Abstract
Propofol is a commonly used general anesthetic agent which has been previously shown to enhance the inhibitory GABAergic transmission in the central nervous system. In addition to the GABAergic element, the excitatory transmission may be another central molecular site impacted by propofol. Increasing evidence implies that the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor represents an excitatory amino acid receptor subtype subjected to the regulation by propofol. Indeed, in this study, we found that a single injection of propofol at an anesthetic dose increased AMPA receptor GluA1 subunit phosphorylation in young (2–3 months old) and aged (20–21 months old) mice in vivo. Propofol caused an increase in GluA1 phosphorylation in the hippocampus but not in the prefrontal cortex. The propofol effect was also site-selective as the drug elevated GluA1 phosphorylation at serine 831 (S831) but not serine 845. Interestingly, while propofol induced a moderate and transient increase in S831 phosphorylation in young mice, the drug caused a substantial and sustained S831 phosphorylation in aged animals. Total GluA1 abundance remained stable in the hippocampus and prefrontal cortex in both young and aged mice in response to propofol. These results provide evidence supporting the sensitivity of GluA1 AMPA receptors to propofol. A single dose of propofol was able to upregulate GluA1 phosphorylation in the confined hippocampus in an age-dependent manner.
Keywords: Glutamate, GluA1, S831, S845, anesthesia, postoperative cognitive dysfunction, righting reflex, hippocampus, prefrontal cortex
1. Introduction
The neurotransmitter glutamate interacts with postsynaptic glutamate receptors to regulate cellular and synaptic activities. A major ionotropic glutamate receptor subtype is the α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) receptor which is broadly expressed in the mammalian brain and mediates fast synaptic transmission (Traynelis et al., 2010). AMPA receptors form functional channels by assembling four subunits (GluA1-4 or formerly GluR1-4) into homo- or heterotetramers. Like many other synaptic proteins, AMPA receptors are regulated by posttranslational phosphorylation (reviewed in Mao et al., 2011; Wang et al., 2014). GluA1 is a major subunit subjected to phosphorylation. In relatively long intracellular C-terminal (CT) tails of this subunit, phosphorylation occurs at two serine residues: serine 831 (S831) and serine 845 (S845) (Roche et al., 1996; Barria et al., 1997; Mammen et al., 1997; Serulle et al., 2007). While S831 is phosphorylated by protein kinase C (PKC) and Ca2+/calmodulin-dependent protein kinase II (CaMKII), S845 is phosphorylated by protein kinase A (PKA) and cGMP-dependent protein kinase II (cGKII). By changing phosphorylation levels at these sites, the responsible protein kinases modulate neurochemical and physiological properties of GluA1 AMPA receptors (Mao et al., 2011; Wang et al., 2014).
Anesthetic agents are believed to impact specific molecular substrates in the central nervous system to induce general anesthesia or other effects. Many anesthetic agents target the inhibitory gamma-aminobutyric acid (GABA)A ion channel to enhance its activity, thereby inducing anesthesia (Hales and Lambert, 1991; Sonner et al., 2003; Irifune et al., 1999; 2003; Jurd et al., 2003). In addition to inhibitory ion channels, excitatory ion channels are among central targets sensitive to anesthetics (Harris et al., 1995). To this end, excitatory AMPA receptor ion channels draw particular attention. Available data show that propofol, a general anesthetic which is widely used for induction and maintenance of general anesthesia and sedation, altered homomeric GluA1 receptor- or heteromeric GluA1/GluA2 receptor-mediated currents in transfected heterologous cells (Yamakura et al., 1995; Krampfl et al., 2005). Propofol also increased GluA1 S845 phosphorylation in cultured rat striatal and cortical neurons (Haines et al., 2008). However, little is known about the effect of propofol on AMPA receptor phosphorylation in rodent brains in vivo. Moreover, it is unclear whether propofol has a different influence on AMPA receptor phosphorylation in young versus aged animals.
In this study, we initiated an effort to investigate the impact of propofol on AMPA receptor phosphorylation in mouse brains with an emphasis to compare the response of AMPA receptors between young and aged animals in vivo. We carried out a time-course study to monitor time-dependent changes in GluA1 phosphorylation at S831 and S845 sites following a systemic injection of propofol at an anesthetic dose that induced reliable loss of righting reflex (LORR). Two forebrain regions that are intimately implicated in cognitive and memory functions, i.e., the prefrontal cortex (PFC) and hippocampus, were analyzed in parallel to define the effect of propofol on GluA1 phosphorylation.
2. Materials and Methods
2.1. Animals
C57BL/6 mice were obtained from Charles River (New York, NY). Young (2–3 months old weighing 21 to 26 g) and aged (20–21 months old weighting 40 to 49 g) mice were used. Given that the mean lifespan of C57BL/6 mice is approximately 27 months (Turturro et al., 1999) as compared to 80 years known for human lifespan, a mouse with 20 months of age corresponds to approximately 60 years of a human age. Animals were individually housed at 23°C and humidity of 50 ± 10% with food and water available ad libitum. The animal room was on a 12/12 h light/dark cycle with lights on at 0700. All animal use procedures were in strict accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee.
2.2. Anesthesia
Propofol (2,6-diisopropylphenol) was purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO) and was freshly prepared on the day of the experiment. Propofol was prepared to a 25 mg/ml solution in intralipid (Sigma). To induce general anesthesia, mice were injected intraperitoneally (i.p.) with propofol at a single dose of 250 mg/kg. Anesthesia was assessed by measuring LORR, which usually occurred between 2 and 4 min after drug injection (as tested every 20 s or as needed). The selection of the anesthetic dose of 250 mg/kg was based on the ED50 value of 140 mg/kg (i.p.) in mice for inducing LORR (Irifune et al., 1999) and the fact that propofol at 250 mg/kg (i.p.) caused LORR in all mice without death (Snyder et al., 2007) and that propofol at this dose (i.p.) increased Tau phosphorylation in the mouse hippocampus (Whittington et al., 2011). Mice that received injection of an equivalent volume of intralipid served as a vehicle control. These control mice were returned to their home cages at room temperature after injection. Mice treated with propofol were initially returned to home cages. Once they lost their righting reflex, they were placed in a heating device maintaining an environmental temperature at 37°C. Body temperature of mice was monitored with a rectal probe (TCAT-2 controller, Harvard Apparatus, Holliston, MA).
2.3. Brain protein extraction
Mice were killed by cervical dislocation at the time indicated. Brains were immediately removed. The PFC and hippocampus were quickly dissected on ice. Brain tissue was homogenized in an ice-cold, isotonic homogenization buffer containing 0.32 M sucrose, 10 mM HEPES, pH 7.4, 2 mM EDTA, a protease inhibitor cocktail (Thermo Scientific, Rochester, NY), and a phosphatase inhibitor cocktail (Thermo Scientific). Homogenates were then centrifuged at 760 g for 10 min. The supernatant was centrifuged again at 10,000 g for 30 min. The pellet 2 (P2) containing crude synaptosomal plasma membranes was washed and centrifuged at 10,000 g (30 min). The washed P2 was solubilized in the homogenization buffer containing 0.5% Triton X-100 and 1% SDS. Protein concentrations were determined with a Pierce BCA assay kit. Samples were stored at −80°C until use.
2.4. Western blot analysis
Western blots were performed as described previously (Guo et al., 2010; Jin et al., 2013). Briefly, proteins were separated on SDS NuPAGE Bis-Tris 4–12% gels (Invitrogen, Carlsbad, CA). They were then transferred to polyvinylidene fluoride membranes. Membranes were incubated with primary antibodies overnight at 4°C. This was followed by an incubation of a secondary antibody (1:2,000). Immunoblots were developed with the enhanced chemiluminescence reagent (GE Healthcare Life Sciences, Piscataway, NJ). MagicMark XP Western protein standards (Invitrogen) were used for protein size determination. Immunoblots were measured using NIH gel analysis software. The values reflect relative density of the bands normalized to actin. Primary antibodies used in this study include the rabbit polyclonal antibodies against GluA1 with phosphorylated S831 (pS831) (PhosphoSolutions, Aurora, CO), GluA1 pS845 (PhosphoSolutions), GluA1 (Millipore, Billerica, MA), or actin (Millipore).
2.5. Behavioral assessment
We monitored righting reflex in mice after propofol administration to assess the state of anesthesia and to compare the anesthetic effect of propofol between young and aged mice. Righting reflex was scored according to the rating scale described previously (Irifune et al., 2003). In this score system, a score of 0 reflected a normal righting reflex; +1 indicated that animals righted themselves within 2 s on all three trials (slightly impaired righting reflex); +2 specified a righting response with a latency period of > 2 s, but < 10 s in three trials (i.e., moderately or severely impaired righting reflex); and +3 corresponded to the loss of righting reflex (no righting responses within 10 s on all three trials).
2.6. Statistics
The results are presented as means ± S.E.M. The righting reflex behavioral data were analyzed by calculating area under the curve (AUC) for the rating values plotted against time, followed by Student’s t-test for group comparison. The Western blot data were evaluated using Student’s t-test or a one-way analysis of variance, as appropriate, followed by a Bonferroni (Dunn) comparison of groups using least-squares-adjusted means. Probability levels of < 0.05 were considered statistically significant.
3. Results
3.1. Normal levels of pS831 and pS845 proteins in the PFC and hippocampus
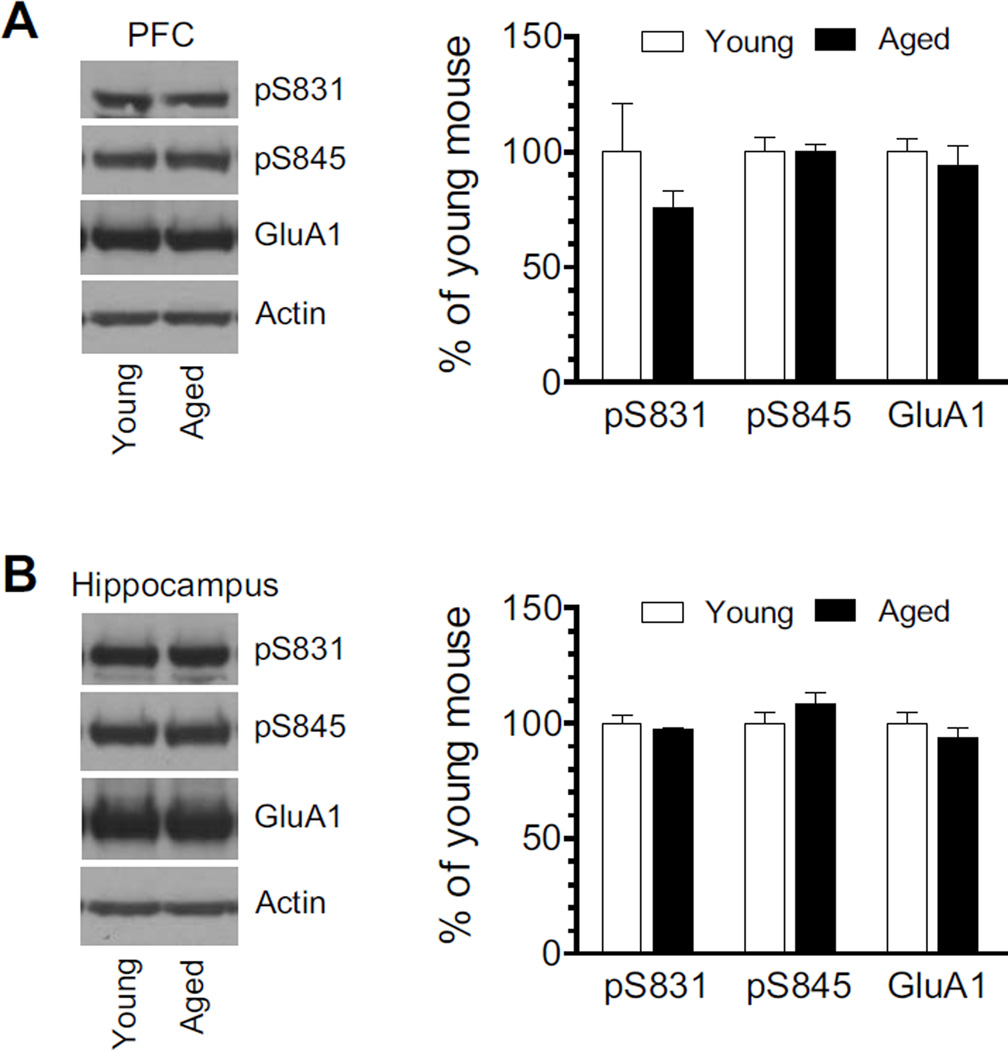
The PFC and hippocampus are key forebrain structures implicated in the cognitive regulation (Arushanyan and Beier, 2008; Salzman and Fusi, 2010; Ray and Zald, 2012). We thus focused on these two regions in our studies testing GluA1 AMPA receptor phosphorylation in response to propofol. We first measured and compared normal levels of GluA1 phosphorylation at S831 and S845 between young and aged mice. As shown in Fig. 1A, pS831 and pS845 protein levels in the PFC were insignificantly different between young and aged mice. Similar results were observed in the hippocampus (Fig. 1B). Total cellular levels of GluA1 in the PFC and hippocampus remained stable at the two ages. Thus, under normal conditions, GluA1 expression and GluA1 phosphorylation at both serine sites are maintained at a similar level between young and aged mice.
Figure 1. GluA1 levels and GluA1 phosphorylation in the PFC and hippocampus of young and aged mice.
(A) pS831, pS845, and GluA1 protein levels in the PFC of young and aged mice. (B) pS831, pS845, and GluA1 protein levels in the hippocampus of young and aged mice. Representative immunoblots (12 µg per lane) are shown left to the quantified data. Data are presented as means ± S.E.M. (n = 3 per group).
3.2. Effects of propofol on GluA1 S831 phosphorylation
We next investigated the effect of propofol on GluA1 S831 phosphorylation. To this end, we subjected mice to a single dose of propofol (250 mg/kg, i.p.). We then killed animals at different time points (0.5, 1, or 2 h after drug injection) to analyze changes in S831 phosphorylation in the two targeted brain regions at the two ages. In young mice, a declining tendency of S831 phosphorylation was shown in the PFC after propofol was administered, although it did not reach a statistically significant level (Fig. 2A). In aged mice, propofol also had a minimal impact on PFC S831 phosphorylation at all three time points surveyed (Fig. 2B). Total cellular GluA1 expression in the PFC was not changed following propofol administration in both young and aged mice. These results indicate that GluA1 S831 phosphorylation in the PFC is resistant to propofol in either young or aged mice.
Figure 2. Effects of propofol on GluA1 S831 phosphorylation in the PFC.
(A) Effects of propofol on S831 phosphorylation in the PFC of young mice. (B) Effects of propofol on S831 phosphorylation in the PFC of aged mice. Note that propofol had no significant effect on cortical S831 phosphorylation at either age. Representative immunoblots are shown left to the quantified data. Mice received a single dose of propofol (250 mg/kg, i.p.) and were killed at different time points (0.5, 1, or 2 h) after drug injection. Data are presented as means ± S.E.M. (n = 4–8 per group).
The same propofol-treated mice were then used to analyze changes in GluA1 S831 phosphorylation in the hippocampus. Interestingly, unlike the minimal S831 response in the PFC, S831 phosphorylation in the hippocampus was sensitive to propofol. In young animals, an amount of pS831 proteins was increased 0.5 h after propofol injection (P < 0.05 versus 0 h; Fig. 3A). A less but still significant increase was seen at 1 h (P < 0.05 versus 0 h). At 2 h, pS831 levels in propofol-treated mice were insignificantly different from the control value. In aged mice, pS831 signals were elevated at 0.5 h (P < 0.05 versus 0 h; Fig. 3B). This elevation noticeably reached a peak at 1 h and remained at a markedly higher level at 2 h. In both young and aged mice, the increase in pS831 protein levels resulted from an accelerated phosphorylation rate at S831 because total GluA1 proteins were not altered after propofol administration. These results established the hippocampus as a sensitive region to propofol in terms of S831 phosphorylation. Moreover, propofol affects S831 phosphorylation in an age-dependent fashion as in aged mice the drug induced a relatively prolonged increase in hippocampal S831 phosphorylation as compared to young animals.
Figure 3. Effects of propofol on GluA1 S831 phosphorylation in the hippocampus.
(A) Effects of propofol on S831 phosphorylation in the hippocampus of young mice. (B) Effects of propofol on S831 phosphorylation in the hippocampus of aged mice. Note that propofol induced a marked and prolonged increase in S831 phosphorylation in aged mice, while propofol produced a transient increase in S831 phosphorylation in young mice. Representative immunoblots are shown left to the quantified data. Mice received a single dose of propofol (250 mg/kg, i.p.) and were killed at different time points (0.5, 1, or 2 h) after drug injection. Data are presented as means ± S.E.M. (n = 5–8 per group). *P < 0.05 versus vehicle (0 h after injection).
3.3. Effects of propofol on GluA1 S845 phosphorylation
Effects of propofol on S845 phosphorylation were tested subsequently. In the PFC, propofol produced insignificant changes in S845 phosphorylation in young and aged mice. As shown in Fig. 4A, at three time points tested, propofol did not alter PFC pS845 protein levels in young animals. Similarly, propofol did not change the amount of pS845 signals in aged animals (Fig 4B). In the hippocampus, there was still no significant change in S845 phosphorylation signals in either young (Fig. 5A) and aged (Fig. 5B) mice. These data reveal that the S845 phosphorylation in cortical and hippocampal neurons at different ages is an insensitive event to propofol.
Figure 4. Effects of propofol on GluA1 S845 phosphorylation in the PFC.
(A) Effects of propofol on S845 phosphorylation in the PFC of young mice. (B) Effects of propofol on S845 phosphorylation in the PFC of aged mice. Representative immunoblots are shown left to the quantified data. Mice received a single dose of propofol (250 mg/kg, i.p.) and were killed at different time points (0.5, 1, or 2 h) after drug injection. Data are presented as means ± S.E.M. (n = 4–7 per group).
Figure 5. Effects of propofol on GluA1 S845 phosphorylation in the hippocampus.
(A) Effects of propofol on S845 phosphorylation in the hippocampus of young mice. (B) Effects of propofol on S845 phosphorylation in the hippocampus of aged mice. Representative immunoblots are shown left to the quantified data. Mice received a single dose of propofol (250 mg/kg, i.p.) and were killed at different time points (0.5, 1, or 2 h) after drug injection. Data are presented as means ± S.E.M. (n = 4–7 per group).
3.4. Propofol-induced LORR
The LORR was measured to compare the behavioral sensitivity to propofol between young and aged mice. The complete LORR was rapidly induced after propofol administration in the two groups of mice (Fig. 6A). After 30 min, young and aged mice showed different recovery rates from the LORR state. As compared to young mice, aged animals showed a slower rate of recovery from LORR. Accordingly, the AUC value of aged animals (measured from 30 to 120 min) was significantly higher than the corresponding value observed in young animals (P < 0.05; Fig. 6B). Thus, propofol at the same dose caused a longer duration of LORR in aged mice relative to young mice.
Figure 6. Effects of propofol on the righting reflex behavior in young and aged mice.
(A) Righting reflex scores from young and aged mice in response to propofol. (B) A comparison of AUC values between young and aged mice. Mice received a single dose of propofol (250 mg/kg, i.p.). Righting reflexes were scored every 10 min after drug injection. Data are presented as means ± S.E.M. (n = 6–7 per group). *P < 0.05 versus young mice.
4. Discussion
This study investigated the effect of propofol on GluA1 phosphorylation in the two different mouse brain regions at different ages in vivo. Systemic administration of propofol at a dose (250 mg/kg, i.p.) that induced reliable LORR increased GluA1 phosphorylation at S831 in the hippocampus, while propofol had no effect on S831 phosphorylation in the PFC. Propofol induced a moderate and reversible increase in S831 phosphorylation in young mice, whereas the agent at an equal dose produced a substantial and sustained elevation of S831 phosphorylation in aged animals. The effect of propofol on S831 phosphorylation was site-selective because the drug did not alter GluA1 phosphorylation at the S845 site. These data support GluA1 AMPA receptors in the hippocampus as a sensitive target of propofol. Exposure to propofol causes a selective S831 phosphorylation in the hippocampus.
An early study found that propofol at the anesthetic dose (250 mg/kg, i.p.) induced a slight decrease in 1) S831 phosphorylation in the striatum but not frontal cortex, and 2) S845 phosphorylation in the frontal cortex but not striatum of young mice (8–10 weeks of age) at 10 min after propofol injection (Snyder et al., 2007). In our study, we found that propofol at the same dose (but 0.5–2 h after drug injection) selectively targeted S831 and impacted the hippocampus. Another important property of propofol is its age-dependent effects. While propofol induced a transient increase in S831 phosphorylation in young mice, propofol produced a prolonged increase in S831 phosphorylation in aged animals. In corresponding to this neurochemical change, behavioral observations of LORR showed a longer duration in aged mice as compared to young ones. It appears that geriatric mice are more sensitive to propofol. This resembles the observations in humans that elderly patients, in general, are more sensitive to anesthetic agents. Less medication is usually required to achieve a desired clinical effect, and drug effect is often prolonged (Kanonidou and Karystianou, 2007). In fact, propofol exhibited a significantly lower clearance in the elderly than in the young patients (Kirkpatrick et al., 1988). The age-dependent nature of propofol effects may have a clinical relevance in promoting a side effect of the drug in the elderly (see below). Of note, in addition to neurons, glial cells express AMPA receptors (Condorelli et al., 1999). Thus, propofol may alter GluA1 S845 phosphorylation in glial cells to regulate their activity.
Propofol increased S845 but not S831 phosphorylation in cultured rat cortical and striatal neurons in vitro (Haines et al., 2008). However, in the current study using animals in vivo, propofol increased S831 phosphorylation in the hippocampus and did not alter S845 phosphorylation in the PFC. Cultured cortical neurons are different from in vivo neurons in many developmental, neurochemical, and physiological aspects. These differences may contribute to different S845 responses to propofol observed in cultured cortical neurons and PFC neurons from adult animal brains. S845 is phosphorylated by PKA and cGKII (Roche et al., 1996; Serulle et al., 2007). Phosphorylation at this site seems to promote GluA1 trafficking to extrasynaptic membranes and primes extrasynaptic receptors for synaptic insertion (Estaban et al., 2003; He et al., 2009; Dias et al., 2012; Incontro et al., 2013). S845 phosphorylation also enhanced the AMPA channel open probability and the current peak (Roche et al., 1996; Banke et al., 2000). The insignificant effect of propofol on S845 phosphorylation observed in this study indicates a limited influence of the drug over the S845-regulated GluA1 trafficking and channel open probability. In contrast to S845, S831 was a site sensitive to propofol. S831 phosphorylation is catalyzed by PKC and CaMKII (Barria et al., 1997; Mammen et al., 1997). Phosphorylation at this site alters biophysical properties of AMPA receptors. For details, S831 phosphorylation increased single channel conductance of recombinant AMPA receptors transfected in HEK293 cells (Derkach et al., 1999; Jenkins and Travnelis, 2012). This effect was also seen in native AMPA receptors expressed on mouse hippocampal neurons (Kristensen et al., 2011). It is believed that phospho-S831, through increasing channel conductance, can potentiate AMPA receptor activity and mediate synaptic plasticity such as long-term potentiation (Boehm et al., 2006; Lee et al., 2010; Makino et al., 2011; Lu and Roche, 2012; Ren et al., 2013). In the current study, we observed a great increase in hippocampal S831 phosphorylation in mice treated with propofol. This suggests that propofol generally enhances AMPA receptor activity in hippocampal neurons. Since CaMKII phosphorylation of S831 only potentiated homomeric GluA1 receptors but not heteromeric GluA1/GluA2 receptors (Oh and Derkach, 2005), propofol may mainly affect the homomeric GluA1 receptor subtype in the hippocampus.
Propofol enhanced homomeric GluA1 receptor currents at high concentrations (0.1–1 mM) in transfected Xenopus oocytes, while GluA2-containing Ca2+-impermeable heteromeric receptors (GluA1/GluA2) were more resistant to propofol and were actually inhibited by 1 mM of propofol (Yamakura et al., 1995). At concentrations ≧ 0.1 mM, propofol decelerated the GluA1 receptor desensitization and increased the relative steady-state current in HEK293 cells (Krampfl et al., 2005). Our finding that propofol enhanced GluA1 S831 phosphorylation may provide a molecular mechanism for these early observations. Of note, it is hard to reason that the propofol-induced increase in S831 phosphorylation and likely augmentation of GluA1 receptor activity could contribute to general anesthesia. This is because anesthesia typically results from general suppression of the excitatory synaptic transmission or enhancement of the inhibitory synaptic transmission or both. Even if propofol inhibited GluA1/GluA2 receptors, propofol produced this effect at a concentration (1 mM) substantially above the EC50 value (0.4 ± 2.2 µM) needed for free and protein-unbound propofol to induce anesthesia (Tonner et al., 1992; Franks and Lieb, 1994; Sewell and Sear, 2002). Propofol has been demonstrated to strongly enhance the inhibitory GABAergic transmission (Hales and Lambert, 1991; Sonner et al., 2003; Irifune et al., 1999; 2003; Jurd et al., 2003) and inhibit voltage-gated sodium channels (Ouyang et al., 2003; Haeseler and Leuwer, 2003) usually in the low µM range. These effects may primarily mediate the anesthetic property of propofol.
Postoperative cognitive dysfunction (POCD) is a neurological disorder characterized by a decline in cognitive function (such as memory and thinking impairments) after surgery (Ramaiah and Lam, 2009). The hallmark of this dysfunction is its increasing risk with age (Silbert et al., 2011). Although it occurs in adult patients of all ages, the elderly (60 years or older) are at a significantly higher risk. The precise etiology of POCD remains elusive. Among possible causes, general anesthesia has drawn attention and has been thought to contribute to the pathophysiology and/or symptomology of cognitive disorders, although direct evidence is lacking. In this study, we found that propofol modified GluA1 phosphorylation preferentially in the hippocampus, an area closely implicated in cognitive and memory function (Arushanyan and Beier, 2008). Moreover, aged mice, like the elderly humans (Kanto, 1988; Kanonidou and Karystianou, 2007), were more vulnerable to propofol. Thus, the propofol-triggered GluA1 pathway in hippocampal neurons may play a role in POCD. While accurate roles of the propofol-GluA1 pathway are unclear, it is likely that propofol stimulates AMPA receptors, especially GluA1-containing/GluA2-lacking (Ca2+-permeable) receptors, to increase cytosolic Ca2+ loading and bring hippocampal neurons more vulnerable to Ca2+-dependent excitotoxicity (Weiss, 2011). However, isoflurane/nitric oxide anesthesia did not induce cell loss in the hippocampus of aged (18-month-old) rats at 3 months post-anesthesia (Mawhinney et al., 2012). Propofol may cause other forms of AMPA receptor dysfunction in hippocampal neurons to promote POCD.
Acknowledgements
This work was supported by NIH grants DA10355 (J.Q.W.) and MH61469 (J.Q.W.) and by a research fund from Saint Luke’s Hospital Foundation of Kansas City and the generosity of the Oppenheimer Brothers Foundation, Beverly Hills California. The authors thank Dr. Bing Xue for technical assistance.
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methylisozazole-4-propionic acid
- AUC
area under the curve
- CaMKII
Ca2+/calmodulin-dependent protein kinase II
- cGKII
cGMP-dependent protein kinase II
- EGTA
ethylene glycol tetraacetic acid
- GABA
gamma-aminobutyric acid
- HEPES
hydroxyethyl piperazineethanesulfonic acid
- LORR
loss of righting reflex
- PFC
prefrontal cortex
- PKA
protein kinase A
- PKC
protein kinase C
- POCD
postoperative cognitive dysfunction
- SDS
sodium dodecyl sulfate
- S.E.M.
standard error of the mean
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- Arushanyan EB, Beier EV. The hippocampus and cognitive impairments. Neurosci. Behav. Physiol. 2008;38:751–758. doi: 10.1007/s11055-008-9043-0. [DOI] [PubMed] [Google Scholar]
- Banke TG, Bowie D, Lee HK, Huganir RL, Schousboe A, Traynelis SF. Control of GluR1 AMPA receptor function by cAMP-dependent protein kinase. J. Neurosci. 2000;20:89–102. doi: 10.1523/JNEUROSCI.20-01-00089.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barria A, Derkach V, Soderling T. Identification of the Ca2+/calmodulin-dependent protein kinase II regulatory phosphorylation site in the α-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate-type glutamate receptor. J. Biol. Chem. 1997;272:32727–32730. doi: 10.1074/jbc.272.52.32727. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Derkach V, Barria A, Soderling TR. Ca2+/calmodulin-kinase II enhances channel conductance of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate type glutamate receptors. Proc. Natl. Acad. Sci. USA. 1999;96:3269–3274. doi: 10.1073/pnas.96.6.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias RB, Ribeiro JA, Sebastiao AM. Enhancement of AMPA currents and GluR1 membrane expression through PKA-coupled adenosine A(2A) receptors. Hippocampus. 2012;22:276–291. doi: 10.1002/hipo.20894. [DOI] [PubMed] [Google Scholar]
- Condorelli DF, Conti F, Gallo V, Kirchhoff F, Seifert G, Steinhauser C, Verkhratsky A, Yuan X. Expression and functional analysis of glutamate receptors in glial cells. Adv. Exp. Med. Biol. 1999;468:49–67. doi: 10.1007/978-1-4615-4685-6_5. [DOI] [PubMed] [Google Scholar]
- Estaban JA, Shi H, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat. Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Franks NP, Lieb WR. Molecular and cellular mechanisms of general anesthesia. Nature. 1994;367:607–614. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- Guo ML, Fibuch EE, Liu XY, Choe ES, Buch S, Mao LM, Wang JQ. CaMKIIα interacts with M4 muscarinic receptors to control receptor and psychomotor function. EMBO J. 2010;29:2070–2081. doi: 10.1038/emboj.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haeseler G, Leuwer M. High-affinity block of voltage-operated rat IIA neuronal sodium channels by 2,6 di-tert-butylphenol, a propofol analogue. Eur. J. Anaesthesiol. 2003;20:220–224. doi: 10.1017/s0265021503000371. [DOI] [PubMed] [Google Scholar]
- Haines M, Mao LM, Yang L, Arora A, Fibuch EE, Wang JQ. Modulation of AMPA receptor GluR1 subunit phosphorylation in neurons by the intravenous anesthetic propofol. Br. J. Anaesth. 2008;100:676–682. doi: 10.1093/bja/aen051. [DOI] [PubMed] [Google Scholar]
- Hales TG, Lambert JJ. The actions of propofol on inhibitory amino acid receptors of bovine adrenomedullary chromaffin cells and rodent central neurons. Br. J. Pharmacol. 1991;104:619–628. doi: 10.1111/j.1476-5381.1991.tb12479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RA, Mihic SJ, Dildy-Mayfield JE, Machu TK. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB J. 1995;9:1454–1462. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc. Natl. Acad. Sci. USA. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incontro S, Ciruela F, Ziff E, Hormann F, Sanchez-Prieto J, Torres M. The type II cGMP dependent protein kinase regulates GluA1 levels at the plasma membrane of developing cerebellar granule cells. Biochim. Biophys. Acta. 2013;1833:1820–1831. doi: 10.1016/j.bbamcr.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irifune M, Sugimura M, Takarada T, Maeoka K, Shimizu Y, Dohi T, Nishikawa T, Kawahara M. Propofol anaesthesia in mice is potentiated by muscimol and reversed by bicuculline. Br. J. Anaesth. 1999;83:665–667. doi: 10.1093/bja/83.4.665. [DOI] [PubMed] [Google Scholar]
- Irifune M, Takarada T, Shimizu Y, Endo C, Katayama S, Dohi T, Kawahara M. Propofol-induced anesthesia in mice is mediated by gamma-aminobutyric acid-A and excitatory amino acid receptors. Anesth. Analg. 2003;97:424–429. doi: 10.1213/01.ANE.0000059742.62646.40. [DOI] [PubMed] [Google Scholar]
- Jenkins MA, Travnelis SF. PKC phosphorylates GluA1-Ser831 to enhance AMPA receptor conductance. Channels (Austin) 2012;6:60–64. doi: 10.4161/chan.18648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin DZ, Guo ML, Xue B, Fibuch EE, Choe ES, Mao LM, Wang JQ. Phosphorylation and feedback regulation of metabotropic glutamate receptor 1 by CaMKII. J. Neurosci. 2013;33:3402–3412. doi: 10.1523/JNEUROSCI.3192-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurd R, Arras M, Lambert S, Drexler B, Siegwart R, Crestani F, Zaugg M, Vogt KE, Ledermann B, Antkoviak B, Rudolph U. General anesthetic actions in vivo strongly attenuated by a point mutation in the GABA(A) receptor beta3 subunit. FASEB J. 2003;17:250–252. doi: 10.1096/fj.02-0611fje. [DOI] [PubMed] [Google Scholar]
- Kanonidou Z, Karystianou G. Anesthesia for the elderly. Hippokratia. 2007;11:175–177. [PMC free article] [PubMed] [Google Scholar]
- Kanto JH. Propofol, the newest induction agent of anesthesia. Int. J. Clin. Pharmacol. Ther. Toxicol. 1988;26:41–57. [PubMed] [Google Scholar]
- Kirkpatrick T, Cockshott ID, Douglas EJ, Nimmo WS. Pharmacokinetics of propofol (diprivan) in elderly patients. Br. J. Anaesth. 1988;60:146–150. doi: 10.1093/bja/60.2.146. [DOI] [PubMed] [Google Scholar]
- Krampfl K, Cordes A, Schlesinger F, Wolfs H, Bufler J. Effects of propofol on recombinant AMPA receptor channels. Eur. J. Pharmacol. 2005;511:1–7. doi: 10.1016/j.ejphar.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir RL, Traynelis SF. Mechanism of Ca(2)/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat. Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, He K, Song L, Huganir RL. Specific roles of AMPA receptor subunit GluR1 (GluA1) phosphorylation sites in regulating synaptic plasticity in the CA1 region of hippocampus. J. Neurophysiol. 2010;103:479–489. doi: 10.1152/jn.00835.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu W, Roche KW. Posttranslational regulation of AMPA receptor trafficking and function. Curr. Opin. Neurobiol. 2012;22:470–479. doi: 10.1016/j.conb.2011.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino Y, Johnson RC, Yu Y, Takamiya K, Huganir RL. Enhanced synaptic plasticity in mice with phosphomimetic mutation of the GluA1 AMPA receptor. Proc. Natl. Acad. Sci. USA. 2011;108:8450–8455. doi: 10.1073/pnas.1105261108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mammen AL, Kameyama K, Roche KW, Huganir RL. Phosphorylation of the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit by calcium/calmodulin-dependent kinase II. J. Biol. Chem. 1997;272:32528–32533. doi: 10.1074/jbc.272.51.32528. [DOI] [PubMed] [Google Scholar]
- Mao LM, Guo ML, Jin DZ, Fibuch EE, Choe ES, Wang JQ. Posttranslational modification biology of glutamate receptors and drug addiction. Front. Neuroanat. 2011;5:19. doi: 10.3389/fnana.2011.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MC, Derkach VA. Dominant role of the GluR2 subunit in regulation of AMPA receptors by CaMKII. Nat. Neurosci. 2005;8:853–854. doi: 10.1038/nn1476. [DOI] [PubMed] [Google Scholar]
- Ouyang W, Wang G, Hemmings HC., Jr Isoflurane and propofol inhibit voltage-gated sodium channels in isolated rat neurohypophysial nerve terminals. Mol. Pharmacol. 2003;64:373–381. doi: 10.1124/mol.64.2.373. [DOI] [PubMed] [Google Scholar]
- Ramajah R, Lam AM. Postoperative cognitive dysfunction in the elderly. Anesthesiol. Clin. 2009;27:485–496. doi: 10.1016/j.anclin.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Ray RD, Zald DH. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2012;36:479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren SQ, Yan JZ, Zhang XY, Bu YF, Pan WW, Yao W, Tian T, Lu W. PKCλ is critical in AMPA receptor phosphorylation and synaptic incorporation during LTP. EMBO J. 2013;32:1365–1380. doi: 10.1038/emboj.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Salzman CD, Fusi S. Emotion, cognition, and mental state representation in amygdala and prefrontal cortex. Annu. Rev. Neurosci. 2010;33:173–202. doi: 10.1146/annurev.neuro.051508.135256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sewell JC, Sear JW. Can molecular similarity-activity models for intravenous general anaesthetics help explain their mechanism of action? Br. J. Anaesth. 2002;88:166–174. doi: 10.1093/bja/88.2.166. [DOI] [PubMed] [Google Scholar]
- Silbert B, Evered L, Scott DA. Cognitive decline in the elderly: is anaesthesia implicated? Best Pract. Res. Clin. Anaesthsiol. 2011;25:379–393. doi: 10.1016/j.bpa.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Snyder GL, Galdi S, Hendrick JP, Hemmings HC., Jr General anesthetics selectively modulate glutamatergic and dopaminergic signaling via site-specific phosphorylation in vivo. Neuropharmacology. 2007;53:619–630. doi: 10.1016/j.neuropharm.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Sonner JM, Zhang Y, Stabernack C, Abaigar W, Xing Y, Laster MJ. GABA(A) receptor blockade antagonizes the immobilizing action of propofol but not ketamine or isofluence in a dose-related manner. Anesth. Analg. 2003;96:706–712. doi: 10.1213/01.ANE.0000048821.23225.3A. [DOI] [PubMed] [Google Scholar]
- Tonner PH, Poppers DM, Miller KW. The general anesthetic potency of propofol and its dependence on hydrostatic pressure. Anesthesiology. 1992;77:926–931. doi: 10.1097/00000542-199211000-00015. [DOI] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol. Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J. Gerontol. A Biol. Sci. Med. Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Guo ML, Jin DZ, Xue B, Fibuch EE, Mao LM. Roles of subunit phosphorylation in regulating glutamate receptor function. EurJ. Pharmacol. 2014;728:183–187. doi: 10.1016/j.ejphar.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss JH. Ca2+ permeable AMPA channels in diseases of the nervous system. Front. Mol. Neurosci. 2011;4:42. doi: 10.3389/fnmol.2011.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington RA, Virag L, Marcouiller G, Papon MA, Khoury NBE, Julien C, Morlin F, Emala CW, Planel E. Propofol directly increases Tau phosphorylation. PLoS ONE. 2011;6:e16648. doi: 10.1371/journal.pone.0016648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakura T, Sakimura K, Shimoji K, Mishina M. Effects of propofol on various AMPA-, kainate- and NMDA-selective glutamate receptor channels expressed in Xenopus oocytes. Neurosci. Lett. 1995;188:187–190. doi: 10.1016/0304-3940(95)11431-u. [DOI] [PubMed] [Google Scholar]