Abstract
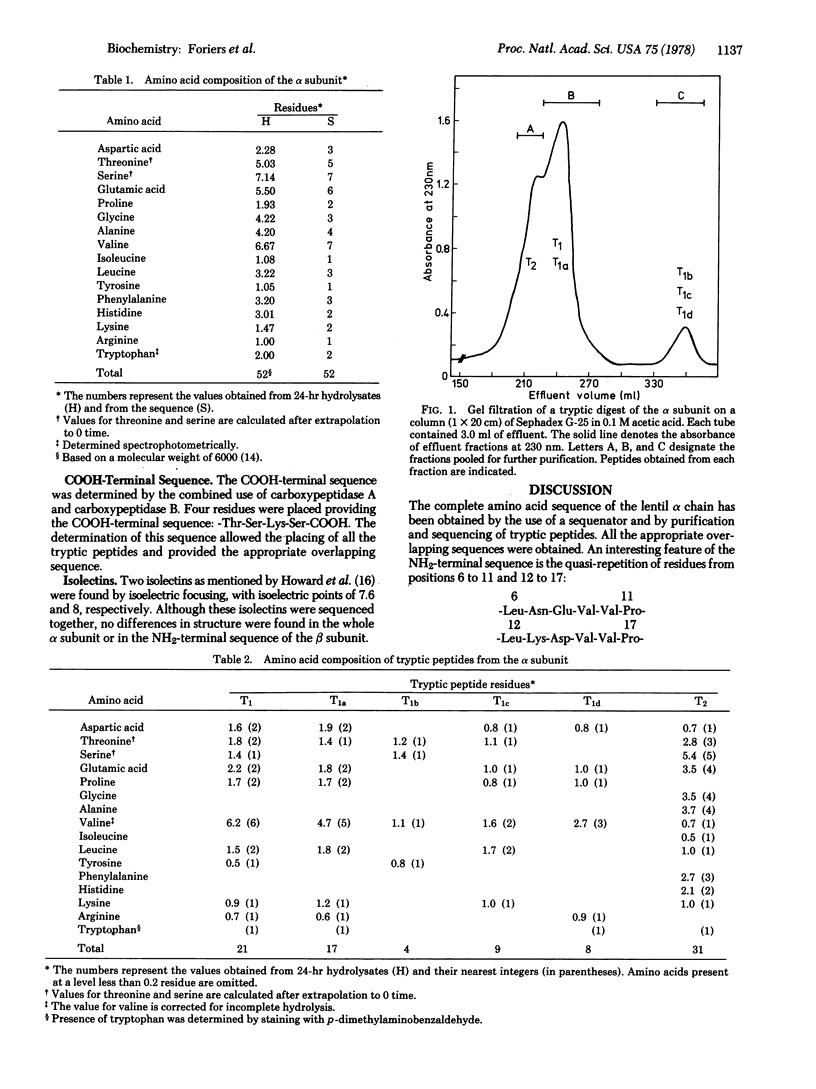
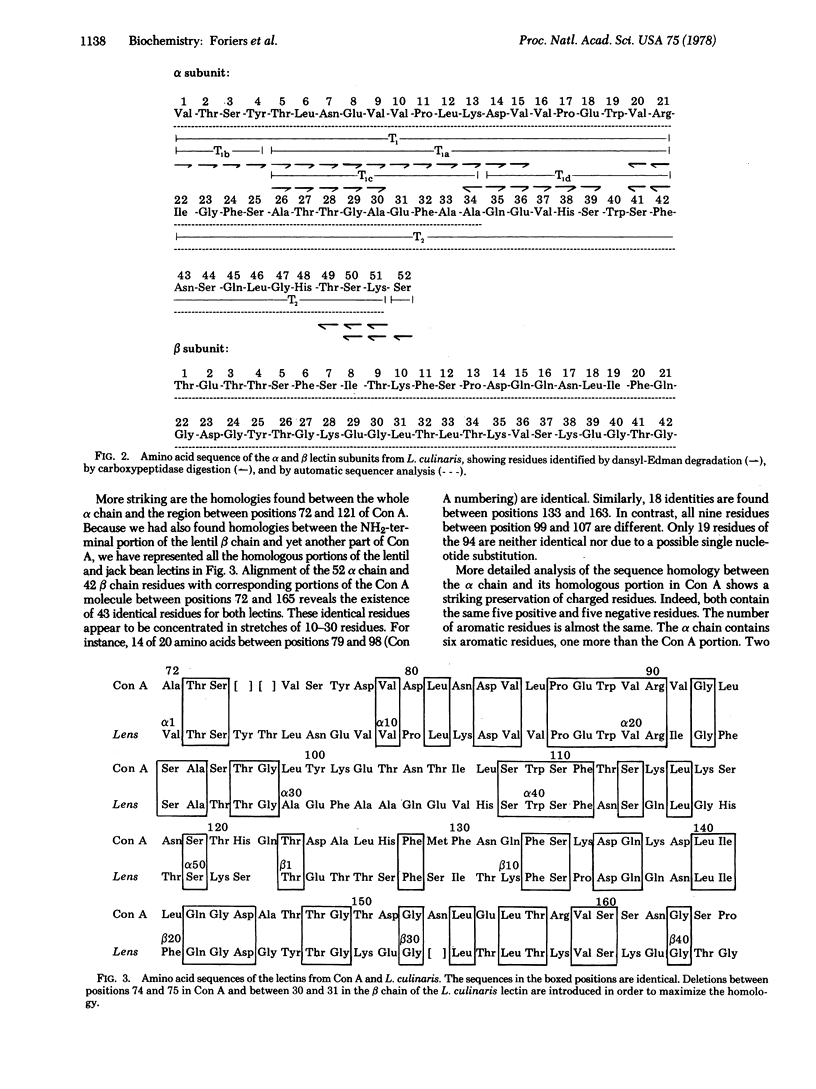
The primary structure of the alpha subunit from Lens culinaris lectin was determined by analysis of tryptic peptides and was shown to consist of 52 amino acid residues. The molecular weight calculated on the basis of the sequence is 5928. The whole chain is homologous with the region between positions 75 and 121 from concanavalin A. The NH2-terminal sequence of the beta chain, determined by automated Edman degradation, is homologous to another portion of the concanavalin A molecule, between positions 123 and 165. Comparison of the 94 residues from the lentil lectin alpha and beta chains with concanavalin A reveals the existence of 43 identities. Thirty-four other homologies could have arisen, each by a single nucleotide substitution. This extensive homology suggests that the lentil lectin alpha and beta chains may be proteolytic fragments from a single polypeptide chain of the same length as concanavalin A.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brauer A. W., Margolies M. N., Haber E. The application of 0.1 M quadrol to the microsequence of proteins and the sequence of tryptic peptides. Biochemistry. 1975 Jul;14(13):3029–3035. doi: 10.1021/bi00684a036. [DOI] [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Prediction of protein conformation. Biochemistry. 1974 Jan 15;13(2):222–245. doi: 10.1021/bi00699a002. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Wang J. L., Waxdal M. J., Edelman G. M. The covalent and three-dimensional structure of concanavalin A. II. Amino acid sequence of cyanogen bromide fragment F3. J Biol Chem. 1975 Feb 25;250(4):1503–1512. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Foriers A., Van Driesschie E., De Neve R., Kanarek L., Strosberg A. D. The subunit structure and N-terminal sequences of the alpha- and beta-subunits of the lentil lectin (Lens culinaris). FEBS Lett. 1977 Mar 15;75(1):237–240. doi: 10.1016/0014-5793(77)80094-x. [DOI] [PubMed] [Google Scholar]
- Foriers A., Wuilmart C., Sharon N., Strosberg A. D. Extensive sequence homologies among lectins from leguminous plants. Biochem Biophys Res Commun. 1977 Apr 25;75(4):980–986. doi: 10.1016/0006-291x(77)91478-4. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Crumpton M. J. Isolation of glycoproteins from pig lymphocyte plasma membrane using Lens culinaris phytohemagglutinin. Biochem Biophys Res Commun. 1972 May 26;47(4):923–930. doi: 10.1016/0006-291x(72)90581-5. [DOI] [PubMed] [Google Scholar]
- Howard I. K., Sage H. J., Stein M. D., Young N. M., Leon M. A., Dyckes D. F. Studies on a phytohemagglutinin from the lentil. II. Multiple forms of Lens culinaris hemagglutinin. J Biol Chem. 1971 Mar 25;246(6):1590–1595. [PubMed] [Google Scholar]
- Lotan R., Lis H., Sharon N. Aggregation and fragmentation of soybean agglutinin. Biochem Biophys Res Commun. 1975 Jan 6;62(1):144–150. doi: 10.1016/s0006-291x(75)80416-5. [DOI] [PubMed] [Google Scholar]
- Neurath H., Walsh K. A. Role of proteolytic enzymes in biological regulation (a review). Proc Natl Acad Sci U S A. 1976 Nov;73(11):3825–3832. doi: 10.1073/pnas.73.11.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- Pisano J. J., Bronzert T. J. Analysis of amino acid phenylthiohydantoins by gas chromatography. J Biol Chem. 1969 Oct 25;244(20):5597–5607. [PubMed] [Google Scholar]
- Radola B. J. Isoelectric focusing in layers of granulated gels. II. Preparative isoelectric focusing. Biochim Biophys Acta. 1975 Mar 28;386(1):181–195. doi: 10.1016/0005-2795(75)90258-5. [DOI] [PubMed] [Google Scholar]
- Sharon N., Lis H. Lectins: cell-agglutinating and sugar-specific proteins. Science. 1972 Sep 15;177(4053):949–959. doi: 10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gibson D., Fanning E. M., Goodfliesh R. M., Gilman J. G., Ballantyne D. L. Quantitative procedures for use with the Edman-Begg sequenator. Partial sequences of two unusual immunoglobulin light chains, Rzf and Sac. Biochemistry. 1971 Dec 21;10(26):4912–4921. doi: 10.1021/bi00802a013. [DOI] [PubMed] [Google Scholar]
- Summers M. R., Smythers G. W., Oroszlan S. Thin-layer chromatography of sub-nanomole amounts of phenylthiohydantoin (PTH) amino acids on polyamide sheets. Anal Biochem. 1973 Jun;53(2):624–628. doi: 10.1016/0003-2697(73)90114-0. [DOI] [PubMed] [Google Scholar]
- Wang J. L., Cunningham B. A., Waxdal M. J., Edelman G. M. The covalent and three-dimensional structural of concanavalin A. I. Amino acid sequence of cyanogen bromide fragments F1 and F2. J Biol Chem. 1975 Feb 25;250(4):1490–1502. [PubMed] [Google Scholar]


