Abstract
New therapies for chronic lymphocytic leukemia (CLL) are needed, particularly those that can eradicate residual disease and elicit anti-CLL immune responses. CD40 ligation on CLL cells, which can be achieved using adenovirus encoding chimeric CD154 (Ad-ISF35), enhances their ability to function as antigen-presenting cells and increases their sensitivity to clearance by immune-effector mechanisms. In this study, we report the results of a first-in-man phase I trial of intranodal direct injection (IDI) of Ad-ISF35 in patients with CLL to evaluate toxicity, safety, and tolerability. Fifteen patients received a single IDI of 1 × 1010 to 33 ×1010 Ad-ISF35 viral particles (vp), with a defined maximum tolerated dose as 1 × 1011 vp. Although the most common adverse events were transient grade 1 to 2 pain at the injection site and flu-like symptoms following IDI, some patients receiving the highest dose had transient, asymptomatic grade 3 to 4 hypophosphatemia, neutropenia, or transaminitis. Increased expression of death receptor, immune costimulatory molecules, and Ad-ISF35 vector DNA was detected in circulating CLL cells. Notably, we also observed preliminary clinical responses, including reductions in leukemia cell counts, lymphadenopathy, and splenomegaly. Six patients did not require additional therapy for more than 6 months, and three achieved a partial remission. In conclusion, Ad-ISF35 IDI was safely delivered in patients with CLLs and induced systemic biologic and clinical responses. These results provide the rationale for phase II studies in CLLs, lymphomas, and CD40-expressing solid tumors.
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by the accumulation of monoclonal B cells in the blood, lymphoid tissues, and marrow (1). Although advances in chemoimmunotherapy have resulted in improved response rates and have prolonged survival (2, 3), such treatments can also impair hematopoiesis and immune function and are not well tolerated by all patients, particularly the elderly (4). Furthermore, most treated patients eventually relapse and require additional therapy and the disease still is considered incurable.
It has been reported that the lymph node and bone marrow microenviroments play an important role in protecting CLL cells from apoptosis (5–8). Evidence exists to postulate that proliferating CLL cells in the lymph nodes are the source of the nonproliferating CLL cells present in the peripheral blood (9). However, most therapies used currently in CLLs do not target residual niches or leukemia cells that may depend heavily on the microenvironment. As such, relapse after chemotherapybased treatment is inevitable and this argues in favor of the development of novel treatment alternatives, including those that promote immune stimulation and activation of the tumor microenvironment.
We have addressed this problem by studying in vitro and in vivo mechanism to promote cellular activation and immune recognition in CLL with an approach that involves transduction of CLL cells with vectors encoding the ligand for CD40 (CD154; ref. 10). Although the leukemia cells express high levels of human lymphocyte antigens (HLA) required for presentation of antigen to T cells, CLL cells are poor antigen-presenting cells. These cells lack expression of the immune costimulatory molecules required for effective T-cell activation and instead appear to suppress T-cell function (11).
CD40 activation using recombinant antibodies or CD154 ligands have been used in patients with cancer (12) and CLL (13–15) showing objective clinical responses. Activation of B cells through CD40 changes its phenotype and induces immunoglobulin class switching and enhances its antigen-presenting capacity (16). Similar changes are also observed when CLL cells are activated via ligation of CD40 (17, 18), which can be achieved through transduction of CLL cells with an adenovirus (Ad) vector encoding CD154 (19). Such transduced and CD40-activated CLL cells can induce autologous T-cell activation and immune recognition, leading to generation of anti-leukemia immune responses (20, 21).
We previously conducted clinical trials evaluating the safety and clinical activity of this approach. For these trials, patients underwent leukapheresis and CLL cells were subsequently transduced ex vivo with an Ad vector encoding either mouse-CD154 or a chimeric-humanized CD154, termed Ad-ISF35 (22, 23). Ad-ISF35 was developed to mitigate generation of immunity against mouse-CD154 and to improve membrane stability. Transduction of CLL cells with Ad-CD154 or Ad-ISF35 generated transduced CLL cells that had phenotypic features of CLL cells that had been activated by contact with CD154-bearing cells. Moreover, as these transduced CLL cells expressed a ligand for CD40, they also could activate bystander, nontransduced CLL cells to undergo such phenotypic changes (19). Clinical studies showed that i.v. infusions of autologous CLL cells that had been transduced with Ad-CD154 or Ad-ISF35 did not cause unacceptable or long-term toxicity, induced activation of “bystander” nontransduced CLL cells similar to that achieved by contact with CD154-/ISF35-bearing cells, almost invariably resulted in acute reductions in leukemia cell blood counts, lymphadenopathy, and splenomegaly, and could induce anti-leukemia immune responses (22, 23).
However, not all patients have sufficient numbers of circulating neoplastic cells to accommodate this approach, which also requires ex vivo processing of cells in specialized facilities that are not widely available. Therefore, we considered whether we could achieve similar biologic and clinical responses by injecting the Ad-ISF35 vector directly into pathologically enlarged lymph nodes of patients with CLL. This hypothesis is supported by preclinical studies in test animals showing that direct injection of Ad-ISF35 into tumor nodules is safe and capable of inducing antitumor responses (24, 25). Thus, we conducted a first-in-man phase I dose-escalation study to evaluate toxicity, tolerability, and safety of Ad-ISF35 intranodal direct injection (IDI) in patients with CLL.
Materials and Methods
Patients and study design
Patients provided written informed consent in accordance with the Declaration of Helsinki to participate in this study. We enrolled and treated 15 patients who met the eligibility criteria, including diagnosis of CLL and progressive disease (PD) requiring treatment according to the International Workshop on Chronic Lymphocytic Leukemia (IWCLL; refs. 26, 27). All patients were offered standard chemoimmunotherapy but declined this treatment in favor of participating in this study. Patients presented at least one single accessible and palpable lymph node in the cervical, supraclavicular, axillary, or inguinal regions. The size of the lymph nodes was larger than 2 × 2 cm in the horizontal and perpendicular axes. Additional inclusion criteria were hemoglobin ≥10 g/dL, platelet count ≥50 × 103/mm3 , total bilirubin, alanine aminotransferase (ALT) and aspartate aminotransferase (AST) and creatinine ≤2× ULN (upper limit of normal), Eastern Cooperative Oncology Group (ECOG) performance status ≤2, and anticipated survival of at least 3 months. Patients were excluded from participating in this clinical trial if they had received treatment with chemotherapy or monoclonal antibodies within 28 days before entering the study. Additional treatments for CLL were not allowed during the time of participation in this trial. In addition, patients with history of secondary malignancies within 5 years of registration were excluded from the protocol (except for patients with history of treated non-melanoma skin cancer).
The study used a standard 3 + 3 dose-escalation scheme. Patients were assigned to 1 of 4 dose cohorts to receive one IDI of 1 × 1010, 3.3 × 1010, 1×1011, or 3.3 × 1011 viral particles (vp) of Ad-ISF35 (provided by Memgen, LLC). The injected node was in the axilla and greater than 2 × 2 cm in size before injection. The study design was reviewed and approved by the U.S. Food and Drug Administration (BB-IND 13046), the Recombinant Advisory Committee (RAC) of the NIH (Bethesda, MD), the University of California San Diego (UCSD, La Jolla, CA) Institutional Biosafety Committee (IBC), and the UCSD Institutional Review Board. This clinical trial was registered in the NIH database (registration NCT00783874; ref. 28).
We evaluated the patient's white blood cell counts (WBC), absolute neutrophil counts (ANC), absolute lymphocyte counts (ALC), platelet counts (plt), hemoglobin (Hb), and size of the spleen, liver, and lymph nodes before and at designated times after Ad-ISF35 IDI. The follow-up period included frequent clinical and laboratory evaluations on days 0,1, 2, 7,14, 21, 30, 60, and 84 after injection and every 3 months thereafter for a total of 1 year. Assessment of response to treatment was evaluated following the IWCLL guidelines (27).
Dose-limiting toxicity (DLT) was defined as any adverse event grade 3 or higher that was considered by the investigator to be possibly related or related to Ad-ISF35 treatment. DLTs were evaluated only during the first 21 days of treatment and their assessment determined dose escalation and defined the maximum tolerated dose (MTD). Toxicity was graded according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 3.0, modified for hematologic toxicities according to the IWCLL guidelines (27). Hematologic adverse events were considered DLT if they were grade 4 or higher and lasted 7 days or more. We defined the MTD as the highest dose where no more than one patient of 6 presented DLTs.
Flow cytometry
Ten million of isolated WBCs/mL in PBS (+1% FBS) were incubated for 30 minutes at 4°C with fluorochrome-conjugated mAbs specific for CD5, CD19, CD54, CD80, CD86, CD20, DR5, CD3, CD4, CD8, mCD154 (used for detection of ISF35 protein expression), CD95, CD23 and κ- or λ-immunoglobulin light chain (Becton Dickinson). Fluorescence data were acquired using a FACSCalibur flow cytometer (Becton Dickinson) and analyzed with Flow-Jo software (Tree Star Inc.).
Measurement of cytokines in patient sera
We examined serum samples for interleukin (IL)-2, IL-3, IL-4, IL-5, IL-6, IL-8, IL-10, IL-12(p70), IL-13, IL-15, monocyte chemoattractant protein-1 (MCP-1), TNF-α, granulocyte monocyte colony-stimulating factor (GM-CSF), and IFN-γ via Luminex xMAP technology following manufacturer's instructions (Millipore). Fluorescence intensity was measured in a Luminex 100TM IS machine and BeadView Multiplex software v1.0 was used for data analysis by Spline curve-fitting method.
Detection of antibodies against adenovirus or human CD154
We measured serum antibodies against Ad-ISF35 or recombinant human CD154 (rhCD154) protein by ELISA. Plates were coated with 4 × 109 vp/mL of Ad-ISF35 or 2 µg/mL of rhCD154 (R&D Systems) for 2 hours at 37°C. Plates were blocked with PBS/Tween 0.01%, bovine serum albumin (BSA) 1%. Sera samples collected on days 0 and 28 were added at different dilutions and incubated for 1 hour at 37°C. Anti-human IgG horseradish peroxidase (HRP)-conjugated antibody (Jackson Laboratories) was added at 1:5,000 dilution for 1 hour at room temperature. After 3 washes with PBS-Tween 0.01%, 3,3′,5,5′-tetramethylbenzidine (TMB) substrate was added, and reaction was stopped by adding 1N phosphoric acid. Optical densities were obtained in a SpectraMax250 plate reader (Molecular Devices). EC50 of each the serum was calculated with GraphPad Prism 4.0.
Neutralization assay
Serial dilutions of serum samples were preincubated with 4 × 108 vp/mL of Ad-ISF35 for 10 minutes at 37°C. Then, the serum samples were added to 2 × 104 HeLa cells in 96-well plates for 24 hours at 37°C. HeLa cells were incubated with PBS-EDTA enzyme-free dissociation buffer (Gibco) for 10 minutes at 37°C and then incubated with 2 µL of antimouse CD154 and phycoerythrin-labeled (BD Pharmigen) for 30 minutes at 4°C. Fluorescence data were acquired using a FACSCalibur flow cytometer (Becton Dickinson) and analysis was carried out using FlowJo software (Tree Star Inc.). Antibody neutralization activity was assessed by decrease in ISF35 (protein encoded by Ad-ISF35) expression in HeLa cells cultured with Ad-ISF35 preincubated with serum dilutions compared with the control cells incubated with Ad-IS35 without serum. Neutralizing activity was defined as at least 2-fold increase compared with pretreatment sample.
Assay for Ad-ISF35 DNA in blood leukocytes
We used a quantitative PCR (qPCR) assay that could detect 5 DNA copies or more of Ad-ISF35 in 100 ng of genomic DNA All the samples were tested in duplicate with a cycle threshold (Ct) variability that was less than 5% for Ad-ISF35 amplification. The following primers were used for Ad-ISF35 amplification (400 nmol/L): forward, 5′CCT CTG GCT GAA GCC CAG 3′; reverse, 5′CTC CCA AGT GAA TGG ATT GT 3′. MGB-FAM probe 5′TTACTCAAGGCGGCAAA3′ was used at 250 nmol/L. The PCR reaction was carried out using TaqMan qPCR Universal Master Mix w/UNG from Applied Biosystems. The qPCR program used was 95°C for 3 minutes; 40 cycles: 95°C for 20 seconds, 52°C for 1 minute; and 20 seconds in Bio-Rad cycler iQ5 PCR machine. β-Actin was used to monitor DNA quality. To calculate the Ad-ISF35 DNA copies present in given sample of DNA or cDNA, we used a standard curve with viral DNA ranging from 5 × 107 to 5 × 1010 viral copies.
ELISPOT assay
We used the IFN-γ ELISPOT plates from R&D Systems, Inc. T cells from pre- and posttreatment (showing the highest number of CD8+ cells) from frozen peripheral blood mononuclear cells (PBMC) were isolated with Dynabeads against CD4 and CD8 (Invitrogen). CLL cells (5 × 106) from pretreatment samples (depleted of CD4 and CD8) were cultured for 24 hours with HeLa cells expressing CD154 (4 × 105) in 12-well plates. CLL cells were removed from cocultures and added into empty wells for 2 hours to allow floating HeLa-CD154 cells to reattach to the plate. CLL cells were washed and incubated with mitomycin C (60µg/mL) for 1 hour at 37°C. Activation of CLL cells was confirmed by flow cytometry measuring the upregulation of CD54 and CD95 markers. A total of 1 × 105 CLL nonactivated (CD4- and CD8-depleted) or CLL CD154-activated cells were cultured for 48 hours with 1×105 T cells plus L-2 (25 IU/mL).
Statistical analysis
Descriptive statistics were used to analyze demographic and baseline characteristics, clinical response variables, immunologic variables, and toxicity data. Univariate analysis was conducted using one-way ANOVA with pre- and posttreatment values. HRs were calculated by using Kaplan–Meier analysis. All analyses were conducted using GraphPad Prism v4.0 and JMP v8.0.
Results
Patient demographics
Five females and 10 males with a median age of 56 years (45–73 years) were enrolled in this study. The median leukemia cell doubling time for all patients was 3.3 months (0.9–22.5 months). The median number of prior treatments was 2 (0–7). Two patients were refractory to fludarabine-based treatment. CLL cells expressed unmutated Ig heavy chain variable region genes (IgVH) in 7 patients and ZAP-70 (29) in 9 patients. Six patients had leukemia cells with adverse cytogenetics, 4 with deletions at 11q, and 2 with deletions at 17p (Table 1).
Table 1.
Patient demographics
| Cohort (dose) | Pt# | Age/sex | IgvH (% Horn) |
%ZAP70+ | %CD38+ | Prior treatments | Cytogenetics | FISH | ECOG | Rai stage |
β2M g/dL |
Responsea | TNTx |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (1 × 1010 vp) | 1 | 49/M | 92.2 | 29.8 | 0 | Idiotype vaccine conjugated with KLH |
46, XY | del13q | 0 | II | 1.6 | PD | 6.8 |
| 2 | 55/M | 96.8 | 36.7 | 0 | None | 46, XY | del13q | 0 | II | 4 | PR | 15.5 | |
| 3 | 67/M | 100 | 28.1 | 0 | AT-101, HDMP + R, A | 46, XY | del17p, del13q | 0 | II | 1.3 | SD | 4.0 | |
| 2 (3.3 × 1010 vp) | 4 | 73/M | 100 | 10.3 | 0 | GT, X-cyte therapy, HDMP + R, AT-101 + R |
NA | del11q, del13q | 0 | IV | 2.5 | SD | 3.8 |
| 5 | 52/M | 94 | 5.2 | 44 | HDMP + R | 48, XY | tri12, del13q | 0 | II | 2.1 | PR | 12.5 | |
| 6b | 53/M | NA | NA | NA | FM, FCR, R, A, 0 | 46, XY | Normal | 0 | II | 2.5 | PD | 4.7 | |
| 3 (1 × 1011 vp) | 7 | 53/F | 98.6 | 4.8 | NA | None | 46, XX | del11q | 1 | II | 2.9 | PR | 7.0 |
| 8b | 48/M | 100 | 33.7 | NA | CP, C, F, FCR, HDMP + R, AT-101 + R |
46, XY | del11q, del13q | 0 | II | 3.7 | PD | 1.1 | |
| 9 | 61/M | 99.3 | 98.7 | 98.7 | FR, R | 47, XY | Tri12 | 0 | II | 2.9 | SD | 2.7 | |
| 4 (3.3 × 1011 vp) | 10 | 55/F | 95.7 | 81.5 | 0 | FR, A, Anti-CD40 antibody, AT-101 + R |
46, XX | Normal | 1 | IV | 1.2 | PD | 3.0 |
| 11 | 54/F | 100 | 8.1 | 82 | None | NA | Normal | 0 | II | 2.2 | SD | 9.8 | |
| 12 | 45/M | NA | 32.8 | 0 | FCR, R | 47, XY | tri12, del17p | 0 | IV | 3.4 | PD | 1.4 | |
| 3E(1 × 1010 vp) | 13 | 57/M | 100 | 92.5 | 19.3 | HDMP+R | 46, XY | del11q | 0 | IV | 2.9 | SD | 5.0 |
| 14 | 54/F | NA | NA | NA | None | 46, XX | del13q | 0 | III | 2.6 | SD | 5.0 | |
| 15 | 63/F | 96.1 | 83.9 | 8.7 | HDMP+R, A, Hsp90 | 46, XX | de!13q | 0 | II | 2.8 | SD | 7.7 |
Abbreviations: % Horn, percentage of gene homology; A, alemtuzumab; AT-101, BCL-2 family member inhibitor; C, chlorambucil; CP, chlorambucil and prednisone; F, fludarabine: FCR, fludarabine, cyclophosphamide, and rituximab; FM, fludarabine and mitoxantrone; FR, fludarabine and rituximab; GT, gene therapy (CD154); HDMP, high-dose methylprednisolone; NA, not available; O, ofatumumab; R, rituximab; X-cyte, T-cell therapy.
Response assessment was conducted on the basis of IWCLL 2008 guidelines criteria.
Patient fludarabine refractory based on IWCLL 2008 definition.
Adverse events
All patients received Ad-ISF35 IDI. Only 2 of 15 patients did not complete the postinjection observation period of 84 days due to disease progression. The most common adverse events were grade 1 or 2 injection site reactions and systemic flu-like symptoms. Four patients had transient grade 3 or 4 neutropenia. One of these patients in cohort 2 presented grade 3 neutropenia that lasted for 6 days. This was not considered as DLT as the adverse event was not grade ≥ 4 and the duration was less than 7 days. In cohort 3, the first 3 patients did not present any DLTs. In cohort 4, 3 patients were enrolled, 2 of them presented DLTs including hypophosphatemia (grade 3 and 4) and AST elevation (grade 3). Because of this, we proceeded to enroll 3 additional patients in cohort 3 (3E). One patient presented with neutropenia grade 4 (not considered DLT as it lasted only 5 days) and one patient with hypophosphatemia grade 3 (considered DLT; Table 2). All adverse effects were subclinical and self-limiting. With these data, we defined cohort 3 dose (1 × 1011 vp) as the MTD. In addition, we have not observed long-term adverse effects associated with Ad-ISF35 IDI after a median follow-up of 2.5 years.
Table 2.
Adverse events
| Event | Grade 1/2 |
Grade 3/4 |
||||||
|---|---|---|---|---|---|---|---|---|
| Cohort 1 (n = 3) |
Cohort 2 (n = 3) |
Cohort 3/3E (n = 6) |
Cohort 4 (n = 3) |
Cohort 1 (n = 3) |
Cohort 2 (n = 3) |
Cohort 3/3E (n = 6) |
Cohort 4 (n = 3) |
|
| Neutropeniaa | 1 (33%) | 2 (67%) | 2 (33%) | 1 (33%)c | 1 (17%)d | 2 (67%)e | ||
| Hypophosphatemia | 1 (33%) | 1 (17%) | 1 (17%)f | 2 (67%)f | ||||
| AST elevation | 1 (33%) | 0 | 1 (17%) | 1 (33%) | 1 (33%)f | |||
| Fatigue | 3 (100%) | 3 (100%) | 6 (100%) | 3 (100%) | ||||
| Injection site reaction | 3 (100%) | 3 (100%) | 6 (100%) | 3 (100%) | ||||
| Flu-like symptoms | 1 (33%) | 3 (100%) | 6 (100%) | 3 (100%) | ||||
| Hyperglycemiab | 1 (33%) | 3 (100%) | 5 (83%) | 3 (100%) | ||||
| Thrombocytopeniaa | 1 (33%) | 3 (100%) | 5 (83%) | 2 (67%) | ||||
| Hyperuricemia | 2 (67%) | 2 (67%) | 4 (67%) | 1 (33%) | ||||
| Sweating | 2 (67%) | 4 (67%) | 2 (67%) | |||||
| Hypocalcemia | 1 (33%) | 2 (67%) | 3 (50%) | 1 (33%) | ||||
| Nausea | 1 (33%) | 1 (17%) | 2 (67%) | |||||
| ALT elevation | 2 (33%) | 2 (67%) | ||||||
| Constipation | 2 (33%) | 1 (33%) | ||||||
| Insomnia | 1 (17%) | 2 (67%) | ||||||
| Anorexia | 2 (33%) | |||||||
| Arthritis (nonseptic) | 1 (33%) | 1 (17%) | ||||||
| Diarrhea | 1 (33%) | 1 (33%) | ||||||
| Hypotension | 1 (33%) | 1 (33%) | ||||||
| Total events/patient | 5.3 | 8.6 | 8.6 | 9.3 | 0 | 0.3 | 0.3 | 1.7 |
| Total events | 16 | 26 | 52 | 28 | 0 | 1 | 2 | 5 |
Based on IWCLL 2008 criteria.
Patient with history of insulin-dependent diabetes mellitus (ID-DM) was not included.
Patient presented neutropenia grade 3 (not considered DLT).
Patient presented neutropenia grade 4 that lasted for 5 days (not considered DLT).
Patients presented neutropenia grade 3 (not considered DLTs).
Adverse events considered DLTs.
Evaluation for Ad-ISF35 DNA presence and ISF35 protein expression in blood leukocytes following IDI of Ad-ISF35
We monitored the presence Ad-ISF35 DNA and ISF35 (protein encoded by Ad-ISF35 vector) expression in PBMCs collected before and after Ad-ISF35 IDI. For detection of Ad-ISF35 DNA, we used a sensitive qPCR assay capable of detecting 5 copies of Ad-ISF35 DNA in 100 ng of genomic DNA (gDNA). Ad-ISF35 was detected in only 3 patients 8 hours after Ad-ISF35 IDI. Each of these patients had PBMCs with less than 9 copies of Ad-ISF35 per 100 ng of gDNA. Ad-ISF35 DNA was not detected at later time points (Fig. 1A). ISF35 protein surface expression was measured by flow cytometry. The results did not show detectable expression of ISF35 protein on the PBMCs of any patient at any time point (data not shown).
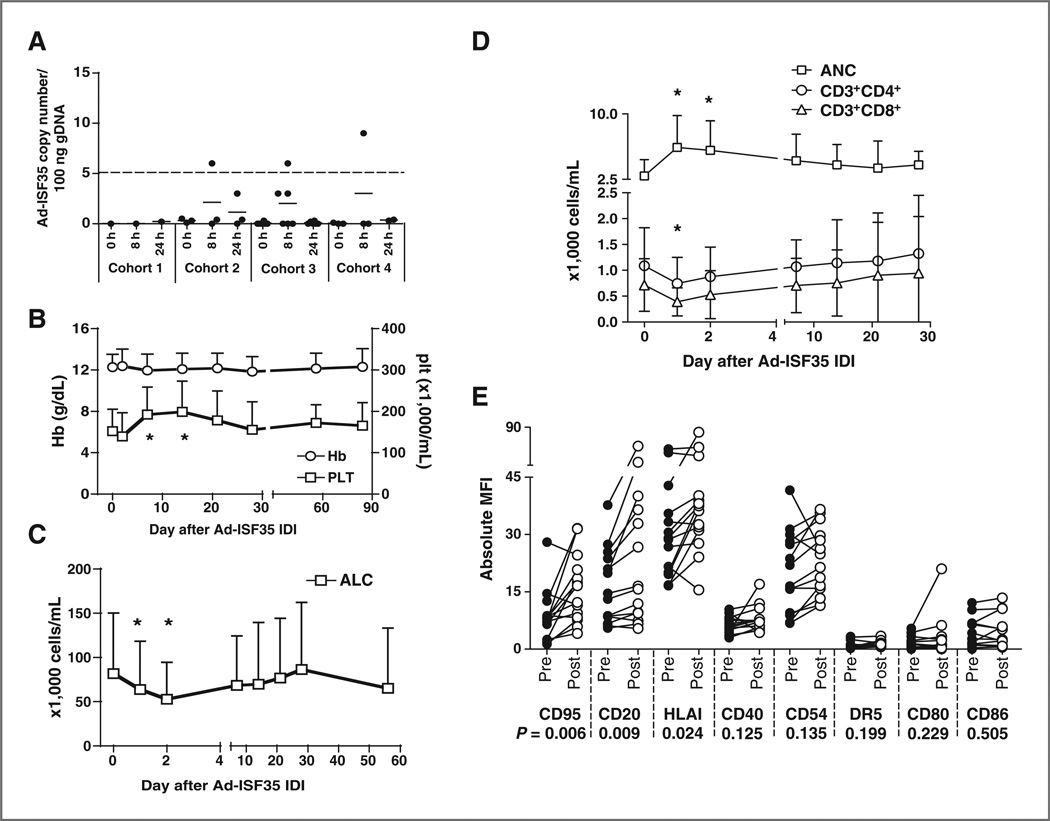
Figure 1.
Laboratory parameters in patients who received Ad-ISF35 IDI. A, DNA isolated from blood leukocytes of patients that received Ad-ISF35 IDI was evaluated for the presence of virus DNA by qPCR. The dotted line indicates the limit of detection of this assay (5 copies of Ad-ISF35 in 100 ng of genomic DNA). B, hemoglobin (Hb) levels and platelet counts (plt). C, absolute lymphocyte counts (ALC). D, T-cell counts (CD3+CD4+ and CD3+CD8+ subsets) and ANC of all patients over time following IDI of Ad-ISF35 are represented in the graphs. E, expression of cell surface markers in CLL cells obtained pre- and post- (1–2 days) Ad-ISF35 IDI. Error bars represent the SD. *, statistical significant difference by t test when compared with baseline level. MFI, mean fluorescence intensity.
Biologic responses to IDI of Ad-ISF35 and evidence of systemic bystander effect
Most patients (n = 14) experienced transient increases in platelet counts at day 7 and 14. None of the patients developed worsening anemia after injection (Fig. 1B). Also, we observed changes in T cells in the majority of patients enrolled in this study. During the first 48 hours, most patients (n = 11) had a transient fall in ALC (Fig. 1C) and absolute T-cell counts (Fig. 1D). The acute drop in T-cell counts was significant when compared with baseline levels in both cell subsets (CD3+CD8+ and CD3+CD4+ cells; P < 0.05). However, the majority of patients (n = 10) later showed an increase of T cells ranging from 6% to 311% during the first 4 weeks after Ad-ISF35 IDI. We observed that the majority of patients experienced an increase in ANC during the first 2 days following Ad-ISF35 IDI (Fig. 1D).
We observed systemic biologic responses to Ad-ISF35 IDI in most patients. In particular, we observed statistically significant upregulation of CD95, CD20, and HLAI during the first 48 hours after Ad-ISF35 IDI (Fig. 1E). Such phenotypic changes are similar to those of CLL cells cocultured with CD154-bearing cells in vitro (20).
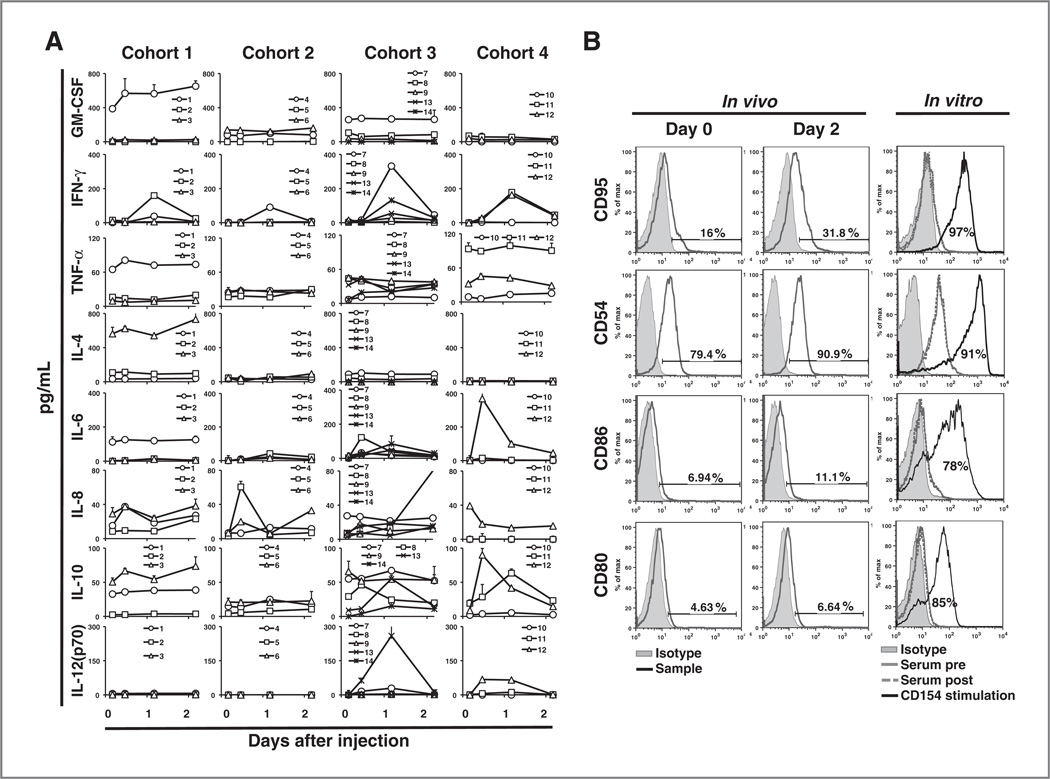
We also noted that levels of IFN-g and IL-6 in the sera of 11 of 14 patients increased 1 day after Ad-ISF35 IDI relative to pretreatment levels. The relative increase in serum IFN-g appeared proportionate to the administered dose of Ad-ISF35 (linear trend analysis, P = 0.0328, r2 = 0.35); patients in cohorts 3 and 4 had the highest posttreatment serum levels of IFN-γ (Fig. 2A).
Figure 2.
Cytokine levels in serum and evidence of bystander effect in leukemic cells from peripheral blood after Ad-ISF35 IDI. A, serum cytokine levels measured over time were assessed using the Luminex assay on each cohort of patients who received Ad-ISF35 IDI. B, CLL cell surface expression of CD95, CD54, CD86, and CD80 was assessed by flow cytometry in vivo and in vitro. Histograms on the left show in vivo data obtained from blood samples collected before (day 0) and 2 days after Ad-ISF35 IDI. Histograms on the right represent the in vitro data obtained after CLL cells were cultured with autologous serum collected before (pre) and 1 day (highest levels of IFN-γ and IL-6) after Ad-ISF35 IDI. As a control, CLL cells were cocultured with HeLa cells expressing CD154 (CD154 stimulation).
We evaluated whether cytokines or other factors present in the serum of patients who received Ad-ISF35 IDI were responsible for the phenotypic changes observed in circulating leukemic cells. CLL cells were cultured with autologous serum samples collected before and 1 day after Ad-ISF35 IDI. As a control, the same CLL cells were stimulated by coculture with CD154-bearing cells (HeLa-CD154). The expression levels of surface immune costimulatory molecules and death receptors were evaluated via flow cytometry. CLL cells cultured with sera collected before or after Ad-ISF35 IDI did not induce increased expression of these surface antigens. In contrast, CLL cells cocultured with HeLa-CD154 expressed higher levels of CD95 or immune costimulatory molecules than CLL cells cocultured with wild-type HeLa (Fig. 2B).
Samples collected before and after Ad-ISF35 IDI from 4 patients were evaluated by ELISPOT assay to detect IFN-γ production from T cells in response to coculture with CD40-stimulated autologous CLL cells. In these patients, we did not observe significant increase in the levels of T-cell/IFN-γ production after Ad-ISF35 IDI (Supplementary Fig. S1).
Antibody responses against Ad-ISF35 and human CD154
We evaluated for antibodies specific for adenovirus or human CD154 (hCD154). Sera collected from patients before Ad-ISF35 IDI had median titer of anti-adenovirus antibodies of 352 [confidence interval (CI), 173–531]. However, sera collected 1 month after therapy had significantly higher titers (median, 622; CI, 151–1,093; P < 0.05). Except for patient #10, the antiadenovirus antibodies induced by Ad-ISF35 IDI did not neutralize the capacity of adenovirus to infect HeLa cells in vitro (Table 3). None of the patients developed detectable antibodies to human CD154.
Table 3.
Anti-adenovirus and neutralizing antibodies titer before (pre) and after (post) Ad-ISF35 injection
| Anti-adenovirus antibodies (EC50 dil−1) |
Anti-adenovirus neutralizing antibodies (EC50 dil−1) |
||||
|---|---|---|---|---|---|
| Cohort | Patient ID | Pre | Posta | Pre | Posta |
| 1 (1 × 1010 vp) | 1 | 244 ± 51 | 664 ± 87 | <50 | 60 |
| 2 | 635 ± 157 | 458 ± 89 | ND | ND | |
| 3 | 352 ± 60 | 317 ± 52 | ND | ND | |
| 2 (3.3 × 1010 vp) | 4 | 1,060 ± 94 | 1,619 ± 138 | 289 | 281 |
| 5 | 32 ± 16 | 30 ± 23 | ND | ND | |
| 6 | 944 ±145 | 622 ±104 | ND | ND | |
| 3 (1 × 1011 vp) | 7 | 891 ± 95 | 1,282 ±152 | <50 | <50 |
| 8 | 113 ± 29 | 186 ± 43 | ND | ND | |
| 9 | 470 ±114 | 475 ± 158 | ND | ND | |
| 4 (3.3 × 1011 vp) | 10 | 384 ± 80 | 1,888 ± 273 | 50 | 170 |
| 11 | 179 ± 46 | 3,571 ± 460 | <50 | 60 | |
| 12 | 178 ± 34 | 243 ± 51 | ND | ND | |
| 3E (1 × 1010 vp) | 13 | 21 ± 88 | 21 ± 88 | ND | ND |
| 14 | 173 ± 34 | 776 ±123 | <50 | <50 | |
| 15 | 837 ±160 | 752 ±123 | ND | ND | |
NOTE: Neutralizing antibodies assay was only conducted in samples showing increased titer after Ad-ISF35 IDI.
Abbreviation: ND, not determined.
Posttreatment evaluation conducted 1 month after Ad-ISF35 injection.
Clinical response to IDI of Ad-ISF35
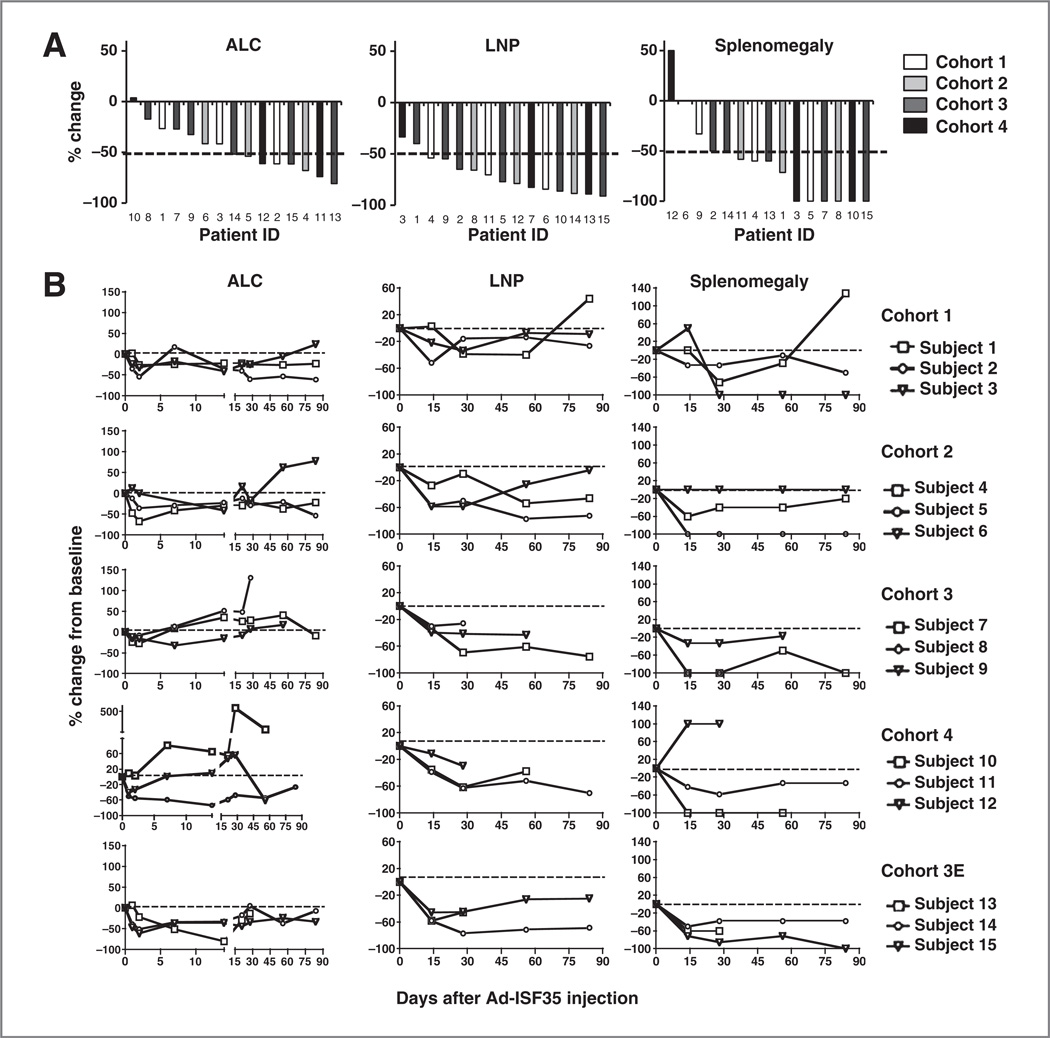
Although Ad-ISF35 IDI was injected into a single axillary lymph node, we observed systemic clinical responses. The magnitude of these responses did not appear to have a clear dose–response relationship. Considering the best response during 84 days after Ad-ISF35 IDI, 87%, 80%, and 53% of patients had respective reductions in lymphadenopathy, splenomegaly, or ALC by more than 50% (Fig. 3A). The response observed in spleen and lymph nodes was durable during the course of the study (Fig. 3B). Three patients achieved partial response (PR), 7 patients had stable disease (SD), and 5 had PD by IWCLL criteria (refs. 26, 27; Table 1). Six patients (patients 1, 2, 5, 7, 11, and 15) had durable responses (median, 8.8 months) and did not require additional treatment for over 6 months. Two of these patients (patients 2 and 5) did not require additional treatment for over a year after Ad-ISF35 IDI. The median time to next treatment (TNTx) for all patients was 5 months.
Figure 3.
Clinical parameters of patients who received Ad-ISF35 IDI. Patients with CLL were enrolled in 4 cohorts using a 3 + 3 dose-escalation design with doses of Ad-ISF35 ranging from 1 × 1010 to 33 × 1010. A, ALC, bidimensional lymph node product (LNP) of measurable and palpable lymph nodes, and palpable spleen size (below the left costal margin) were recorded from day 0 (Ad-ISF35 IDI) to day 90. Data were normalized to day 0 and the best response change is defined as the maximum reduction/minimum increase from baseline. The dotted line indicates 50% reduction. B, ALC, LNP, and splenomegaly are shown overtime. Cohort 3E corresponds to the extension of cohort 3 after toxicities in cohort 4 were observed. The dotted line indicates 0% change from baseline.
We conducted Kaplan–Meier analysis to determine hazard ratios (HR) comparing the duration of response (≥6 months), with variables such as number of prior therapies (≤1), ZAP-70 (≤20% positive cells), B2M levels (≤2.5 mg/L), IgG levels (≤500 mg/dL), splenomegaly (≤5 cm), and bidimensional lymph node product (total ≤70 cm2). This analysis showed that only the number of prior therapies (≤1) had a statistically significant correlation with the duration of response (HR, 0.1491; CI, 0.04071–0.5464; P = 0.0041; Table 4). Therefore, patients who had no more than one prior treatment for CLL were 6.7 times more likely to have a durable response (TNTx ≥ 6 months).
Table 4.
Factors influencing duration of response (TNTx ≥6 months) after Ad-ISF35 IDI
| HRa (95% CI) | P | |
|---|---|---|
| No. of previous treatment (≤1) | 0.1491 (0.04071–0.5464) | 0.0041 |
| ZAP-70 (≤20% positive cells) | 0.6217 (0.1900–1.941) | 0.4001 |
| B2M (≤2.5 mg/L) | 1.029 (0.3536–3.011) | 0.9543 |
| IgG levels (≤500 mg/dL) | 1.799 (0.6586–5.830) | 0.2266 |
| Splenomegaly (≤5 cm) | 1.974 (0.7330–6.671) | 0.1589 |
| LNP (total ≤70 cm2) | 2.104 (0.6982–9.480) | 0.1556 |
NOTE: Splenomegaly measured in cm below the right costal margin.
Abbreviations: B2M, (β2 microglobulin; LNP, bidimensional lymph node product.
HRs calculated with Kaplan–Meier analysis.
Discussion
We examined the safety of gene immunotherapy using Ad-ISF35 IDI in patients with CLL. The injection of Ad-ISF35 into an axillary lymph node was safely delivered and without long-term adverse effects. Most of adverse effects were anticipated and of low-grade (grade 1 or 2). These included flu-like symptoms and erythema or pain at the site of injection. Such adverse effects have been observed in patients injected with adenovirus vectors directly into tumor nodules (30–32) and most likely represent an inflammatory response to adenovirus infection/transduction. Many of the adverse events noted in this study also were similar to those observed in patients infused with autologous CLL cells transduced ex vivo with Ad-mCD154 or Ad-ISF35 (22, 23). In these ex vivo studies, patients who received the highest dose of transduced cells generally had a higher incidence of grade 2 versus grade 1 adverse events. We observed a similar dose relationship in patients receiving the 2 higher doses of Ad-ISF35, suggesting that there might be a relationship between the injected dose of Ad-ISF35 and frequency or intensity of adverse events.
We observed grade 3/4 neutropenia, hypophosphatemia, and AST elevation in 6 patients treated in cohorts 2, 3E, and 4. In 3 of these patients (n = 2 in cohort 4 and n = 1 in cohort 3E), hypophosphatemia and AST elevation were considered DLTs and thus defined the MTD as 1 × 1011 vp (cohort 3 dose). All the adverse events observed were subclinical and transient. The grade 3 to 4 hypophosphatemia observed could be related to increased serum levels of IFN-γ and IL-6 during the first 24 hours following Ad-ISF35 IDI. Hypophosphatemia has been associated with high serum levels of such inflammatory cytokines in patients with sepsis and in mice injected with IL-6, TNF-α, or IL-1β (33).
Some patients in the cohorts that received the higher doses also experienced transient elevations in serum hepatic transaminases. Although such transient and asymptomatic elevation of transaminases has been observed in patients who received i.v. injections of adenovirus vectors (22), the patient who had grade 3 elevation in AST elevation did not have detectable Ad-ISF35 in the blood. As such, it is conceivable that the transient elevations in hepatic transaminases instead could be associated with high serum levels of inflammatory cytokines (34). These cytokines could have been secreted in response to adenovirus infection as well as in response to CD40-CD154 stimulation (35).
Several patients had decreased ALC and elevated ANC during the first 48 hours after Ad-ISF35 IDI, only to develop neutropenia 2 to 3 weeks later. Early increases in blood neutrophils associated with decreases in blood lymphocytes have been observed in mice infused with syngeneic dendritic cells stimulated by CD154 and IL-2 and pulsed with lymphoma antigens (36). Changes in ANC could be a direct response to modulation of the CD40-CD154 pathway as it has been observed in our mouse model (25, 26) and in patients with CD154 deficiency and hyper-IgM syndrome (37–39). On the other hand, neutropenia could be secondary to elevated levels of IFN-γ and/or IL-6 (40, 41). Nevertheless, each patient recovered with no evidence of infection or other related complications.
We observed fluctuation in T-cell counts after Ad-ISF35 IDI. Initially, during the first 48 hours after injection, the T cells decreased significantly and this could be in response to viral infection, cytokines, cell migration, or activation-induced cell death (42). At later time points, the majority of patients showed increase in T cells up to 300%, which was similar to what we have observed in previous studies using Ad-CD154 (22). We studied whether T cells isolated after therapy were reactive against autologous CLL cells using ELISPOT assay for IFN-γ. However, in 4 patients available for evaluation, we did not observe reactivity of T cells against CD40-stimulated CLL cells. This finding is different than our previous experience in patients receiving ex vivo transduced autologous CLL cells with Ad-CD154 (22). It is unclear the reasons for the lack of evidence of T-cell stimulation in response to AD-ISF35 IDI measured by IFN-γ ELISPOT. Potential explanations could be the different route of administration (ex vivo vs. IDI), sample size, and time of analysis of the samples. Other immunemediated processes including microenvironment cell activation of macrophages (43) could be responsible for the objective responses observed in some patients in this study. It is conceivable to hypothesize that multiple intranodal injections could enhance immunologic responses including T-cell reactivity against CLL antigens. We will address these mechanistic questions in future studies.
We did not find evidence for Ad-ISF35 DNA in the PBMCs except in 3 patients, who had trace levels of detectable Ad-ISF35 DNA (close to the low-limit of sensitivity) 8 hours after injection. In addition, we did not detect expression of ISF35 protein on circulating CLL cells at any time following Ad-ISF35 IDI. Nevertheless, all patients experienced a systemic biologic response. We observed phenotypic changes in the circulating CLL cells that included expression of immune costimulatory molecules and death receptors. These changes were similar to those observed in vitro after leukemia cells are cultured with CD154-expressing cells (19).
It is unlikely that the clinical and biologic activity observed after Ad-ISF35 IDI are mediated by direct transduction of cells distant from the injected lymph node. Our data in an A20 lymphoma mouse model have shown that after intratumoral injections, Ad-ISF35 is primarily found in tumor tissues with no evidence of accumulation or persistence in peripheral organs with a rapid virus clearance 24 hours after injection (25, 26). In the same mouse model, we have detected the transduction not only in lymphoma cells but also in other important cells from the microenvironment such as fibroblasts, macrophages, and epithelial cells (manuscript in preparation). This suggests the possibility that phenotypic changes of microenvironment of cells may play a role in response to intratumoral Ad-ISF35 injection.
Similarly, our data suggest that serum cytokines or other soluble factors are not entirely responsible for the phenotypic changes observed after Ad-ISF35 IDI (Fig. 2B). Therefore, the presence of systemic objective clinical response observed in conjunction with phenotypic changes and the lack of detectable Ad-ISF35 in circulating leukemia cells suggest that these effects are due to the stimulation generated by either contact with ISF35-bearing cells or other nontransduced activated cells, providing the so-called bystander-effect. Lymph node biopsies before and after Ad-ISF35 injection could have provided additional insight into this process. However, they were not conducted in this study to avoid additional local toxicities and adverse events that could interfere with the primary endpoint of this phase I study, which was to evaluate safety and tolerability. We are planning to conduct these biopsies in subsequent clinical trials.
One concern was whether Ad-ISF35 IDI would induce autoantibodies reactive with hCD154. Such autoantibodies conceivably could induce adverse effects that are similar to those noted for some patients treated with antibodies specific for CD154 (44, 45). However, none of the patients developed antibodies reactive with hCD154. Nevertheless, 40% of treated patients were induced to increase their titers of antibodies of anti-adenovirus, indicating that Ad-ISF35 IDI could elicit an anti-adenovirus antibody response even in patients who have immune deficiency associated with CLL. Moreover, patients with CLL typically have hypogammaglobulinemia (median serum IgG levels in this study was 496 mg/dL) and typically respond poorly to administered vaccines (46). One of the treated patient also developed antibodies to adenovirus that could neutralize Ad-ISF35. It is uncertain whether the development of such antibodies can mitigate the activity of subsequent injections of Ad-ISF35. This will be evaluated in subsequent clinical studies examining the safety and activity of repeated administrations of Ad-ISF35 IDI.
Although the primary purpose of the current study was to examine the safety of Ad-ISF35 IDI, we observed objective clinical responses in the majority of the patients treated. Patients experienced a 50% or more reduction in leukemia cell counts (7 of 15), lymphadenopathy (13 of 15), or splenomegaly (12 of 15). Six patients did not require additional treatment for 6 months or more and 3 patients achieved a PR. Moreover, responses achieved in the spleen and lymph nodes were durable and the majority of patients requiring additional treatment did so because of persistent lymphocytosis. This suggests that Ad-ISF35 can promote changes in the microenvironment of lymph nodes and spleen that could translate into a clinical benefit. Further studies to corroborate these observations are needed.
Clinical responses in CLL can be compartmentalized. Patients can have excellent reduction of lymphocytosis but no response in large lymph nodes like in the case of patients treated with alemtuzumab (47), or good reduction in lymphadenopathy and spleen, but lack of response or lymphocytosis progression like in patients receiving tyrosine kinase inhibitors (48,49). In the patients reported in this phase I study, we observed better and more durable responses in reduction of lymphadenopathy and splenomegaly. Despite the fact that most patients had some degree of response (Fig. 3), only 3 of them fulfill all the criteria needed to achieve a clinical response based on the international guidelines.
Previously untreated patients or those with less than one previous treatment showed the best clinical responses to Ad-ISF35 IDI (almost 7 times more likely to have durable responses). This supports the notion that early immunologic intervention in patients with CLL could result in better clinical outcomes. Although whether prior treatment with drugs or agents that suppress immune function mitigates the activity of Ad-ISF35 IDI needs to be evaluated in subsequent clinical trials.
In summary, this first-in-man phase I clinical study shows that gene immunotherapy using Ad-ISF35 IDI is feasible and safe. We defined the dose of 1 × 1011 vp per injection as the MTD. Even though the study was designed to determine safety and tolerability of a single Ad-ISF35 IDI, we also observed systemic biologic and clinical effects. This is despite the fact that we could not detect expression of ISF35 protein in mononuclear cells isolated from the blood of patients following Ad-ISF35 IDI. This suggests that such responses were due to a bystander-effect in which leukemia cells are induced to undergo phenotypic changes when in contact with ISF35-bearing cells or other nontransduced activated cells. Our results also indicate that Ad-ISF-35 IDI–induced antibody, cytokine responses, and changes in the phenotype of circulating CLL cells that are consistent with immune activation similar to that induced by i.v. administration of autologous Ad-ISF35–transduced CLL cells. This suggests a greater interchange of cells between the blood, spleen, and lymphoid compartments of patients with CLL.
To our knowledge, this is the first clinical study conducted in patients with CLL showing that direct intratumoral/intranodal modulation by an immune-based strategy can result in clinical activity. This argues that the tumor microenvironment is a relevant target in this disease.
Additional studies of the safety and potential activity of multiple Ad-ISF35 intranodal injections for patients with CLL, related B-cell malignancies, and solid tumors expressing CD40 are warranted.
Supplementary Material
Acknowledgments
The authors thank Jose Sandoval-Sus and Theresa Bishop for data preparation, Laura Rassenti and CRC Tissue core staff for technical support, and Karen Messer for helpingwith the statistical analysis of our data. Memgen LLC provided Ad-ISF35.
Grant Support
This study was supported by Alliance for Cancer Gene Therapy (J.E. Castro, J. Melo-Cardenas, M. Urquiza, J.S. Barajas-Gamboa, R.S. Pakbaz, and T.J. Kipps), P01-CA81534 grant—CLL Research Consortium (J.E. Castro and T.J. Kipps), and FDA-OOPD-R01-3427 grant (J.E. Castro and T.J. Kipps).
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Disclosure of Potential Conflicts of Interest
T.J. Kipps receives stock options as payment for providing consulting services to Memgen, LLC and was not involved in the selection or treatment of patients in this clinical trial. He contributed in analyzing data and writing/editing of the manuscript. The University of California owns the patent for Ad-ISF35 and licenses it to Memgen, LLC. No potential conflicts of interests were disclosed by the other authors.
References
- 1.Kipps TJ. Chronic lymphocytic leukemia and related diseases. In: Kaushansky K, Lichtman MA, Beutler B, Kipps TJ, Seligsohn U, Prchal JT, editors. Williams hematology. 8th ed. New York: McGraw-Hill; 2010. pp. 1431–1482. [Google Scholar]
- 2.Tam CS, O'Brien S, Wierda W, Kantarjian H, Wen S, Do KA, et al. Long-term results of the fludarabine, cyclophosphamide, and rituximab regimen as initial therapy of chronic lymphocytic leukemia. Blood. 2008;112:975–980. doi: 10.1182/blood-2008-02-140582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallek M, Fischer K, Fingerle-Rowson G, Fink AM, Busch R, Mayer J, et al. Addition of rituximab to fludarabine and cyclophosphamide in patients with chronic lymphocytic leukaemia: a randomised, openlabel, phase 3 trial. Lancet. 2010;376:1164–1174. doi: 10.1016/S0140-6736(10)61381-5. [DOI] [PubMed] [Google Scholar]
- 4.Ysebaert L, Gross E, Kühlein E, Blanc A, Corre J, Fournie JJ, et al. Immune recovery after fludarabine-cyclophosphamide-rituximab treatment in B-chronic lymphocytic leukemia: implication for maintenance immunotherapy. Leukemia. 2010;24:1310–1316. doi: 10.1038/leu.2010.89. [DOI] [PubMed] [Google Scholar]
- 5.Kurtova AV, Balakrishnan K, Chen R, Ding W, Schnabl S, Quiroga MP, et al. Diverse marrow stromal cells protect CLL cells from spontaneous and drug-induced apoptosis: development of a reliable and reproducble system to assess stromal cell adhesion-mediated drug resistance. Blood. 2009;114:4441–4450. doi: 10.1182/blood-2009-07-233718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsuda H, Nishimura H, Sawada T, Takatsuki K. The roles of lymph node stromal cells in proliferation of lymphoid leukaemia cells. Br J Cancer. 1990;61:362–364. doi: 10.1038/bjc.1990.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karras JG, Tsukada N, et al. Protection of CLL B cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood. 2002;100:1795–1801. [PubMed] [Google Scholar]
- 8.Meads MB, Hazlehurst LA, Dalton WS. The bone marrow microenvironment as a tumor sanctuary and contributor to drug resistance. Clin Cancer Res. 2008;14:2519–2526. doi: 10.1158/1078-0432.CCR-07-2223. [DOI] [PubMed] [Google Scholar]
- 9.Herishanu Y, Perez-Galan P, Liu D, Biancotto A, Pittaluga S, Vire B, et al. The lymph node microenvironment promotes B-cell receptor signaling, NF-kappaB activation, and tumor proliferation in chronic lymphocytic leukemia. Blood. 2011;117:563–574. doi: 10.1182/blood-2010-05-284984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kipps TJ, Chu P, Wierda WG. Immunogenetic therapy for B-cell malignancies. Semin Oncol. 2000;27:104–109. [PubMed] [Google Scholar]
- 11.Ramsay AG, Johnson AJ, Lee AM, Gorgün G, Le Dieu R, Blum W, et al. Chronic lymphocytic leukemia T cells show impaired immunological synapse formation that can be reversed with an immunomodulating drug. J Clin Invest. 2008;118:2427–2437. doi: 10.1172/JCI35017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fonsatti E, Maio M, Altomonte M, Hersey P. Biology and clinical applications of CD40 in cancer treatment. Semin Oncol. 2010;37:517–523. doi: 10.1053/j.seminoncol.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Luqman M, Klabunde S, Lin K, Georgakis GV, Cherukuri A, Holash J, et al. The antileukemia activity of a human anti-CD40 antagonist antibody, HCD122, on human chronic lymphocytic leukemia cells. Blood. 2008;112:711–720. doi: 10.1182/blood-2007-04-084756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]
- 15.Vonderheide RH, Dutcher JP, Anderson JE, Eckhardt SG, Stephans KF, Razvillas B, et al. Phase I study of recombinant human CD40 ligand in cancer patients. J Clin Oncol. 2001;19:3280–3287. doi: 10.1200/JCO.2001.19.13.3280. [DOI] [PubMed] [Google Scholar]
- 16.Cerutti A, Puga I, Cols M. Innate control of B cell responses. Trends Immunol. 2011;32:202–211. doi: 10.1016/j.it.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ranheim EA, Kipps TJ. Activated T cells induce expression of B7/BB1 on normal or leukemic B cells through a CD40-dependent signal. J Exp Med. 1993;177:925–935. doi: 10.1084/jem.177.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buhmann R, Nolte A, Westhaus D, Emmerich B, Hallek M. CD40-activated B-cell chronic lymphocytic leukemia cells for tumor immunotherapy: stimulation of allogeneic versus autologous T cells generates different types of effector cells. Blood. 1999;93:1992–2002. [PubMed] [Google Scholar]
- 19.Kato K, Cantwell MJ, Sharma S, Kipps TJ. Gene transfer of CD40-ligand induces autologous immune recognition of chronic lymphocytic leukemia B cells. J Clin Invest. 1998;101:1133–1141. doi: 10.1172/JCI1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dicker F, Kater AP, Fukuda T, Kipps TJ. Fas-ligand (CD178) and TRAIL synergistically induce apoptosis of CD40-activated chronic lymphocytic leukemia B cells. Blood. 2005;105:3193–3198. doi: 10.1182/blood-2003-10-3684. [DOI] [PubMed] [Google Scholar]
- 21.Fukuda T, Chen L, Endo T, Tang L, Lu D, Castro JE, et al. Antisera induced by infusions of autologous Ad-CD154-leukemia B cells identify ROR1 as an oncofetal antigen and receptor for Wnt5a. Proc Natl Acad Sci U S A. 2008;105:3047–3052. doi: 10.1073/pnas.0712148105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wierda WG, Cantwell MJ, Woods SJ, Rassenti LZ, Prussak CE, Kipps TJ. CD40-ligand (CD154) gene therapy for chronic lymphocytic leukemia. Blood. 2000;96:2917–2924. [PubMed] [Google Scholar]
- 23.Wierda WG, Castro JE, Aguillon R, Sampath D, Jalayer A, McMannis J, et al. A phase I study of immune gene therapy for patients with CLL using a membrane-stable, humanized CD154. Leukemia. 2010;24:1893–1900. doi: 10.1038/leu.2010.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Melo-Cardenas J, Urquiza M, Aguillon-Prada R, Kipps TJ, Castro JE. Ad-ISF35 intratumoral administration induces a bystander effect and immune-mediated tumor rejection with a safe vector biodistribution and toxicology profile in a NHL mouse model. Blood. 2010;116:628–629. [Google Scholar]
- 25.Melo-Cardenas J, Urquiza M, Kipps T, Castro J. Intratumoral delivery of CD154 homolog (Ad-ISF35) induces tumor regression: analysis of vector biodistribution, persistence and gene expression. Cancer Gene Ther. 2012;19:336–344. doi: 10.1038/cgt.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Grever M, Kay N, Keating MJ, O'Brien S, et al. National Cancer Institute-sponsored Working Group guidelines for chronic lymphocytic leukemia: revised guidelines for diagnosis and treatment. Blood. 1996;87:4990–4997. [PubMed] [Google Scholar]
- 27.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Dohner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Institutes of Health-clinicaltrials.gov. [Database on the Internet]. [cited 2012]. Available from: http://clinicaltrials.gov/ct2/show/NCT00850057?term=NCT00783874&rank=1.
- 29.Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. N Engl J Med. 2004;351:893–901. doi: 10.1056/NEJMoa040857. [DOI] [PubMed] [Google Scholar]
- 30.Khorana AA, Rosenblatt JD, Sahasrabudhe DM, Evans T, Ladrigan M, Marquis D, et al. A phase I trial of immunotherapy with intratumoral adenovirus-interferon-gamma (TG1041) in patients with malignant melanoma. Cancer Gene Ther. 2003;10:251–259. doi: 10.1038/sj.cgt.7700568. [DOI] [PubMed] [Google Scholar]
- 31.Li JL, Liu HL, Zhang XR, Xu JP, Hu WK, Liang M, et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 2009;16:376–382. doi: 10.1038/gt.2008.179. [DOI] [PubMed] [Google Scholar]
- 32.Mazzolini G, Alfaro C, Sangro B, Feijoo E, Ruiz J, Benito A, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23:999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 33.Barak V, Schwartz A, Kalickman I, Nisman B, Gurman G, Shoenfeld Y. Prevalence of hypophosphatemia in sepsis and infection: the role of cytokines. Am J Med. 1998;104:40–47. doi: 10.1016/s0002-9343(97)00275-1. [DOI] [PubMed] [Google Scholar]
- 34.Gomez CR, Nomellini V, Baila H, Oshima K, Kovacs EJ. Comparison of the effects of aging and IL-6 on the hepatic inflammatory response in two models of systemic injury: scald injury versus i.p. LPS administration. Shock. 2009;31:178–184. doi: 10.1097/SHK.0b013e318180feb8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wierda WG, Kipps TJ. Gene therapy of hematologic malignancies. Semin Oncol. 2000;27:502–511. [PubMed] [Google Scholar]
- 36.Roskrow MA, Dilloo D, Suzuki N, Zhong W, Rooney CM, Brenner MK. Autoimmune disease induced by dendritic cell immunization against leukemia. Leuk Res. 1999;23:549–557. doi: 10.1016/s0145-2126(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 37.Rezaei N, Aghamohammadi A, Ramyar A, Pan-Hammarstrom Q, Hammarstrom L. Severe congenital neutropenia or hyper-IgM syndrome? A novel mutation of CD40 ligand in a patient with severe neutropenia. Int Arch Allergy Immunol. 2008;147:255–259. doi: 10.1159/000142050. [DOI] [PubMed] [Google Scholar]
- 38.Atarod L, Aghamohammadi A, Moin M, Kanegane H, Rezaei N, Rezaei Kalantari K, et al. Successful management of neutropenia in a patient with CD40 ligand deficiency by immunoglobulin replacement therapy. Iran J Allergy Asthma Immunol. 2007;6:37–40. [PubMed] [Google Scholar]
- 39.Andrews FJ, Katz F, Jones A, Smith S, Finn A. CD40 ligand deficiency presenting as unresponsive neutropenia. Arch Dis Child. 1996;74:458–459. doi: 10.1136/adc.74.5.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prat C, Sancho JM, Dominguez J, Xicoy B, Gimenez M, Ferra C, et al. Evaluation of procalcitonin, neopterin, C-reactive protein, IL-6 and IL-8 as a diagnostic marker of infection in patients with febrile neutropenia. Leuk Lymphoma. 2008;49:1752–1761. doi: 10.1080/10428190802258956. [DOI] [PubMed] [Google Scholar]
- 41.Soker M, Colpan L, Ece A, Devecioglu C, Haspolat K. Serum levels of IL-1 beta, sIL-2R, IL-6, IL-8, and TNF-alpha in febrile children with cancer and neutropenia. Med Oncol. 2001;18:51–57. doi: 10.1385/MO:18:1:51. [DOI] [PubMed] [Google Scholar]
- 42.Scholz C, Starck L, Willimsky G, Blankenstein T, Dorken B, Daniel PT. Adenoviral transduction of tumor cells induces apoptosis in co-cultured T lymphocytes. Gene Therapy. 2002;9:1438–1446. doi: 10.1038/sj.gt.3301796. [DOI] [PubMed] [Google Scholar]
- 43.Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang W, Sinha J, Newman J, Reddy B, Budhai L, Furie R, et al. The effect of anti-CD40 ligand antibody on B cells in human systemic lupus erythematosus. Arthritis Rheum. 2002;46:1554–1562. doi: 10.1002/art.10273. [DOI] [PubMed] [Google Scholar]
- 45.Boumpas DT, Furie R, Manzi S, Illei GG, Wallace DJ, Balow JE, et al. A short course of BG9588 (anti-CD40 ligand antibody) improves serologic activity and decreases hematuria in patients with proliferative lupus glomerulonephritis. Arthritis Rheum. 2003;48:719–727. doi: 10.1002/art.10856. [DOI] [PubMed] [Google Scholar]
- 46.Jurlander J, de Nully Brown P, Skov PS, Henrichsen J, Heron I, Obel N, et al. Improved vaccination response during ranitidine treatment, and increased plasma histamine concentrations, in patients with B cell chronic lymphocytic leukemia. Leukemia. 1995;9:1902–1909. [PubMed] [Google Scholar]
- 47.Moreton P, Kennedy B, Lucas G, Leach M, Rassam SM, Haynes A, et al. Eradication of minimal residual disease in B-cell chronic lymphocytic leukemia after alemtuzumab therapy is associated with prolonged survival. J Clin Oncol. 2005;23:2971–2979. doi: 10.1200/JCO.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 48.Furman RR, Byrd JC, Brown JR, Coutre SE, Benson DM, Wagner-Johnston ND, et al. CAL-101, an isoform-selective inhibitor of phosphatidylinositol 3-kinase P110 delta, demonstrates clinical activity and pharmacodynamic effects in patients with relapsed or refractory chronic lymphocytic leukemia. Blood. 2010;116:31a. [Google Scholar]
- 49.Burger JA, O’Brien S, Fowler N, Advani R, Sharman JP, Furman RR, et al. The Bruton's tyrosine kinase inhibitor, PCI-32765, is well tolerated and demonstrates promising clinical activity in chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): an update on ongoing phase 1 studies. Blood. 2010;116:32. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.