or Holter monitoring; reversible wall motion abnormality on stress echocardiogram; ankle-arm index < 0.9; abdominal aortic aneurysm detected by ultrasonography, computed tomography scan, or radiograph; carotid or femoral bruits.
During the post-trial period, we were able to passively follow 17, 411 men and 15,393 women for mortality status and 9,537 men and 12,086 women for morbidity status (see Supplemental Figure 1). The larger differential between the sexes for morbidity status occurred because of the lack of follow-up of VA participants, who were mostly male. (See Cushman et al.18 for details).
Intermediate Outcomes
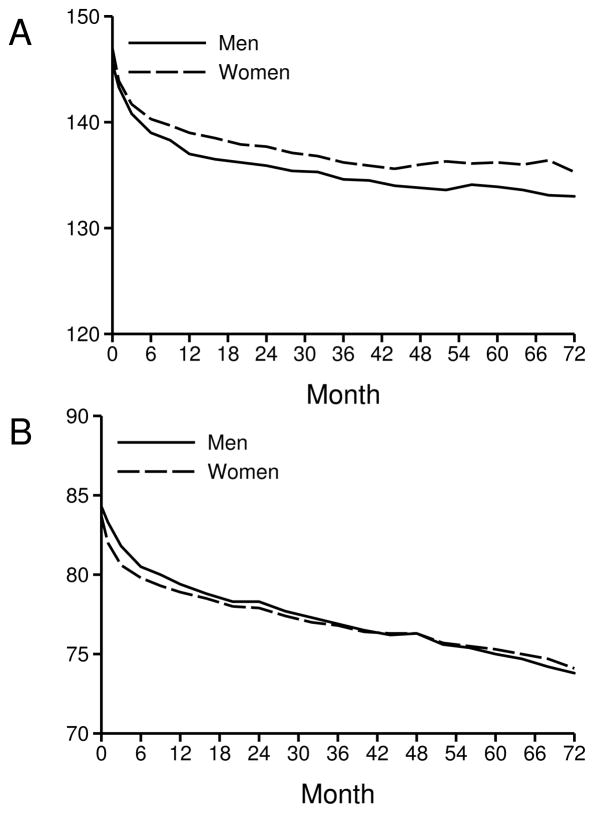
BP decreased substantially during the first year of the study and showed modest further decreases over subsequent years in both genders in all treatment groups, although the decreases in systolic BP were slightly less in women than in men across treatment groups (Figure 2 and Table 2). Together with higher baseline BPs, this resulted in significantly higher systolic BPs in women than in men during the study. Diastolic BPs showed only minor differences between genders. In women, the mean reduction in systolic BP from baseline was 2–3 mmHg greater with chlorthalidone than with lisinopril and 0.4–1 mmHg greater with chlorthalidone than with amlodipine. The mean attained systolic BP in women was 2–3 mmHg lower on chlorthalidone vs lisinopril, and differed by <1mmHg on chlorthalidone vs amlodipine. Treatment related differences were smaller in men: follow-up systolic BPs were 1.3–1.5 mmHg lower on chlorthalidone than on either amlodipine or lisinopril. Follow-up diastolic BPs in men were similar in all 3 treatment groups.
Figure 2.
Average systolic and diastolic blood pressure by gender
Systolic A
Diastolic B
Table 2.
Blood pressure and fasting glucose results at baseline and follow-up
| BP and fasting glucose results | Men | Women | ||||||
|---|---|---|---|---|---|---|---|---|
| Chlorthalidone | Amlodipine | Lisinopril | All | Chlorthalidone | Amlodipine | Lisinopril | All | |
| Participants, n | ||||||||
| Baseline | 8,084 | 4,768 | 4,867 | 17,719 | 7,171 | 4,280 | 4,187 | 15,638 |
| Year-1 | 6,945 | 4,101 | 4,148 | 15,194 | 5,919 | 3,508 | 3,372 | 12,799 |
| Year-2 | 6,377 | 3,727 | 3,744 | 13,848 | 5,362 | 3,155 | 2,956 | 11,473 |
| Year-4 | 5,256 | 3,113 | 3,051 | 11,420 | 4,124 | 2,524 | 2,274 | 8,922 |
| Systolic Blood Pressure mmHg, mean (sd) | ||||||||
| Baseline | 145.5(15.9) | 145.8(15.7) | 145.6(15.7) | 145.6(15.8) | 147.1(15.4) | 146.7(15.6) | 147.3(15.3) | 147.0(15.4) |
| Year-1 | 136.1(15.5) | 137.8(14.6) | 138.7(18.0) | 137.3(16.0) | 137.7(16.2) | 139.3(15.2) | 141.6(19.0) | 139.2(16.8) |
| Year-2 | 135.1(15.3) | 136.7(14.4) | 137.3(17.6) | 136.1(15.8) | 136.9(16.5) | 137.6(15.7) | 139.9(18.1) | 137.9(16.8) |
| Year-4 | 132.9(15.5) | 134.2(14.5) | 134.4(17.0) | 133.7(15.7) | 135.3(16.0) | 135.6(15.5) | 137.0(17.5) | 135.8(16.2) |
| Mean change in SBP from baseline, mmHg, mean(SD) | ||||||||
| Year-1 | −9.1(18.4) | −7.8(18.8) | −6.7(20.4) | −8.1(19.1) | −9.0(19.1) | −7.19(18.8) | −5.6(21.2) | −7.6(19.7) |
| Year-2 | −10.1(19.0) | −8.9(18.4) | −7.8(20.3) | −9.2(19.2) | −9.8(19.8) | −8.8(19.8) | −7.0(21.2) | −8.8(20.2) |
| Year-4 | −12.0(19.6) | −11.3(19.3) | −10.8(20.6) | −11.5(19.8) | −11.2(19.9) | −10.8(20.0) | −9.6(21.4) | −10.7(20.3) |
| Diastolic blood pressure mmHg, mean(sd) | ||||||||
| Baseline | 84.3(10.1) | 84.2(10.2) | 84.4(10.0) | 84.3(10.1) | 83.8(10.0) | 83.6(10.1) | 83.8(10.0) | 83.7(10.0) |
| Year-1 | 79.7(9.6) | 79.0(9.5) | 79.9(10.5) | 79.6(9.8) | 78.9(9.6) | 78.3(9.6) | 80.0(10.5) | 79.0(9.9) |
| Year-2 | 78.5(9.6) | 78.0(9.4) | 78.6(10.5) | 78.4(9.8) | 78.2(9.5) | 77.4(9.8) | 78.7(10.1) | 78.0(9.7) |
| Year-4 | 76.4(9.8) | 75.7(9.5) | 76.5(10.4) | 76.2(9.9) | 76.5(9.4) | 75.8(9.6) | 76.8(10.3) | 76.4(9.7) |
| Mean change in DBP from baseline, mg/dL, mean(SD) | ||||||||
| Year-1 | −4.3(10.6) | −5.0(10.9) | −4.4(11.2) | −4.5(10.9) | −4.7(11.2) | −5.2(10.8) | −3.6(11.5) | −4.5(11.2) |
| Year-2 | −5.6(11.1) | −6.1(11.1) | −5.5(11.3) | −5.7(11.1) | −5.4(11.3) | −6.1(11.4) | −4.8(11.7) | −5.5(11.4) |
| Year-4 | −7.5(11.6) | −8.3(11.5) | −7.7(11.9) | −7.8(11.6) | −6.9(11.5) | −7.6(11.4) | −6.5(12.1) | −7.0(11.6) |
| Blood Pressure <140/90 mmHg, n(%) | ||||||||
| Baseline | 2,344(29.0) | 1,339(28.1) | 1,352(27.8) | 5,035(28.4) | 1,810(25.2) | 1,156(27.0) | 1,031(24.6) | 3,997(25.6) |
| Year-1 | 4,123(59.4) | 2,318(56.5) | 2,213(53.4) | 8,654(57.0) | 3,309(55.9) | 1,880(53.6) | 1,588(47.1) | 6,777(53.0) |
| Year-2 | 4,047(63.5) | 2,162(58.0) | 2,114(56.5) | 8,323(60.1) | 3,109(58.0) | 1,785(56.6) | 1,513(51.2) | 6,407(55.8) |
| Year-4 | 3,658(69.6) | 2,077(66.7) | 2,001(66.0) | 7,736(67.8) | 2,630(63.8) | 1,630(64.6) | 1,359(60.0) | 5,619(63.0) |
| Fasting glucose, mg/dL † | ||||||||
| Participants at baseline, n | 6,003 | 3,523 | 3,657 | 13,183 | 5,093 | 3,015 | 3,000 | 11,108 |
| Year-2 | 3,320 | 1,917 | 1,892 | 7,129 | 2,517 | 1,501 | 1,353 | 5,371 |
| Year-4 | 2,828 | 1,612 | 1,566 | 6,006 | 2,021 | 1,260 | 1,093 | 4,374 |
| Mean (SD) Baseline | 121.8(55.9) | 121.1(53.5) | 121.1(52.5) | 121.4(54.3) | 125.7(61.4) | 125.7(60.9) | 125.3(60.1) | 125.6(60.9) |
| Baseline if have 2 yr follow- up* | 118.0(48.8) | 118.0(47.9) | 119.3(48.7) | 118.3(48.5) | 122.5(57.4) | 122.7(58.4) | 120.4(54.8) | 122.0(57.0) |
| Year-2 | 125.9(55.5) | 121.2(51.8) | 121.5(53.8) | 123.4(54.1) | 129.9(63.9) | 124.0(57.6) | 120.6(55.3) | 125.9(60.2) |
| Year-4 | 125.0(53.1) | 122.3(48.8) | 122.3(51.0) | 123.5(51.4) | 128.3(59.6) | 126.1(56.6) | 120.3(51.5) | 125.7(56.9) |
| Change from baseline, mg/dL, mean(sd) | ||||||||
| Year-2 | 7.2(48.4) | 2.4(45.7) | 2.4(47.4) | 4.6(47.5) | 8.1(53.3) | 2.0(52.9) | 0.23(49.5) | 4.4(52.4) |
| Year-4 | 5.1(54.5) | 4.4(48.7) | 2.6(48.5) | 4.2(51.4) | 7.1(59.1) | 4.2(56.8) | 1.5(49.0) | 4.8(56.1) |
| Fasting glucose ≥ 126 mg/dL, n(%) | ||||||||
| Baseline | 1,650(27.5) | 997(28.3) | 1,043(28.5) | 3,690(28.0) | 1,573(31.0) | 926(30.7) | 916(30.5) | 3,415(30.7) |
| Year-2 | 1,083(32.6) | 559(29.2) | 560(29.6) | 2,202(30.9) | 844(33.5) | 461(30.7) | 369(27.3) | 1,674(31.2) |
| Year-4 | 911(32.2) | 488(30.3) | 464(29.6) | 1,863(31.0) | 670(33.2) | 395(31.4) | 300(27.5) | 1,365(31.2) |
The mean changes are calculated using only those participants who have both a value at baseline and a value at the indicated year of follow-up. All other means are calculated for all participants at the designated time point.
The number of participants with fasting glucose values is smaller than the numbers for the other measurements because the participants frequently arrived non-fasting, were asked to return fasting, and did not. The mean at baseline was also calculated for fasting glucose for only those participants who had a fasting glucose at year-2 follow-up. This is to make it easier for the reader to understand the mean changes which are calculated only for participants with measurements at both time points.
While BP control rates were lower with lisinopril compared with chlorthalidone or amlodipine, more than 60% of participants were controlled to <140/90 mmHg at year 4, and mean BP was <140/90 mmHg in both gender subgroups. The percent of participants with controlled BP (<140/90 mmHg) was lower in women than in men by 1% to 4% at baseline across treatment groups, and this difference increased to 6% in the chlorthalidone and lisinopril groups, but decreased to 2% in the amlodipine group after 4 years of follow-up. BP control rates at year 4 were lowest with lisinopril in both gender subgroups; control rates with chlorthalidone and amlodipine were similar in women, but greater with chlorthalidone than amlodipine in men.
Diabetes prevalence based on only examining fasting blood glucose levels at baseline (rather than glucose levels and/or history of diabetes as in Table 1) was higher in women versus men, 30.7% versus 28.0% (Table 2).23 During follow-up, fasting blood glucose increased the least in the lisinopril arm, and the most in the chlorthalidone arm in both gender subgroups (Table 2). The percentage of participants with fasting blood glucose >126 mg/dL at 2 and 4 years of follow-up increased by 2%–5% with chlorthalidone, increased by 1%–2% with amlodipine, and changed by between +1% and −3% with lisinopril in both gender groups. During follow-up, changes in serum K+, serum cholesterol, and serum creatinine were similar to what has been reported previously in both genders (data not shown).10 For the post-trial period, data are not available on glucose, potassium, cholesterol or creatinine levels, or blood pressure.
In-Trial Primary and Secondary Outcomes
Six-year event rates for the primary outcome of nonfatal MI and fatal CHD were lower in women than in men, 9.1 vs. 13.9 per 100 participants (Table 3). Between treatments, no significant differences were noted in either gender subgroup. Rates for the secondary end-points of all-cause mortality, combined CHD, combined CVD, stroke, heart failure (treated/fatal/hospitalized), and cancer were all lower in women. Rates for components of the secondary endpoints, including angina, coronary revascularization, and peripheral arterial disease, were also lower in women, while rates for hospitalized/fatal HF and ESRD were similar in women and men.
Table 3a.
Clinical outcomes by antihypertensive treatment group (women)
| Outcomes | 6-year Rate per 100 persons (SE), n | From Cox Regression Hazard Ratio and 95% Confidence Interval | ||||
|---|---|---|---|---|---|---|
| Chlorthalidone | Amlodipine | Lisinopril | Total | A/C | L/C | |
| Total randomized, n | 7,171 | 4,280 | 4,187 | 15,638 | ||
| Primary Endpoint | ||||||
| CHD (nonfatal MI + fatal CHD) | 9.2(0.5)477 | 8.9(0.6), 282 | 9.2(0.6), 287 | 9.1(0.3), 1046 | 0.99(0.86–1.14) | 1.05(0.91–1.21) |
| Secondary Endpoints | ||||||
| Mortality outcomes | ||||||
| All-Cause Mortality | 15.7(0.6), 867 | 15.1(0.7), 491 | 15.3(0.7), 499 | 15.4(0.4), 1857 | 0.96(0.86–1.07) | 1.00 (0.90–1.11) |
| CV Mortality | 7.9(0.4), 416 | 7.9(0.5), 244 | 7.8(0.5), 246 | 7.9(0.3), 906 | 0.97(0.83–1.13) | 1.00(0.86–1.17) |
| CHD | 4.0(0.3), 206 | 3.8(0.4), 116 | 3.7(0.4), 116 | 3.9(0.2), 438 | 0.94(0.76–1.18) | 0.97(0.78–1.21) |
| Stroke | 1.2(0.2), 69 | 1.3(0.2), 35 | 1.7(0.3), 54 | 1.4(0.1), 158 | 0.86(0.58–1.28) | 1.38(0.98–1.96) |
| Heart Failure | 1.4(0.2), 63 | 1.4(0.2), 45 | 1.4(0.3), 37 | 1.4(0.1), 145 | 1.12(0.77–1.62) | 0.92(0.62–1.37), |
| Cancer | 2.8(0.3), 155 | 3.1(0.3), 107 | 2.6(0.3), 86 | 2.8(0.2), 348 | 1.19(0.93–1.51)* | 1.03(0.80–1.32) |
| Combined fatal/nonfatal outcomes | ||||||
| Combined CVD | 26.8(0.7), 1526 | 27.3(0.9), 944 | 28.4(0.9), 963 | 27.3(0.5), 3433 | 1.03(0.95–1.12) | 1.11(1.02–1.20) |
| Stroke | 5.3(0.3), 281 | 4.5(0.4), 139 | 6.3(0.5), 196 | 5.3(0.2), 616 | 0.84(0.69–1.02) | 1.21(1.01–1.45) |
| Renal disease | 1.8(0.2), 83 | 1.9(0.3), 55 | 2.2(0.3), 62 | 2.0(0.2), 200 | 1.08(0.77–1.51) | 1.27(0.91–1.76) |
| Cancer | 6.8(0.4), 366 | 6.7(0.5), 229 | 7.3(0.5), 226 | 6.9(0.3), 821 | 1.06(0.90–1.25) | 1.09(0.93–1.29) |
| Heart Failure (treated/hospitalized/fatal) | 7.9(0.5), 379 | 9.6(0.6), 302 | 8.3(0.5), 268 | 8.5(0.3), 949 | 1.32(1.13–1.53) | 1.22(1.04–1.42) |
| Hospitalized/fatal heart failure | 6.8(0.4), 325 | 8.0(0.5), 251 | 6.7(0.5), 206 | 7.1(0.3), 782 | 1.28(1.08–1.50) | 1.09(0.91–1.29) |
| Hospitalized Angina | 6.3(0.3), 369 | 6.6(0.5), 221 | 7.3(0.5), 243 | 6.6(0.2), 833 | 0.98(0.83–1.16) | 1.14(0.97–1.34) |
| Coronary revascularization | 6.4(0.4), 349 | 6.8(0.5), 227 | 6.5(0.5), 208 | 6.5(0.3), 784 | 1.08(0.92–1.28) | 1.03(0.87–1.22) |
| Peripheral arterial disease | 3.1(0.3), 175 | 2.5(0.3), 90 | 3.7(0.4), 117 | 3.1(0.2), 382 | 0.85(0.66–1.10) | 1.15(0.91–1.45) |
Abbreviations: CVD, cardiovascular disease; CV, cardiovascular; MI, myocardial infarction; SE, standard error.
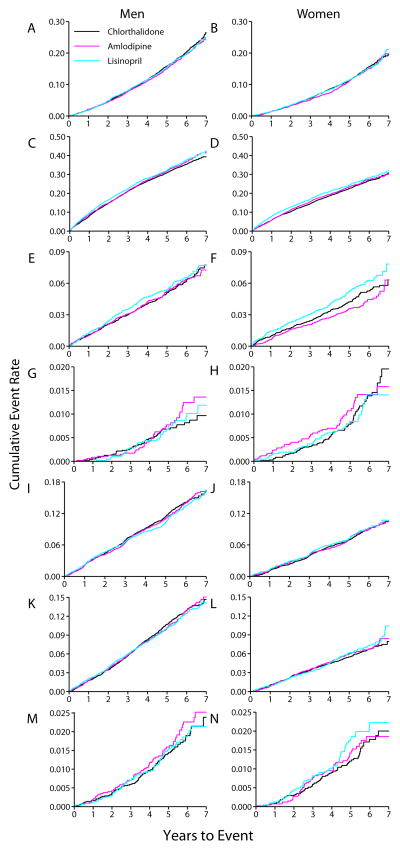
Between treatments, no significant differences were found for all-cause mortality or its subcomponents, CV or combined CHD mortality, in either gender (Figures 3A&B). In women, stroke mortality rates tended to be lower (HR=0.86 P=0.45, for amlodipine versus chlorthalidone) and higher, (HR=1.38, P=0.07, for lisinopril versus chlorthalidone). In men, these differences were smaller and also not statistically significant and there were no significant gender-treatment interactions (P>.36). Combined CVD and heart failure (treated/fatal/hospitalized) were significantly increased in the lisinopril versus chlorthalidone arms in both women and men whereas both heart failure outcomes were in the amlodipine versus chlorthalidone arms. Stroke was significantly increased for women only in the lisinopril versus chlorthalidone comparison. There were no significant treatment gender interactions. Individual components of the combined CVD endpoint did not differ by gender. Regarding other secondary outcomes, rates for cancer or ESRD were not different in the amlodipine and lisinopril arms versus the chlorthalidone-arm for either women or men.
Figure 3.
Kaplan-Meier curves for morbidity and mortality rates by gender and treatment group
Total mortality A B
Combined CVD C D
Hospitalized/fatal stroke E F
HF mortality G H
Endpoint CHD I J
Hospitalized/fatal cancer K L
ESRD M N
Abbreviations: CHD, coronary heart disease; CVD, cardiovascular disease; HF, heart failure; ESRD, End-stage renal disease.
Extension Results
No significant differences appeared in CV mortality for amlodipine (hazard ratio [HR], 1.01; 95% confidence interval [CI], 0.93–1.10) or lisinopril (HR, 0.96; CI, 0.88–1.05), each compared with chlorthalidone, overall, or for men or women (Supplemental Table 1 and Supplemental Figure 2). The only significant differences in secondary outcomes were for hospitalized and fatal HF, which was higher with amlodipine (HR, 1.12; CI, 1.02–1.22), and stroke mortality, which was higher with lisinopril (HR, 1.20; CI, 1.01–1.41), each compared with chlorthalidone. While men had a significantly higher HF risk (HR=1.17, P=.03), there were no significant interactions by sex.
The only significant treatment gender interaction noted was for cancer mortality for amlodipine versus chlorthalidone. For women, the HR was 1.20, 95% CI (1.02–1.40); for men, it was 0.93, 95% CI (0.82–1.05), P for interactions =.01. With regard to comparison of rates by gender, men had higher overall total, CVD, and CHD 10-year mortality rates than women, whereas the stroke and HF 10-year morality rates were similar, overall, and within each treatment group. For combined fatal/nonfatal outcomes, men had higher 10-year rates of CHD, CVD, stroke, cancer, and ESRD, but similar 10-year rates for HF.
Discussion
Subgroup analyses of ALLHAT extend the findings of this trial and those of other studies by confirming the consistency of the results in both genders.10, 24–28 In ALLHAT, no differences in CVD outcomes by gender were detected for the in-trial or extension periods.
For the in-trial period, there were significantly higher rates of HF, stroke, and combined CVD for lisinopril compared to chlorthalidone, and significantly higher rates of HF for amlodipine compared to chlorthalidone. These findings did not persist through the extension period with the exception of the HF result for amlodipine versus chlorthalidone.18 Though men had a significant 17% increased risk for amlodipine versus chlorthalidone, this was not significantly different from the result for women. Also, the finding of significantly higher stroke mortality during the extension period overall (HR, 1.20, CI, 1.10–14.1) for lisinopril versus chlorthalidone18 did not differ significantly by sex. These results could be consistent with many other post-trial results wherein the medications used, including the use of diuretics, likely became more similar across the randomized groups, or could be due to chance.
In a previous summary of the ALLHAT findings,29 it was noted that BP differences may account for some but not all of the advantages seen with chlorthalidone. ALLHAT has reported analyses using achieved BP levels as time-dependent covariates in a Cox proportional hazard regression model showing that after adjustment for BP, the differences in risk of stroke and HF between treatment arms remain statistically significant, with only slight reduction in the hazard ratios.10,14, 17, 30 Also, in this previous summary,29 it was noted that at doses equivalent to that used in ALLHAT (chlorthalidone, average of 20 mg/day), it is likely that attributes of chlorthalidone extend to the class of thiazide and thiazide-type diuretics.
There was one significant treatment by gender interaction for the extended follow-up for cancer mortality (P=.01 for interaction) for amlodipine versus chlorthalidone--women had an HR=1.20 (95%CI, 1.02–1.40, P=.02) whereas men had an HR=0.93 (95% CI, 0.82–1.05, P=.26). For the in-trial period, the results were similar – significant treatment x gender interaction (P=.03 for interaction) for amlodipine versus chlorthalidone – women had an HR=1.19 (95% CI, 0.93–1.51, P=.16) whereas men had an HR=0.82 (0.68–0.97, P=.02). Given the many analyses performed, this may just be the play of chance.
These findings are consistent with results of a prospectively designed overview by the Blood Pressure Lowering Treatment Trialists’ Collaboration of 31 randomized controlled trials of antihypertensive treatment with CVD outcomes that included 87,349 women and 103,268 men.3 The Collaboration, which included ALLHAT, tested whether there were important differences between genders in the effects of different BP-lowering regimens.3 Separate overviews were carried out for trials comparing active agents with placebo, more intensive with less intensive BP-lowering regimens, and one active agent with another. For all treatment comparisons, mean baseline BPs were slightly higher for women compared with men, but achieved BP reductions were comparable in both sexes. There was no evidence of a difference in the effects of BP-lowering treatment regimens between women and men for any CVD outcome except stroke. For stroke, there was some evidence that women, but not men, derive greater benefit from CCB-based regimens than ACE inhibitor-based regimens. Evidence for this interaction was of borderline (P=.05) statistical significance and, in view of the large number (42) of subgroup analyses made in the study, was attributed by the authors to chance. Overall, there was no evidence that women and men derived different levels of outcome benefit from BP reduction or that regimens based on different drug classes were more effective in one sex than the other. Based on these observations, the authors concluded that a patient’s gender should not influence decisions about the need for BP-lowering therapy, the magnitude of BP reduction to be sought, or the selection of drug class.
These findings reinforce the conclusions of earlier overviews of aggregated data from randomized trials that compared active treatment with placebo or less systematically treated controls and reported results by gender.31–33 The earlier analyses concluded that treatment effects (as assessed by hazard ratios) on morbidity and mortality did not differ between women and men. While absolute benefit of treatment did differ between genders for some outcomes (e.g. greater for coronary heart disease and mortality in men) but not others (e.g. stroke), the gender differences in absolute benefit were entirely attributable to differences in underlying risk. With regard to choice of first-step drugs, early trial evidence suggested no significant differences in major outcomes between diuretic-based and beta-blocker-based treatment in either gender.
Other outcome trials of BP treatment that reported results by gender confirm the ALLHAT findings. With regard to CCB-based treatment, the placebo-controlled Systolic Hypertension in Europe trial reported no interaction by gender for either stroke or cardiac outcomes,34 and one direct comparator trial, the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial25 reported no gender difference in relative risks for the primary composite CV outcome in the study as a whole, which were near unity, or between treatment arms (verapamil vs. beta-blocker or diuretic). A pre-specified subgroup analysis from the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial found a relative excess of the primary composite CV endpoint with valsartan-based compared with amlodipine-based treatment in women but not in men.35, 36 Much of this outcome benefit was attributed to greater BP reduction with amlodipine in women. However, the authors questioned whether there is a genuine gender difference in the cardiac protection afforded by the amlodipine-based vs. valsartan-based treatment because the trend toward less HF in valsartan-treated patients was significant only in men.
The results of ALLHAT differ from those of a smaller trials carried out in a less diverse population in which many fewer events were reported. The Australian National Blood Pressure-2 (ANBP-2) trial showed a lack of benefit of ACE inhibitor (enalapril)-based treatment compared with diuretic-based treatment in women, but not in men.37 There was a nonsignificant interaction by gender in the context of an overall marginally significant difference (P=.05) favoring the ACE inhibitor for all CV events/total mortality. Only 524 events were observed in women in ANBP-2, suggesting that the apparent lack of benefit of ACE inhibitor treatment reflected inadequate power to detect a beneficial effect of renin-angiotensin-aldosterone system blockade in hypertensive women, in whom event rates are lower than in men. The different results may also reflect differences in baseline characteristics of the populations included in ANBP-2 compared with ALLHAT. ANBP-2 included an older population with higher baseline systolic and diastolic BPs and lower prevalence of CHD, cerebrovascular disease, diabetes and smoking.
Perspectives
The similarity of the treatment effects in ALLHAT by gender is robust because of the large sample size equally distributed between men and women. The ALLHAT conclusion reported for the whole cohort that neither lisinopril nor amlodipine is superior to chlorthalidone for initial therapy of hypertension also applies to the pre-specified subgroups defined by gender. These drugs did not surpass the diuretic chlorthalidone in lowering BP, tolerability, or in preventing major clinical complications of hypertension.
Supplementary Material
Table 3b.
Clinical outcomes by antihypertensive treatment group (men)
| Outcomes | 6-year Rate per 100 persons (SE), n | From Cox Regression Hazard Ratio and 95% Confidence Interval | ||||
|---|---|---|---|---|---|---|
| Chlorthalidone | Amlodipine | Lisinopril | Total | A/C | L/C | |
| Total randomized | 8,084 | 4,768 | 4,867 | 17,719 | ||
| Primary Endpoint | ||||||
| CHD (nonfatal MI + fatal CHD) | 14.1(0.5), 878 | 14.0(0.6), 508 | 13.5(0.6), 493 | 13.9(0.3), 1879 | 0.98(0.88–1.10) | 0.94(0.84–1.05) |
| Secondary Endpoints | ||||||
| Mortality outcomes | ||||||
| All-Cause Mortality | 20.4(0.5), 1345 | 19.7(0.7), 763 | 19.8(0.7), 797 | 20.0(0.4), 2905 | 0.95(0.88–1.04) | 0.98(0.90–1.06) |
| CV Mortality | 9.1(0.4), 581 | 9.9(0.5), 370 | 9.6(0.5), 362 | 9.5(0.3), 1313 | 1.05(0.93–1.20) | 1.03(0.90–1.17) |
| CHD | 5.4(0.3), 338 | 5.5(0.40), 202 | 5.5(0.4), 205 | 5.4(0.2), 745 | 0.99(0.84–1.18) | 1.00(0.84–1.18) |
| Stroke | 1.6(0.2), 91 | 1.6(0.2), 58 | 1.6(0.2), 61 | 1.6(0.1), 210 | 1.05(0.76–1.44) | 1.11(0.81–1.52) |
| Heart Failure | 0.8(0.1), 51 | 1.2(0.2), 38 | 1.0(0.2), 33 | 1.0(0.1), 122 | 1.27(0.84–1.91) | 1.06(0.69–1.63) |
| Cancer* | 6.1(0.3), 369 | 4.6(0.4), 175 | 5.5(0.4), 208 | 5.5(0.2), 752 | 0.82(0.68–0.97)* | 0.94(0.80–1.11) |
| Combined fatal/nonfatal outcomes | ||||||
| Combined CVD | 35.4(0.7), 2376 | 36.8(0.9), 1454 | 37.8(0.9), 1515 | 36.4(0.4), 5345 | 1.04(0.98–1.11) | 1.07(1.00–1.15) |
| Stroke | 6.3(0.3), 380 | 6.4(0.4), 231 | 6.5(0.4), 250 | 6.4(0.2), 861 | 1.00(0.86–1.18) | 1.09(0.93–1.28) |
| Renal disease | 1.9(0.2), 105 | 2.3(0.3), 73 | 1.9(0.3), 64 | 2.0(0.1), 242 | 1.13(0.85–1.52) | 1.00(0.74–1.35) |
| Cancer | 12.9(0.5), 799 | 13.0(0.6), 463 | 12.4(0.6), 461 | 12.8(0.3), 1723 | 0.98(0.88–1.10) | 0.96(0.86–1.08) |
| Heart Failure (treated/hospitalized/fatal) | 7.9(0.4), 466 | 11.0(0.6), 385 | 9.2(0.5), 333 | 9.1(0.3), 1184 | 1.41(1.24–1.61) | 1.18(1.03–1.36) |
| Hospitalized/fatal heart failure | 6.5(0.4), 375 | 8.9(0.5), 307 | 7.2(0.5), 253 | 7.3(0.3), 935 | 1.40(1.20–1.62) | 1.12(0.96–1.31) |
| Hospitalized Angina | 10.7(0.4), 681 | 10.2(0.5), 400 | 11.5(0.6), 438 | 10.8(0.3), 1519 | 0.98(0.87–1.11) | 1.06(0.94–1.20) |
| Coronary revascularization | 11.9(0.5), 737 | 12.9(0.6), 472 | 13.5(0.6), 495 | 12.6(0.3), 1704 | 1.10(0.98–1.23) | 1.12(1.00–1.25) |
| Peripheral arterial disease | 4.9(0.3), 328 | 4.7(0.4), 169 | 5.0(0.4), 193 | 4.9(0.2), 690 | 0.88(0.73–1.05) | 0.97(0.81–1.16) |
Abbreviations: CVD, cardiovascular disease; CV, cardiovascular; MI, myocardial infarction; SE, standard error.
Cancer mortality: significant treatment x gender interaction (P=.03 for interaction) for A vs C. Female: HR 1.19 (0.93–1.51, P=.16). Male: HR 0.82 (0.68–0.97, P=.02).
Novelty and Significance.
What Is New?
This analysis includes over 15000 women, the largest female cohort in a trial to examine the comparative efficacy of antihypertensive medications
Participants were actively followed for an average of 4.9 years and passively followed for an additional average of 4 years, making this one of the longest follow-up times for this type of trial.
What Is Relevant?
Hypertension is highly prevalent, difficult to control, and associated with a high incidence of CVD complications in older women, the population that was enrolled in ALLHAT
Epidemiological evidence shows that women are less likely than men to meet BP control targets
Summary
The ALLHAT conclusion reported for the whole cohort that neither lisinopril nor amlodipine is superior to chlorthalidone for initial therapy of hypertension also applies to the pre-specified subgroups defined by gender.
Acknowledgments
Sources of Funding
This study was supported by contracts NO1-HC-35130 and HHSN268201100036C with the National Heart, Lung, and Blood Institute. The ALLHAT investigators acknowledge study medications contributed by Pfizer, Inc., (amlodipine and doxazosin), AstraZeneca (atenolol and lisinopril), and Bristol-Myers Squibb (pravastatin) and financial support provided by Pfizer, Inc.
Footnotes
Disclosures
Dr. Cushman has received honoraria from Amgen, Daiichi Sankyo, Novartis, Noven, Sanofi, Takeda, Theravance; and has received research support from GlaxcoSmithKline and Novartis. Dr. Davis has received honoria from Amgen and Takeda. Dr. Habib has received research support from Astra-Zeneca, GlaxcoSmithKline, and Sanofi Aventis. Dr. Margolis has received research support from Bristol-Myers Squibb. Dr. Oparil has received honoraria from Eli Lilly, Schering Plough, Novartis, Daiichi Sankyo, Boehringer Ingelheim, Forest Pharmaceuticals, NicOx, Forest Laboratories, Pfizer, Omron Healthcare; and has received research support from Bristol-Myers Squibb, Takeda, Daiichi Sankyo, Gilead, Merck, Amgen, Boehringer Ingelheim, Sanofi Aventis, Abott Laboratories, and GlaxoSmithKline. Dr. Wright has received honoraria from Sanofi Aventis, CVRx, Daiichi Sankyo, and Novartis.
Drs. Ford, Furberg, Haywood, and Whelton have no financial interests to disclose
Clinical trial registration: www.clinicaltrials.gov, NCT00000542
References
- 1.Lawes CM, Vander Hoorn S, Rodgers A International Society of Hypertension. Global burden of blood-pressure-related disease, 2001. Lancet. 2008;371:1513–1518. doi: 10.1016/S0140-6736(08)60655-8. [DOI] [PubMed] [Google Scholar]
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turnbull F, Woodward M, Neal B, Barzi F, Ninomiya T, Chalmers J, Perkovic V, Li N, MacMahon S Blood Pressure Lowering Treatment Trialists’ Collaboration. Do men and women respond differently to blood pressure-lowering treatment? Results of prospectively designed overviews of randomized trials. Eur Heart J. 2008;29:2669–2680. doi: 10.1093/eurheartj/ehn427. [DOI] [PubMed] [Google Scholar]
- 4.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D. Prevalence of hypertension in the US adult population. Results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension. 1995;25:305–313. doi: 10.1161/01.hyp.25.3.305. [DOI] [PubMed] [Google Scholar]
- 5.Wassertheil-Smoller S, Anderson G, Psaty BM, Black HR, Manson J, Wong N, Francis J, Grimm R, Kotchen T, Langer R, Lasser N. Hypertension and its treatment in postmenopausal women: baseline data from the Women’s Health Initiative. Hypertension. 2000;36:780–789. doi: 10.1161/01.hyp.36.5.780. [DOI] [PubMed] [Google Scholar]
- 6.Lloyd-Jones DM, Evans JC, Levy D. Hypertension in adults across the age spectrum: current outcomes and control in the community. JAMA. 2005;294:466–472. doi: 10.1001/jama.294.4.466. [DOI] [PubMed] [Google Scholar]
- 7.Keyhani S, Scobie JV, Hebert PL, McLaughlin MA. Gender disparities in blood pressure control and cardiovascular care in a national sample of ambulatory care visits. Hypertension. 2008;51:1149–1155. doi: 10.1161/HYPERTENSIONAHA.107.107342. [DOI] [PubMed] [Google Scholar]
- 8.Davis BR, Cutler JA, Gordon DJ, Furberg CD, Wright JT, Jr, Cushman WC, Grimm RH, LaRosa J, Whelton PK, Perry HM, Alderman MH, Ford CE, Oparil S, Francis C, Proschan M, Pressel S, Black HR, Hawkins CM. Rationale and design for the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Am J Hypertens. 1996;9:342–360. doi: 10.1016/0895-7061(96)00037-4. [DOI] [PubMed] [Google Scholar]
- 9.Grimm RH, Jr, Margolis KL, Papademetriou V, Cushman WC, Ford CE, Bettencourt J, Alderman MH, Basile JN, Black HR, Dequattro V, Eckfeldt J, Hawkins CM, Perry HM, Jr, Proschan M for the ALLHAT Collaborative Research Group. Baseline Characteristics of Participants in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2001;37:19–27. doi: 10.1161/01.hyp.37.1.19. [DOI] [PubMed] [Google Scholar]
- 10.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2002;288:2981–2997. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 11.The ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group. Major cardiovascular events in hypertensive patients randomized to doxazosin vs chlorthalidone: The Antihypertensive and Lipid-lowering Treatment to Prevent Heart Attack Trial (ALLHAT) JAMA. 2000;283:1967–1975. [PubMed] [Google Scholar]
- 12.Pressel SL, Davis BR, Wright JT, Jr, Geraci TS, Kingry C, Ford CE, Piller LB, Bettencourt J, Kimmel B, Lusk C, Parks H, Simpson LM, Nwachuku C, Furberg CD ALLHAT Collaborative Research Group. Operational aspects of terminating the doxazosin arm of The Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Control Clin Trials. 2001;22:29–41. doi: 10.1016/s0197-2456(00)00109-4. [DOI] [PubMed] [Google Scholar]
- 13.Vidt DG. Alpha-blockers and congestive heart failure: early termination of an arm of the ALLHAT trial. Cleve Clin J Med. 2000;67:429–433. doi: 10.3949/ccjm.67.6.429. [DOI] [PubMed] [Google Scholar]
- 14.Davis BR, Cutler JA, Furberg CD, Wright JT, Farber MA, Felicetta JV, Stokes JD ALLHAT Collaborative Research Group. Relationship of antihypertensive treatment regimens and change in blood pressure to risk for heart failure in hypertensive patients randomly assigned to doxazosin or chlorthalidone: further analyses from the Antihypertensive and Lipid-Lowering treatment to prevent Heart Attack Trial.summary for patients in Ann Intern Med. 2002 Sep 3;137(5 Part 1):I38; PMID: 12204046. Arch Intern Med. 2002;137:313–320. doi: 10.7326/0003-4819-137-5_part_1-200209030-00006. [DOI] [PubMed] [Google Scholar]
- 15.Piller LB, Davis BR, Cutler JA, Cushman WC, Wright JT, Jr, Williamson JD, Leenen FH, Einhorn PT, Randall OS, Golden JS, Haywood LJ for the ALLHAT Collaborative Research Group. Validation of Heart Failure Events in the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants Assigned to Doxazosin and Chlorthalidone. Curr Control Trials Cardiovasc Med. 2002;3:10. doi: 10.1186/1468-6708-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barzilay JI, Davis BR, Bettencourt J, Margolis KL, Goff DC, Jr, Black H, Habib G, Ellsworth A, Force RW, Wiegmann T, Ciocon JO, Basile JN ALLHAT Collaborative Research Group. Cardiovascular outcomes using doxazosin vs. chlorthalidone for the treatment of hypertension in older adults with and without glucose disorders: a report from the ALLHAT study. J Clin Hypertens. 2004;6:116–125. doi: 10.1111/j.1524-6175.2004.03216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Diuretic versus alpha-blocker as first-step antihypertensive therapy: final results from the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Hypertension. 2003;42:239–246. doi: 10.1161/01.HYP.0000086521.95630.5A. [DOI] [PubMed] [Google Scholar]
- 18.Cushman WC, Davis BR, Pressel SL, Cutler JA, Einhorn PT, Ford CE, Oparil S, Probstfield JL, Whelton PK, Wright JT, Jr, Alderman MH, Basile JN, Black HR, Grimm RH, Jr, Hamilton BP, Haywood LJ, Ong ST, Piller LB, Simpson LM, Stanford C, Weiss RJ ALLHAT Collaborative Research Group. Mortality and morbidity during and after the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. J Clin Hypertens (Greenwich) 2012;14:20–31. doi: 10.1111/j.1751-7176.2011.00568.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Piller LB, Baraniuk S, Simpson LM, Cushman WC, Massie BM, Einhorn PT, Oparil S, Ford CE, Graumlich JF, Dart RA, Parish DC, Retta TM, Cuyjet AB, Jafri SZ, Furberg CD, Saklayen MG, Thadani U, Probstfield JL, Davis BR ALLHAT Collaborative Research Group. Long-term follow-up of participants with heart failure in the antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT) Circulation. 2011;124:1811–1818. doi: 10.1161/CIRCULATIONAHA.110.012575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barzilay JI, Davis BR, Pressel SL, Cutler JA, Einhorn PT, Black HR, Cushman WC, Ford CE, Margolis KL, Moloo J, Oparil S, Piller LB, Simmons DL, Sweeney ME, Whelton PK, Wong ND, Wright JT, Jr ALLHAT Collaborative Research Group. Long-term effects of incident diabetes mellitus on cardiovascular outcomes in people treated for hypertension: the ALLHAT Diabetes Extension Study. Circ Cardiovasc Qual Outcomes. 2012;5:153–162. doi: 10.1161/CIRCOUTCOMES.111.962522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rahman M, Ford CE, Cutler JA, Davis BR, Piller LB, Whelton PK, Wright JT, Jr, Barzilay JI, Brown CD, Colon PJS, Fine LJ, Grimm RH, Jr, Gupta AK, Baimbridge C, Haywood LJ, Henriquez MA, Ilamaythi E, Oparil S, Preston R for the ALLHAT Collaborative Research Group. Long-Term Renal and Cardiovascular Outcomes in Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Participants by Baseline Estimated GFR. Clin J Am Soc Nephrol. 2012;7:989–1002. doi: 10.2215/CJN.07800811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cushman WC, Ford CE, Cutler JA, Margolis KL, Davis BR, Grimm RH, Black HR, Hamilton BP, Holland J, Nwachuku C, Papademetriou V, Probstfield J, Wright JT, Jr, Alderman MH, Weiss RJ, Piller L, Bettencourt J, Walsh SM for the ALLHAT Collaborative Research Group. Success and predictors of blood pressure control in diverse North American settings: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, Barzilay J, Cushman WC, Davis BR, Iiamathi E, Kostis JB, Leenen FH, Louis GT, Margolis KL, Mathis DE, Moloo J, Nwachuku C, Panebianco D, Parish DC, Pressel S, Simmons DL, Thadani U ALLHAT Collaborative Research Group. Clinical outcomes in antihypertensive treatment of type 2 diabetes, impaired fasting glucose concentration, and normoglycemia: Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) Arch Intern Med. 2005;165:1401–1409. doi: 10.1001/archinte.165.12.1401. [DOI] [PubMed] [Google Scholar]
- 24.Brown MJ, Palmer CR, Castaigne A, de Leeuw PW, Mancia G, Rosenthal T, Ruilope LM. Morbidity and mortality in patients randomised to double-blind treatment with a long-acting calcium-channel blocker or diuretic in the International Nifedipine GITS study: Intervention as a Goal in Hypertension Treatment (INSIGHT) Lancet. 2000;356(9227):366–372. doi: 10.1016/S0140-6736(00)02527-7. [DOI] [PubMed] [Google Scholar]
- 25.Black HR, Elliott WJ, Grandits G, Grambsch P, Lucente T, White WB, Neaton JD, Grimm RH, Jr, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber MA, Williams G, Wittes J, Zanchetti A, Anders RJ CONVINCE Research Group. Principal results of the Controlled Onset Verapamil Investigation of Cardiovascular End Points (CONVINCE) trial. JAMA. 2003;289:2073–2082. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 26.Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study Investigators. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 27.Hansson L, Lindholm LH, Ekbom T, Dahlof B, Lanke J, Schersten B, Wester PO, Hedner T, de Faire U. Randomised trial of old and new antihypertensive drugs in elderly patients: cardiovascular mortality and morbidity the Swedish Trial in Old Patients with Hypertension-2 study. Lancet. 1999;354:1751–1756. doi: 10.1016/s0140-6736(99)10327-1. [DOI] [PubMed] [Google Scholar]
- 28.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 29.Wright JT, Jr, Probstfield JL, Cushman WC, Pressel SL, Cutler JA, Davis BR, Einhorn PT, Rahman M, Whelton PK, Ford CE, Haywood LJ, Margolis KL, Oparil S, Black HR, Alderman MH ALLHAT Collaborative Research Group. ALLHAT findings revisited in the context of subsequent analyses, other trials, and meta-analyses. Arch Intern Med. 2009;169:832–842. doi: 10.1001/archinternmed.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davis BR, Piller LB, Cutler JA, Furberg C, Dunn K, Franklin S, Goff D, Leenen F, Mohiuddin S, Papademetriou V, Proschan M, Ellsworth A, Golden J, Colon P, Crow R Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial Collaborative Research Group. Role of diuretics in the prevention of heart failure: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Circulation. 2006;113:2201–2210. doi: 10.1161/CIRCULATIONAHA.105.544031. [DOI] [PubMed] [Google Scholar]
- 31.MacMahon SW, Cutler JA, Furberg CD, Payne GH. The effects of drug treatment for hypertension on morbidity and mortality from cardiovascular disease: a review of randomized controlled trials. Prog Cardiovasc Dis. 1986;29:99–118. doi: 10.1016/0033-0620(86)90038-1. [DOI] [PubMed] [Google Scholar]
- 32.Simons-Morton DG, Cutler JA, Allender PS. Hypertension treatment trials and stroke occurrence revisited. A quantitative overview. Ann Epidemiol. 1993;3:555–562. doi: 10.1016/1047-2797(93)90116-l. [DOI] [PubMed] [Google Scholar]
- 33.Gueyffier F, Boutitie F, Boissel JP, Pocock S, Coope J, Cutler J, Ekbom T, Fagard R, Friedman L, Perry M, Prineas R, Schron E. Effect of antihypertensive drug treatment on cardiovascular outcomes in women and men. A meta-analysis of individual patient data from randomized, controlled trials. The INDANA Investigators. Ann Intern Med. 1997;126:761–767. doi: 10.7326/0003-4819-126-10-199705150-00002. [DOI] [PubMed] [Google Scholar]
- 34.Staessen JA, Thijs L, Celis H, Gasowski J, Wang JG, Fagard RH Systolic Hypertension in Europe Trial Investigators (the Syst-Eur Trial) Dihydropyridine calcium-channel blockers for antihypertensive treatment in older patients--evidence from the Systolic Hypertension in Europe Trial. S Afr Med J. 2001;91:1060–1068. [PubMed] [Google Scholar]
- 35.Zanchetti A, Julius S, Kjeldsen S, McInnes GT, Hua T, Weber M, Laragh JH, Plat F, Battegay E, Calvo-Vargas C, Cieslinski A, Degaute JP, Holwerda NJ, Kobalava J, Pedersen OL, Rudyatmoko FP, Siamopoulos KC, Storset O. Outcomes in subgroups of hypertensive patients treated with regimens based on valsartan and amlodipine: An analysis of findings from the VALUE trial. J Hypertens. 2006;24:2163–2168. doi: 10.1097/01.hjh.0000249692.96488.46. [DOI] [PubMed] [Google Scholar]
- 36.Julius S, Kjeldsen SE, Weber M, Brunner HR, Ekman S, Hansson L, Hua T, Laragh J, McInnes GT, Mitchell L, Plat F, Schork A, Smith B, Zanchetti A VALUE trial group. Outcomes in hypertensive patients at high cardiovascular risk treated with regimens based on valsartan or amlodipine: the VALUE randomised trial. Lancet. 2004;363:2022–2031. doi: 10.1016/S0140-6736(04)16451-9. [DOI] [PubMed] [Google Scholar]
- 37.Wing LM, Reid CM, Ryan P, Beilin LJ, Brown MA, Jennings GL, Johnston CI, McNeil JJ, Macdonald GJ, Marley JE, Morgan TO, West MJ Second Australian National Blood Pressure Study Group. A comparison of outcomes with angiotensin-converting--enzyme inhibitors and diuretics for hypertension in the elderly. N Engl J Med. 2003;348:583–592. doi: 10.1056/NEJMoa021716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.