Abstract
BACKGROUND
Circulating cell-free DNA (ccf-DNA) is becoming an important biomarker for cancer diagnostics and therapy monitoring. The isolation of ccf-DNA from plasma as a “liquid biopsy” may begin to replace more invasive tissue biopsies for the detection and analysis of cancer-related mutations. Conventional methods for the isolation of ccf-DNA from plasma are costly, time-consuming, and complex, preventing the use of ccf-DNA biomarkers for point-of-care diagnostics and limiting other biomedical research applications.
METHODS
We used an AC electrokinetic device to rapidly isolate ccf-DNA from 25 μL unprocessed blood. ccf-DNA from 15 chronic lymphocytic leukemia (CLL) patients and 3 healthy individuals was separated into dielectrophoretic (DEP) high-field regions, after which other blood components were removed by a fluidic wash. Concentrated ccf-DNA was detected by fluorescence and eluted for quantification,PCR,and DNA sequencing. The complete process, blood to PCR, required <10 min. ccf-DNA was amplified by PCR with immunoglobulin heavy chain variable region (IGHV)-specific primers to identify the unique IGHV gene expressed by the leukemic B-cell clone, and then sequenced.
RESULTS
PCR and DNA sequencing results obtained by DEP from 25 μL CLL blood matched results obtained by use of conventional methods for ccf-DNA isolation from 1 mL plasma and for genomic DNA isolation from CLL patient leukemic B cells isolated from 15–20 mL blood.
CONCLUSIONS
Rapid isolation of ccf-DNA directly from a drop of blood will advance disease-related biomarker research, accelerate the transition from tissue to liquid biopsies, and enable point-of-care diagnostic systems for patient monitoring.
Circulating cell-free DNA (ccf-DNA)4 is now considered an important biomarker for early detection of cancer (1–4) and residual disease (5), monitoring of chemotherapy (6), and other aspects of cancer management (1, 7–13). The isolation of ccf-DNA from plasma as a “liquid biopsy” will begin to replace more invasive tissue biopsies as a means to detect and analyze cancer mutations (1, 7, 9–12). Unfortunately, conventional methods and techniques for the isolation of ccf-DNA from plasma are extremely time consuming and complex. These are major drawbacks that greatly limit many biomedical research applications and rule out the use of ccf-DNA biomarkers for point-of-care (POC) diagnostic applications. Other limitations of these conventional sample preparation methods and processes include that (a) the procedures generally require starting with ≥1 mL plasma; (b) obtaining the plasma from blood requires centrifugation and pi-petting steps; (c) the large number of manipulations increases the chance for technician errors; (d) the extended time and multiple processing steps add considerable cost to the diagnostic test; (e) the ccf-DNA recovery efficiency decreases as sample size decreases and as the concentration of ccf-DNA in the sample decreases; (f) ccf-DNA can be degraded by mechanical shearing during the many processing steps; and (g) the degradation and loss of higher nano-particulate forms of ccf-DNA limit our knowledge of its true in vivo nature.
In the case of hematologic cancers such as chronic lymphocytic leukemia (CLL) and lymphomas, DNA can be obtained from the transformed cancer cells (14, 15) or isolation of ccf-DNA from plasma (16). The B cells of CLL patients can be segregated into 1 of at least 2 major subsets on the basis of whether the immunoglobulin (Ig) variable region has somatic mutations (17). Patients with CLL cells that express unmutated Ig heavy chain variable region (IGHV)5 genes tend to have an aggressive clinical course relative to patients who have CLL cells that express IGHV with somatic mutations (18–20). For CLL diagnostics and management, DNA is isolated from the peripheral blood mononuclear cells (PBMCs). PBMCs are usually purified from the CLL patient blood samples by density centrifugation with Ficoll-Hypaque 1077. This is a long and labor-intensive process, which adds considerable cost to patient management. PCR and DNA sequencing are performed on the isolated B-cell DNA to determine the mutation status for the expressed IGHV gene (21–23).
Promising electrokinetic technologies, in particular dielectrophoresis (DEP), have long been known to provide effective separation of cells, nanoparticles, DNA, and other biomolecules (24–26). Until recently, DEP techniques remained impractical for general use with high-conductance solutions (5–15 mS/cm), which include important clinical samples such as whole blood, plasma, and serum (25, 26). In earlier work, sample dilution to low-conductance conditions (<1 mS/cm) was required before effective DEP separations could be carried out (26–29). Although some progress was made by using DEP under high-conductance conditions, these efforts have been limited to separations of cells and micrometer-sized entities by negative DEP forces with hybrid electrokinetic devices (27, 30–32). The devices still could not be used with whole blood samples and, more importantly, did not provide isolation of DNA from the sample. We have developed an electrokinetic technique that allows nanoscale entities, including high molecular weight DNA and nanoparticles, to be isolated from high-conductance (>10 mS/cm) solutions (33–35) and whole blood samples (36), and ccf-DNA from blood samples (37). In this study, we show fluorescent analysis, PCR, and Sanger sequencing results for ccf-DNA isolated by DEP from 25-μL samples of unprocessed CLL patient blood. PCR and Sanger sequencing results for the DEP process are compared to results obtained by use of conventional sample preparation of ccf-DNA from 1 mL CLL patient plasma and to DNA sequencing results obtained directly from leukemic B cells. The ability to rapidly isolate ccf-DNA, RNA, and other nanoparticulate biomarkers directly from blood in their in vivo forms will provide an advantage to basic biomedical research to expedite discoveries and treatments for a variety of diseases.
Materials and Methods
SAMPLE ACQUISITION
We collected blood samples from 15 CLL patients and 3 healthy volunteers (institutional review board no. 080918) in collection tubes containing lithium heparin (Becton Dickinson). For the dielectrophoresis experiments, 300 μL blood was taken from the top of each undisturbed blood sample within 4–5 h of collection. The remaining blood was then centrifuged for 10 min at 1100 RCF, and the supernatant (plasma) was pi-petted into a microcentrifuge tube.
QIAGEN DNA EXTRACTION FROM PLASMA
We used the QIAamp Circulating Nucleic Acid kit to extract ccf-DNA from 1 mL plasma from each of the CLL patients and healthy donors. After addition of a lysing buffer and 30-min incubation, the plasma mixture was pulled through a silica binding column with a vacuum manifold, followed by 3 washing steps on the vacuum manifold. After a 10-min incubation at 56 °C to dry the membrane, the DNA was eluted into the provided elution buffer by centrifugation for 1 min at 20 000 RCF and stored at 4 °C.
DNA EXTRACTION ON AC ELECTROKINETIC MICROARRAY
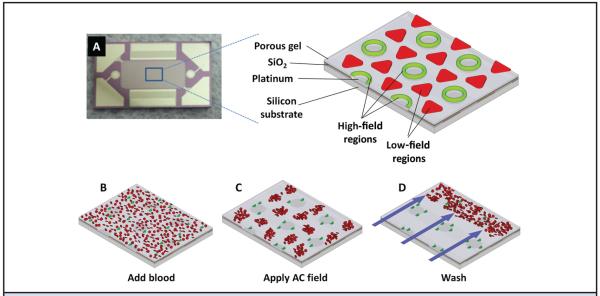
New AC electrokinetic microarray devices (Biological Dynamics) allow the rapid isolation of ccf-DNA directly from a small volume of unprocessed blood. Fig. 1A shows the 10- × 20-mm microarray device containing 1000 microelectrodes, each 60 μm in diameter. The expanded view shows a section of the chip that is fabricated on a silicon base with platinum microelectrodes insulated by SiO2 and coated with a porous hydrogel layer of poly(2-hydroxyethyl methacrylate) (polyHEMA). The expanded view also shows the location of the DEP highfield regions (green) and the DEP low-field regions (red) which form when the AC field is applied. In the first step of the process, a blood sample containing the ccf-DNA is placed into the microarray device and an AC electric field is then applied (Fig. 1B). At a specific AC frequency and voltage, the ccf-DNA, which is more polarizable than the surrounding media, experiences positive DEP (p-DEP), which causes it to concentrate into the DEP high-field regions over the circular microelectrode structures. The blood cells, which are less polarizable, experience negative DEP (n-DEP), which causes them to move into the DEP low-field regions between the microelectrodes (Fig. 1C). Concentration of the ccf-DNA into the DEP high-field regions requires only 3 min, after which a fluid wash removes the blood cells and other blood components from the microarray (Fig. 1D). After the washing step, the ccf-DNA, if fluorescently stained, can be analyzed on-chip by fluorescence microscopy and the sample can be eluted for subsequent PCR and DNA sequencing analysis.
Fig. 1.
AC electrokinetic microarray device and scheme for isolation of ccf-DNA from blood. (A), AC electrokinetic microarray device used to carry out the isolation of ccf-DNA. Expanded view shows the device materials composition: porous gel, platinum microelectrodes, SiO2 layer and silicon base; and the location of the DEP high-field (green) and the DEP low-field (red) regions when an AC field is applied. (B), Microarray with whole blood (red circles) containing fluorescent DNA (green dots). (C), Application of the AC electric field causing the fluorescent DNA (green dots) to be concentrated in the DEP high-field regions on the microelectrodes, while the blood cells (red circles) move into the DEP low-field regions between the microelectrodes. (D), AC field remains on while a fluidic wash removes the blood cells from the microarray with DNA remaining concentrated in the DEP high-field regions.
The microarrays were coated with a porous poly-HEMA hydrogel layer. We spin-coated a 5% poly-HEMA solution in ethanol (PolySciences, Inc.) at 6000 rpm for 30 s by use of a commercial spin-coater (Brewer Science). We then baked the coated chip at 60 °C, in air, for 45 min. We pretreated each microarray device (chip) by adding 25 μL of 0.5× PBS (Lonza) to the flow cell and applying a 2-VRMS, 5-Hz sinusoidal waveform for 15 s to improve the hydrogel porosity. We removed the 0.5× PBS and added 25 μL blood to the flow cell. An 11-V peak-to-peak (Vp-p), 10-kHz sinusoidal waveform was then applied to the chip for 3 min with no fluid flow. The same electric field was maintained while the chip was washed for 5 min at 200 μL/min with 1× TE (Sigma-Aldrich). The electric field was then turned off, allowing captured DNA to diffuse into the 1× TE solution. The 25 μL fluid was removed within 30 s and stored in a microcentrifuge tube. For each CLL patient and healthy donor, this process was repeated 4 times, each time on a new microelectrode device. The 25-μL eluted sample from each of the 4 runs was combined into a single microcentrifuge tube (100 μL total volume) and stored at 4 °C.
To visualize collection on the microelectrode array, we stained the CLL and healthy donor samples with SYBR Green I fluorescent double-stranded DNA dye (Life Technologies). SYBR Green I (1.5 μL, 100×) was added to 28.5 μL blood and allowed to incubate at room temperature for 5 min. This solution (25μL) was added to the device and run as described above. After the 3-min electric field collection and 5-min washing, we acquired bright-field and fluorescent images of the microelectrode pads by use of a charge-coupled device (CCD) camera with a 10× objective, fluorescein isothiocyanate filter, and 470-nm light-emitting diode excitation source. DNA with SYBR Green I from these imaged devices was not eluted or used in subsequent analyses.
DNA QUANTIFICATION
We quanitified the DNA collected by use of both the DEP and Qiagen protocols by use of Quant-iT PicoGreen (Life Technologies), a double-stranded DNA dye. Each sample was diluted and combined with the PicoGreen reagent, and the resulting fluorescence was measured with a fluorescence plate reader (Tecan).
GEL ELECTROPHORESIS
To verify that the collected DNA was from leukemic B cells, we amplified it by PCR using Phusion High-Fidelity DNA Polymerase (New England Biolabs). The forward primers used were specific to the VH1, VH3, and VH4 regions, and the reverse primer was specific for the JH region. PCR thermal cycling conditions were a 5-min initial denaturation at 98 °C followed by 40 cycles of 98 °C denaturation for 15 s, 66 °C annealing for 15 s, and 72 °C extension for 15 s. The PCR product was analyzed by gel electrophoresis on a 2% agarose gel containing ethidium bromide (Life Technologies). The gels were viewed in a transilluminator, and images were captured by use of a CCD camera. The images were analyzed with ImageJ soft-ware to determine the fluorescence in the region where the main 500- to 550-bp CLL target fragments should appear, regardless of whether a discrete band was observed. In ImageJ, the red channel was separated and used, whereas the blue and green channel data were discarded. We removed the background fluorescence of the image by use of the “subtract background” tool. A 40-pixel-wide by 22-pixel-tall region was selected around the 500- to 550-bp region of each gel lane, and an “integrate density” measurement was taken. Remaining PCR product was cleaned with the QIAquick PCR purification kit (Qiagen) and sequenced by Sanger sequencing.
B-CELL ISOLATION AND IGHV ANALYSIS
PBMCs were isolated by density centrifugation with Ficoll-Hypaque 1077 (Sigma-Aldrich) and suspended in fetal calf serum containing 10% dimethyl sulfoxide (Sigma-Aldrich) for storage in liquid nitrogen. DNA was extracted by use of the QIAamp DNA Mini kit (Qiagen) and eluted in 30 μL nuclease-free water. We assessed IGHV gene characterization and mutation status as previously described (38). Most PCR products were sequenced directly, although in some cases, amplified products were cloned into pGEM-T (Promega). Nucleotide sequences were analyzed by use of the ImMunoGeneTics (IMGT) directory (European Bioinformatics Institute ImMunoGeneTics Informations System available at http://imgt.cines.fr) (Leukemia 2011 Langerak, Davi, ERIC guidelines) (22). Sequences with <98% homology with the corresponding germline IGHV gene were considered mutated. We determined the heavy chain complementarity-determining region (23) as defined by the number of amino acids between codon 94 at the end of framework 3 and the conserved Trp of position 102 at the beginning of framework 4.
Results
ccf-DNA ISOLATION FROM CLL SAMPLES
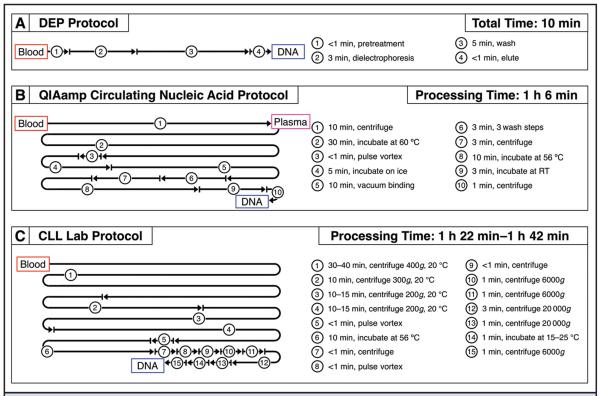
In this study, AC electrokinetic microarrays were used to isolate ccf-DNA from 15 CLL patient blood samples and 3 healthy blood samples. The study shows for the first time that an on-chip dielectrophoresis technique allows: (a) isolation of ccf-DNA directly from unprocessed blood, (b) on-chip fluorescence analysis of ccf-DNA in under 5 min, and (c) elution of ccf-DNA for subsequent analysis in <10 min. The manipulations for the DEP process comprise 2 simple steps: the addition of the blood sample into the microarray device and removal of the eluted sample upon completion of the process. To compare the DEP process to a conventional ccf-DNA sample preparation method, the Qiagen QIAamp Circulating Nucleic Acid procedure was used to isolate ccf-DNA from plasma from the same 15 CLL patients and 3 healthy individuals. The Qiagen procedures are frequently used for isolation of ccf-DNA from cancer patient plasma samples (7, 10, 13). Sanger sequencing results for ccf-DNA isolated with both the DEP and Qiagen procedures were compared to those obtained from isolated leukemic B cells. Fig. 2 shows a comparison of the processing time and number of manipulations required for the DEP procedure (Fig. 2A), the Qiagen QIAamp Circulating Nucleic Acid procedure (Fig. 2B), and the CLL Laboratory procedure (Fig. 2C). The processing times for the Qiagen procedure and the CLL Laboratory procedure include only the actual time necessary to run a specific processing step (i.e., 10 min for centrifugation). They do not include the time necessary for setup, carrying out transfers such as pipetting, and other manipulations. When these processes are performed manually, the additional manipulations can add an hour or more to the total time required for the Qiagen and CLL Laboratory processes.
Fig. 2.
Comparison of processing times and steps for the 3 different DNA sample preparation methods. (A), DEP procedure used to isolate ccf-DNA from 25 μL blood. (B), Qiagen QIAamp Circulating Nucleic Acid procedure used to isolate ccf-DNA from 1 mL plasma. (C), Procedure used to isolate genomic DNA from CLL patient B lymphocytes (starting with 15–20 mL patient blood).
ON-CHIP FLUORESCENCE DETECTION OF ccf-DNA
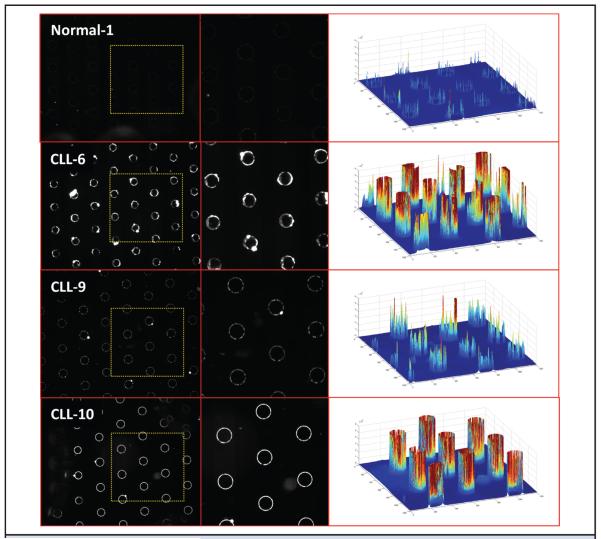
For on-chip fluorescence detection of the ccf-DNA, SYBR Green I (Invitrogen) stain is added to the blood samples before the application of the DEP field. After DEP is carried out for 3 min and blood cells are removed by a fluidic wash, the fluorescently stained ccf-DNA, which is concentrated in the DEP high-field regions (on the microelectrodes), is detected. Fig. 3 shows the fluorescent image results for ccf-DNA isolated by DEP from 1 healthy blood sample (Normal-1) and 3 representative CLL blood samples (CLL-6, CLL-9, CLL-10). On the far right are 3-dimensional fluorescence intensity images, which allow visualization of the relative amounts of ccf-DNA that were isolated. Overall, the fluorescent DNA concentrations were higher in most of the CLL patient samples compared with the fluorescent DNA concentrations obtained from the healthy blood samples.
Fig. 3. Fluorescence detection of ccf-DNA in CLL patient and healthy blood samples.
On-chip fluorescence imaging results from 25-μL blood samples showing SYBR Green–stained ccf-DNA that was concentrated into the DEP high-field regions after the DEP field was applied for 3 min. Images of 1 healthy blood sample (Normal-1) and 3 representative CLL blood samples (CLL-6, CLL-9, CLL-10). Yellow dotted square areas in the images on the left side are enlarged in the center column images. The right side column shows 3-dimensional fluorescence intensity images created by MATLAB, which provide better visualization of the relative amounts of ccf-DNA that were isolated on the DEP high-field areas over the microelectrodes.
DNA CONCENTRATION IN THE ELUTED SAMPLES
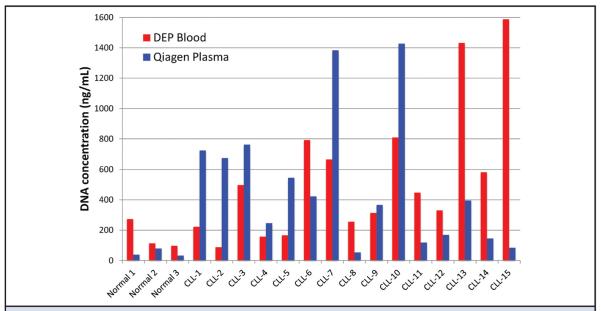
Quant-iT PicoGreen (Invitrogen) fluorescence analysis was used to measure the concentration of ccf-DNA in samples eluted from the AC electrokinetic microarray and the Qiagen QIAamp Circulating Nucleic Acid procedure. For these experiments, SYBR Green I DNA dye was not added to the blood samples before DEP. Fig. 4 shows the ccf-DNA concentration results for the eluted samples starting with 25 μL blood for the DEP process (red bars) and 1 mL plasma for the Qiagen process (blue bars). No correlation could be found between the DEP blood results and Qiagen plasma results for the CLL samples. For the CLL samples (n = 15), the mean (SD) concentration of DNA isolated by the DEP process, 557 (450) ng/mL, was very similar (P = 0.73) to that for the Qiagen process, 502 (436) ng/mL. For the healthy samples (n = 3), the mean concentration of DNA isolated by the DEP process, 162 (97.6) ng/mL, was higher, though not significantly (P = 0.13), than that for the Qiagen process, 50.3 (25.5) ng/mL. The eluted samples for both the DEP process and the Qiagen QIAamp Circulating Nucleic Acid process were amplified by use of primers for the IGHV1, IGHV3, and IGHV4 regions. For the DEP process, eluted ccf-DNA from the equivalent of 5 μL of the original CLL blood sample was amplified. For the Qiagen process, eluted ccf-DNA from the equivalent of 100 μL of the original 1-mL plasma samples was amplified. Table 1 compares the IGHV PCR band intensity results for ccf-DNA isolated from blood by use of the DEP process with the results obtained for ccf-DNA isolated by use of the Qiagen process. In Table 1, the PCR product band intensities for correct IGHV type (IGHV1, IGHV3, IGHV4) are in bold, and secondary IGHV bands, which have intensities >20% of those for the correct IGHV type, are in italic. The correct IGHV PCR amplification products were obtained for ccf-DNA from all 15 CLL patient samples by use of both the DEP process and the Qiagen process. Both the DEP and Qiagen processes also produced secondary IGHV PCR bands; 9 bands for DEP and 9 bands for Qiagen.
Fig. 4. Concentration of ccf-DNA in the DEP and Qiagen eluted samples.
Bar graph of the ccf-DNA concentrations in the final eluted samples that were obtained directly from blood by use of the DEP process (red bars) and of the final eluted samples that were obtained from plasma by use of the Qiagen process (blue bars). The DNA concentrations were determined by fluorescence analysis with Quant-iT PicoGreen (Invitrogen) assay for double-stranded (ds) DNA and normalized to the original sample volume.
Table 1.
IGHV PCR band intensities for DEP blood and Qiagen plasma.a
| DEP |
Qiagen |
|||||
|---|---|---|---|---|---|---|
| Patient | VH1 | VH3 | VH4 | VH1 | VH3 | VH4 |
| CLL-1 | 2110 | 2520 | 74 500 | 7760 | 4000 | 88 700 |
| CLL-2 | 45 200 | 2170 | 2040 | 89 800 | 3220 | 24 900 |
| CLL-3 | 78 900 | 1780 | 2430 | 86 100 | 2210 | 2170 |
| CLL-4 | 62 000 | 37 400 | 17 500 | 5040 | 61 400 | 2410 |
| CLL-5 | 1440 | 10 900 | 71 200 | 4330 | 4970 | 91 000 |
| CLL-6 | 1930 | 60 500 | 32 200 | 2430 | 60 500 | 2290 |
| CLL-7 | 55 100 | 5150 | 5040 | 87 400 | 1800 | 1510 |
| CLL-8 | 54 500 | 1860 | 1420 | 50 600 | 3440 | 19 600 |
| CLL-9 | 1880 | 59 800 | 1310 | 6680 | 59 100 | 11 900 |
| CLL-10 | 2240 | 65 400 | 45 700 | 2890 | 30 300 | 20 700 |
| CLL-11 | 2140 | 46 400 | 1360 | 2990 | 53 400 | 2020 |
| CLL-12 | 18 400 | 55 500 | 43 900 | 19 600 | 64 700 | 1830 |
| CLL-13 | 42 100 | 9240 | 71 100 | 38 400 | 2850 | 79 300 |
| CLL-14 | 1900 | 14 500 | 72 200 | 1840 | 27 500 | 79 500 |
| CLL-15 | 1920 | 23 600 | 70 300 | 30 400 | 26 300 | 75 900 |
Results in bold are IGHV (1, 3, or 4) that correlate with the previously obtained data from CLL Lab. Italicized results do not match the IGHV in the database, but have 20% or more intensity of correct (bold) bands. Both DEP and Qiagen produced 9 secondary IGHV bands.
DNA SEQUENCING RESULTS
Once the IGHV regions for each of the CLL samples were analyzed by use of PCR, the resulting PCR products were sequenced (Sanger sequencing) and compared to those obtained from isolated B cells. This step verified that the isolated ccf-DNA came from the leukemia cell population. The results in Table 2 show that for all 15 CLL samples, the sequences from the ccf-DNA isolated by DEP and by the Qiagen process matched those from isolated B cells.
Table 2.
| DEP blood |
Qiagen plasma |
Genomic DNA |
||||
|---|---|---|---|---|---|---|
| ID | V-gene/allele | V-region ID, % | V-gene/allele | V-region ID, % | V-gene/allele | V-region ID, % |
| CLL-1 | IGHV4-39*01 F | 95.5 | IGHV4-39*01 F | 95.5 | IGHV4-39*01 F | 95.5 |
| CLL-2 | IGHV1-69*01 F | 100.0 | IGHV1-69*01 F | 100.0 | IGHV1-69*01 F | 100.0 |
| CLL-3 | IGHV3-30*03 F | 95.1 | IGHV3-30*03 F | 95.1 | IGHV3-30*03 F | 95.1 |
| CLL-4 | IGHV1-2*02 F | 100.0 | IGHV1-2*02 F | 100.0 | IGHV1-2*02 F | 100.0 |
| CLL-5 | IGHV4-4*07 F | 100.0 | IGHV4-4*07 F | 100.0 | IGHV4-4*07 F | 100.0 |
| CLL-6 | IGHV1-8*01 F | 100.0 | IGHV1-8*01 F | 100.0 | IGHV1-8*01 F | 100.0 |
| CLL-7 | IGHV3-21*02 F | 88.2 | IGHV3-21*02 F | 88.2 | IGHV3-21*02 F | 88.2 |
| CLL-8 | IGHV3-33*01 F | 94.1 | IGHV3-33*01 F | 94.1 | IGHV3-33*01 F | 94.1 |
| CLL-9 | IGHV1-69*01 F | 100.0 | IGHV1-69*01 F | 100.0 | IGHV1-69*01 F | 100.0 |
| CLL-10 | IGHV3-53*04 F | 97.2 | IGHV3-53*04 F | 97.2 | IGHV3-53*04 F | 97.2 |
| CLL-11 | IGHV3-64*01 F | 94.8 | IGHV3-64*01 F | 94.8 | IGHV3-64*01 F | 94.8 |
| CLL-12 | IGHV3-48*03 F | 99.0 | IGHV3-48*03 F | 99.0 | IGHV3-48*03 F | 99.0 |
| CLL-13 | IGHV4-39*01 F | 96.9 | IGHV4-39*01 F | 96.9 | IGHV4-39*01 F | 96.9 |
| CLL-14 | IGHV4-34*01 F | 99.3 | IGHV4-34*01 F | 99.3 | IGHV4-34*01 F | 99.3 |
| CLL-15 | IGHV4-39*01 F | 100.0 | IGHV4-39*01 F | 100.0 | IGHV4-39*01 F | 100.0 |
DNA sequencing results for ccf-DNA isolated from CLL blood samples by DEP and ccf-DNA isolated from CLL patient plasma samples by Qiagen process compared with the results obtained using an established method performed on genomic DNA obtained from CLL patient leukemic cells.
IGHV4-39, IGHV1-69, IGHV3-30, IGHV1-2, IGHV4-4, IGHV1-8, IGHV3-21, IGHV3-33, IGHV1-69, IGHV3-53, IGHV3-64, IGHV3-48, IGHV4-34, immunoglobulin heavy variables 4–39, 1–69, 3–30, 1–2, 4–4, 1–8, 3–21, 3–33, 1–69, 3–53, 3–64, 3–48, and 4–34, respectively.
Discussion
ccf-DNA and ccf-RNA have the potential to become important biomarkers for diagnostics and patient management in almost all hematologic and solid tumor cancers. ccf-DNA/RNA isolated from plasma, constituting a liquid biopsy, may serve as an alternative to more invasive tissue biopsies in the detection and analysis of cancer mutations. Unfortunately, the time, complexity, and cost of employing conventional methods to isolate ccf-DNA/RNA from plasma can limit the use of these procedures, especially for POC diagnostic applications. This study demonstrates the ability of an on-chip dielectrophoresis technique to isolate ccf-DNA directly from unprocessed blood. The DEP process comprises only 2 steps and can be completed in <10 min from 25 μL blood. In contrast, the Qiagen procedure typically involves obtaining 1–2 mL of plasma from 2–3 mL blood and subsequently subjecting the plasma to a series of manipulations to obtain ccf-DNA over the course of 1–2 h. The present process for isolating genomic DNA from B lymphocytes requires 15–20 mL of blood and takes several hours to complete. The DEP process enables the use of unprocessed blood samples and reduces the cost and complexity of ccf-DNA isolation relative to the Qiagen and standard CLL Laboratory protocols, while providing comparable PCR and DNA sequencing results.
An additional advantage of the DEP process is the ability to carry out fluorescence detection of ccf-DNA within minutes of application of the blood sample to the chip. The use of fluorescence to rapidly determine ccf-DNA concentrations in clinical blood samples could ultimately provide a first level “alarm” for POC diagnostics. In the case of solid tumors, researchers have demonstrated a correlation between ccf-DNA concentrations in patient plasma and survivability for lung and colon cancers (39, 40). However, the isolation of ccf-DNA in these studies required long and involved processes. We have previously demonstrated rapid semiquantitative fluorescence detection results for the DEP isolation of DNA spiked into serum that spanned 8 to 500 ng/mL, which would be a useful dynamic range for measuring ccf-DNA in clinical samples (37).
In addition to on-chip fluorescence analysis, the concentrations of ccf-DNA from the DEP process and the Qiagen process were determined by fluorescence after elution. Both the DEP and Qiagen methods showed, on average, higher ccf-DNA concentrations for CLL patients than for healthy patients. In many cases, the ccf-DNA concentrations of CLL samples were substantially higher than those of healthy samples. However, we did not detect a correlation between the CLL sample ccf-DNA concentrations obtained by the DEP and Qiagen methods for each patient. This lack of correlation may be due, at least in part, to the fact that the DEP method isolates DNA directly from blood while the Qiagen process uses plasma. It is very likely that the ccf-DNA isolated directly from blood is more representative of the actual in vivo ccf-DNA size range than is ccf-DNA isolated from the plasma. The numerous processing steps required for the extraction of ccf-DNA from plasma cause shearing and degradation of higher molecular weight ccf-DNA into small fragments.
The primary goal of this study was achieved in demonstrating that the PCR and DNA sequencing results obtained by the DEP process were comparable to those obtained by the Qiagen and CLL Laboratory processes. The IGHV analysis revealed that in all 15 CLL patients, the IGHV sequencing results obtained by use of ccf-DNA isolated by both DEP and the Qiagen process matched exactly the original IGHV patient-specific sequencing results obtained from B-cell DNA. To the best of our knowledge, this work represents the first study of CLL or any other cancer carried out by use of ccf-DNA isolated directly from an unprocessed blood sample.
In summary, the DEP technique shows potential for enabling rapid, simple, and cost-effective liquid biopsy and POC cancer diagnostics. In addition, the DEP technique may become a useful tool for biomedical research. Currently, the true in vivo nature and actual concentrations of ccf-DNA/RNA, exosomes, and other nanoparticulate biomarkers in blood are not well known. The ability to rapidly isolate, in their unperturbed states, the cellular nanoparticulates released into the bloodstream by injured, necrotic, and transformed cells is critical to a better understanding of the disease process itself. Unquestionably, conventional sample preparation procedures, which involve processing plasma from blood and subsequently subjecting plasma to numerous time-consuming/labor-intensive physical manipulations, may lead to loss and degradation of the biomarkers. The use of this DEP technique for rapid isolation of ccf-DNA/RNA directly from blood samples may also provide biomarkers in their unperturbed state, en abling improved biomarker isolation for research and better diagnostic tools.
Supplementary Material
Acknowledgments
The authors thank Dr. Raj Krishan, Dr. David Charlot, and Gene Tu at Biological Dynamics (San Diego, CA) for their help and cooperation, and for allowing early access to the AC electrokinetic instrument and microarrays used in this study.
Research Funding: This study was supported by PO1-CA081534 for the CLL Research Consortium. The DEP technology used in the study was the result of original research carried out by M.J. Heller's lab at the UCSD Moores Cancer Center under NIH NCI NanoTumor Center Grant (U54-CA119335). M. Heller, Biological Dynamics.
Role of Sponsor: The funding organizations played no role in the design of study, choice of enrolled patients, review and interpretation of data, or preparation or approval of manuscript.
Footnotes
Nonstandard abbreviations: ccf-DNA, circulating cell-free DNA; POC, point-of-care; CLL, chronic lymphocytic leukemia; Ig, immunoglobulin; PBMC, peripheral blood mononuclear cell; DEP, dielectrophoresis; RCF, relative centrifugal force; AC, alternating current; polyHEMA, poly(2-hydroxyethyl methacrylate).
Author Contributions: All authors confirmed they have contributed to the intellectual content of this paper and have met the following 3 requirements: (a) significant contributions to the conception and design, acquisition of data, or analysis and interpretation of data; (b) drafting or revising the article for intellectual content; and (c) final approval of the published article.
Authors' Disclosures or Potential Conflicts of Interest: Upon manuscript submission, all authors completed the author disclosure form. Disclosures and/or potential conflicts of interest:
Employment or Leadership: J. McCanna, Biological Dynamics Inc.
Consultant or Advisory Role: M. Heller, Biological Dynamics.
Stock Ownership: J. McCanna, Biological Dynamics Inc.; M. Heller, Biological Dynamics.
Honoraria: None declared.
Expert Testimony: None declared.
Patents: M. Heller, US Appl. No. 12/936,147.
Human genes: For IGHV genes, specific genes are listed both here and in Table 2; IGHV4-39, IGHV1-69, IGHV3-30, IGHV1-2, IGHV4-4, IGHV1-8, IGHV3-21, IGHV3-33, IGHV1-69, IGHV3-53, IGHV3-64, IGHV3-48, IGHV4-34, immunoglobulin heavy variables 4–39, 1–69, 3–30, 1–2, 4–4, 1–8, 3–21, 3–33, 1–69, 3–53, 3–64, 3–48, and 4–34, respectively.
References
- 1.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–37. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 2.Fleischhacker M, Schmidt B. Circulating nucleic acids (CNAs) and cancer—a survey. Biochim Biophys Acta. 2007;1775:181–232. doi: 10.1016/j.bbcan.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Ziegler A, Zangemeister-Wittke U, Stahel RA. Circulating DNA: a new diagnostic gold mine? Cancer Treat Rev. 2002;28:255–71. doi: 10.1016/s0305-7372(02)00077-4. [DOI] [PubMed] [Google Scholar]
- 4.Sozzi G, Conte D, Leon M, Ciricione R, Roz L, Ratcliffe C, et al. Quantification of free circulating DNA as a diagnostic marker in lung cancer. J Clin Oncol. 2003;21:3902–8. doi: 10.1200/JCO.2003.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Sozzi G, Conte D, Mariani L, Lo Vullo S, Roz L, Lombardo C, et al. Analysis of circulating tumor DNA in plasma at diagnosis and during follow-up of lung cancer patients. Cancer Res. 2001;61:4675–8. [PubMed] [Google Scholar]
- 6.Gautschi O, Bigosch C, Huegli B, Jermann M, Marx A, Chasse E, et al. Circulating deoxyribonucleic acid as prognostic marker in non-small-cell lung cancer patients undergoing chemotherapy. J Clin Oncol. 2004;22:4157–64. doi: 10.1200/JCO.2004.11.123. [DOI] [PubMed] [Google Scholar]
- 7.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, et al. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 8.Gormally E, Caboux E, Vineis P, Hainaut P. Circulating free DNA in plasma or serum as biomarker of carcinogenesis: practical aspects and biological significance. Mutat Res. 2007;635:105–17. doi: 10.1016/j.mrrev.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 9.Diehl F, Li M, Dressman D, He Y, Shen D, Szabo S, et al. Detection and quantification of mutations in the plasma of patients with colorectal tumors. Proc Natl Acad Sci U S A. 2005;102:16368–73. doi: 10.1073/pnas.0507904102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90. doi: 10.1038/nm.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swisher EM, Wollan M, Mahtani SM, Willner JB, Garcia R, Goff BA, et al. Tumor-specific p53 sequences in blood and peritoneal fluid of women with epithelial ovarian cancer. Am J Obstet Gynecol. 2005;193:662–7. doi: 10.1016/j.ajog.2005.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Board RE, Wardley AM, Dixon JM, Armstrong AC, Howell S, Renshaw L, et al. Detection of PIK3CA mutations in circulating free DNA in patients with breast cancer. Breast Cancer Res Treat. 2010;120:461–7. doi: 10.1007/s10549-010-0747-9. [DOI] [PubMed] [Google Scholar]
- 13.Leary RJ, Sausen M, Kinde I, Papadopoulos N, Carpten JD, Craig D, et al. Detection of chromosomal alterations in the circulation of cancer patients with whole-genome sequencing. Sci Transl Med. 2012;4:162ra154. doi: 10.1126/scitranslmed.3004742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 15.Ghia EM, Jain S, Widhopf GF, 2nd, Rassenti LZ, Keating MJ, Wierda WG, et al. Use of IGHV3–21 in chronic lymphocytic leukemia is associated with high-risk disease and reflects antigen-driven, post-germinal center leukemogenic selection. Blood. 2008;111:5101–8. doi: 10.1182/blood-2007-12-130229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deligezer U, Erten N, Akisik EE, Dalay N. Circulating fragmented nucleosomal DNA and caspase-3 mRNA in patients with lymphoma and myeloma. Exp Mol Pathol. 2006;80:72–6. doi: 10.1016/j.yexmp.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Fais F, Ghiotto F, Hashimoto S, Sellars B, Valetto A, Allen SL, et al. Chronic lymphocytic leukemia B cells express restricted sets of mutated and unmutated antigen receptors. J Clin Invest. 1998;102:1515–25. doi: 10.1172/JCI3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamblin TJ, Davis Z, Gardiner A, Oscier DG, Stevenson FK. Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood. 1999;94:1848–54. [PubMed] [Google Scholar]
- 19.Damle RN, Wasil T, Fais F, Ghiotto F, Valetto A, Allen SL, et al. Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood. 1999;94:1840–7. [PubMed] [Google Scholar]
- 20.Kipps TJ, Rassenti LZ, Duffy S, Johnson T, Kobayashi R, Carson DA. Immunoglobulin V gene expression in CD5 B-cell malignancies. Ann NY Acad Sci. 1992;651:373–83. doi: 10.1111/j.1749-6632.1992.tb24638.x. [DOI] [PubMed] [Google Scholar]
- 21.Guarini A, Gaidano G, Mauro FR, Capello D, Mancini F, De Propris MS, et al. Chronic lymphocytic leukemia patients with highly stable and indolent disease show distinctive phenotypic and genotypic features. Blood. 2003;102:1035–41. doi: 10.1182/blood-2002-12-3639. [DOI] [PubMed] [Google Scholar]
- 22.Giudicelli V, Chaume D, Jabado-Michaloud J, Le-franc MP. Immunogenetics sequence annotation: the strategy of IMGT based on IMGT-ONTOLOGY. Stud Health Technol Inform. 2005;116:3–8. [PubMed] [Google Scholar]
- 23.Kabat EA, Wu TT. Identical V region amino acid sequences and segments of sequences in antibodies of different specificities. Relative contributions of VH and VL genes, minigenes, and complementarity-determining regions to binding of antibody-combining sites. J Immunol. 1991;147:1709–19. [PubMed] [Google Scholar]
- 24.Becker FF, Wang XB, Huang Y, Pethig R, Vykoukal J, Gascoyne PR. Separation of human breast cancer cells from blood by differential dielectric affinity. Proc Natl Acad Sci U S A. 1995;92:860–4. doi: 10.1073/pnas.92.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asbury CL, van den Engh G. Trapping of DNA in nonuniform oscillating electric fields. Biophys J. 1998;74:1024–30. doi: 10.1016/s0006-3495(98)74027-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng J, Sheldon EL, Wu L, Heller MJ, O'Connell JP. Isolation of cultured cervical carcinoma cells mixed with peripheral blood cells on a bioelectronic chip. Anal Chem. 1998;70:2321–6. doi: 10.1021/ac971274g. [DOI] [PubMed] [Google Scholar]
- 27.Alazzam A, Stiharu I, Bhat R, Meguerditchian AN. Interdigitated comb-like electrodes for continuous separation of malignant cells from blood using dielectrophoresis. Electrophoresis. 2011;32:1327–36. doi: 10.1002/elps.201000625. [DOI] [PubMed] [Google Scholar]
- 28.Morgan H, Hughes MP, Green NG. Separation of submicron bioparticles by dielectrophoresis. Biophys J. 1999;77:516–25. doi: 10.1016/S0006-3495(99)76908-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Asbury CL, Diercks AH, van den Engh G. Trapping of DNA by dielectrophoresis. Electrophoresis. 2002;23:2658–66. doi: 10.1002/1522-2683(200208)23:16<2658::AID-ELPS2658>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 30.Han KH, Frazier AB. Lateral-driven continuous dielectrophoretic microseparators for blood cells suspended in a highly conductive medium. Lab Chip. 2008;8:1079–86. doi: 10.1039/b802321b. [DOI] [PubMed] [Google Scholar]
- 31.Gao J, Sin ML, Liu T, Gau V, Liao JC, Wong PK. Hybrid electrokinetic manipulation in high-conductivity media. Lab Chip. 2011;11:1770–5. doi: 10.1039/c1lc20054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pratt ED, Huang C, Hawkins BG, Gleghorn JP, Kirby BJ. Rare cell capture in microfluidic devices. Chem Eng Sci. 2011;66:1508–22. doi: 10.1016/j.ces.2010.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krishnan R, Sullivan BD, Mifflin RL, Esener SC, Heller MJ. Alternating current electrokinetic separation and detection of DNA nanoparticles in high-conductance solutions. Electrophoresis. 2008;29:1765–74. doi: 10.1002/elps.200800037. [DOI] [PubMed] [Google Scholar]
- 34.Krishnan R, Heller MJ. An AC electrokinetic method for enhanced detection of DNA nanoparticles. J Biophotonics. 2009;2:253–61. doi: 10.1002/jbio.200910007. [DOI] [PubMed] [Google Scholar]
- 35.Krishnan R, Dehlinger DA, Gemmen GJ, Mifflin RL, Esener SC, Heller MJ. Interaction of nanoparticles at the DEP microelectrode interface under high conductance conditions. Electrochem Commun. 2009;11:1661–6. doi: 10.1016/j.elecom.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sonnenberg A, Marciniak JY, Krishnan R, Heller MJ. Dielectrophoretic isolation of DNA and nano-particles from blood. Electrophoresis. 2012;33:2482–90. doi: 10.1002/elps.201100700. [DOI] [PubMed] [Google Scholar]
- 37.Sonnenberg A, Marciniak JY, McCanna J, Krishnan R, Rassenti L, Kipps TJ, et al. Dielectrophoretic isolation and detection of cfc-DNA nanoparticulate biomarkers and virus from blood. Electrophoresis. 2013;34:1076–84. doi: 10.1002/elps.201200444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Widhopf GF, Rassenti LZ, Toy TL, Gribben JG, Wierda WG, Kipps TJ. Chronic lymphocytic leukemia B cells of more than 1% of patients express virtually identical immunoglobulins. Blood. 2004;104:2499–504. doi: 10.1182/blood-2004-03-0818. [DOI] [PubMed] [Google Scholar]
- 39.van der Drift MA, Hol BE, Klaassen CH, Prinsen CF, van Aarssen YA, Donders R, et al. Circulating DNA is a non-invasive prognostic factor for survival in non-small cell lung cancer. Lung Cancer. 2010;68:283–7. doi: 10.1016/j.lungcan.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 40.Guadalajara H, Dominguez-Berzosa C, Garcia-Arranz M, Herreros MD, Pascual I, Sanz-Baro R, et al. The concentration of deoxyribonucleic acid in plasma from 73 patients with colorectal cancer and apparent clinical correlations. Cancer Detect Prev. 2008;32:39–44. doi: 10.1016/j.cdp.2008.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.