Abstract
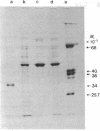
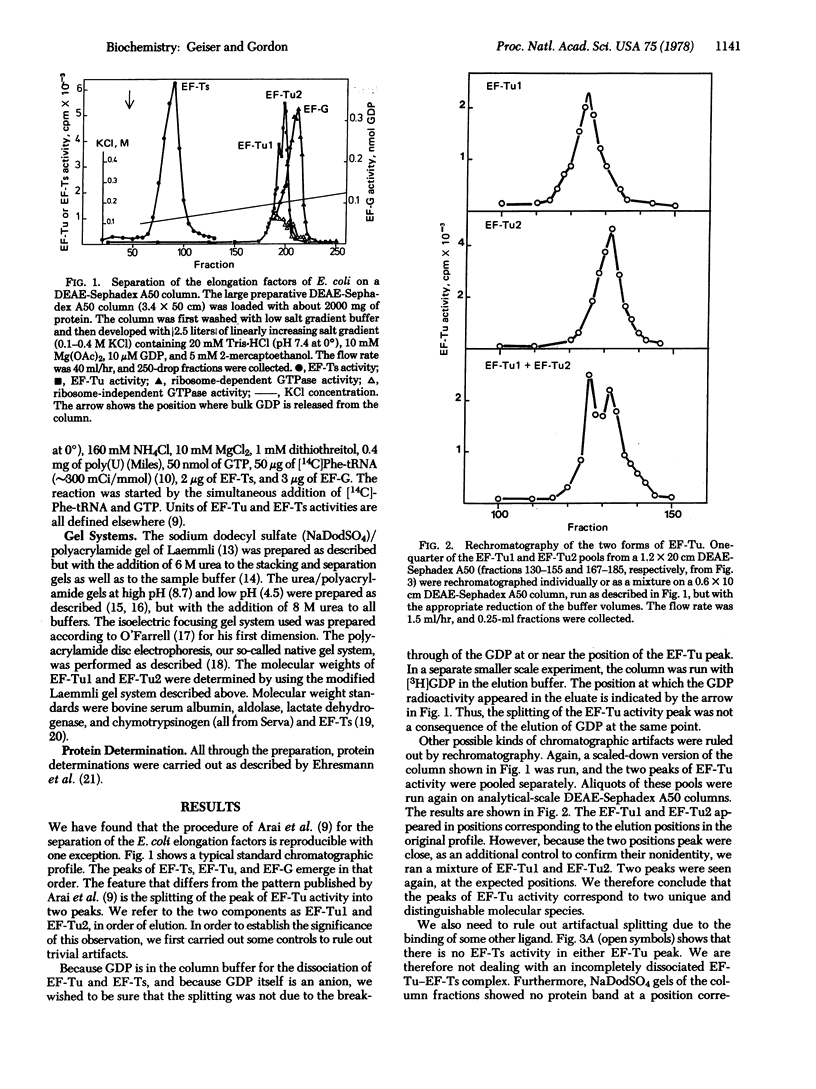
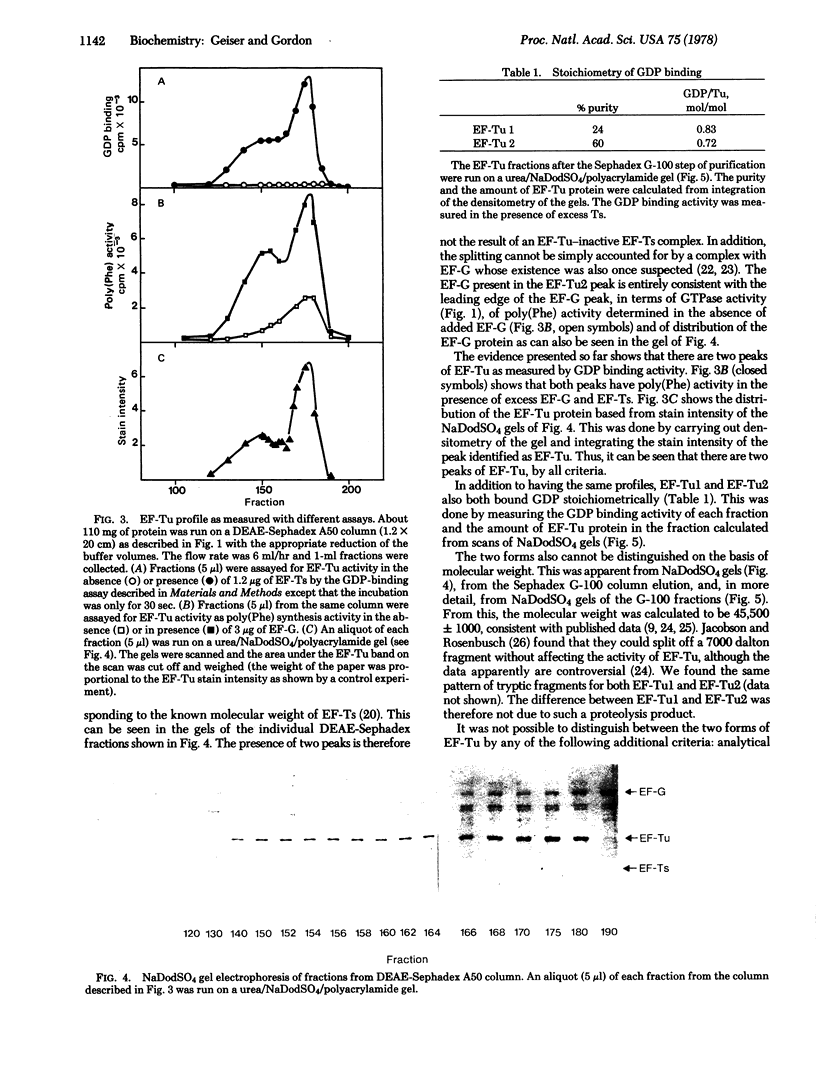
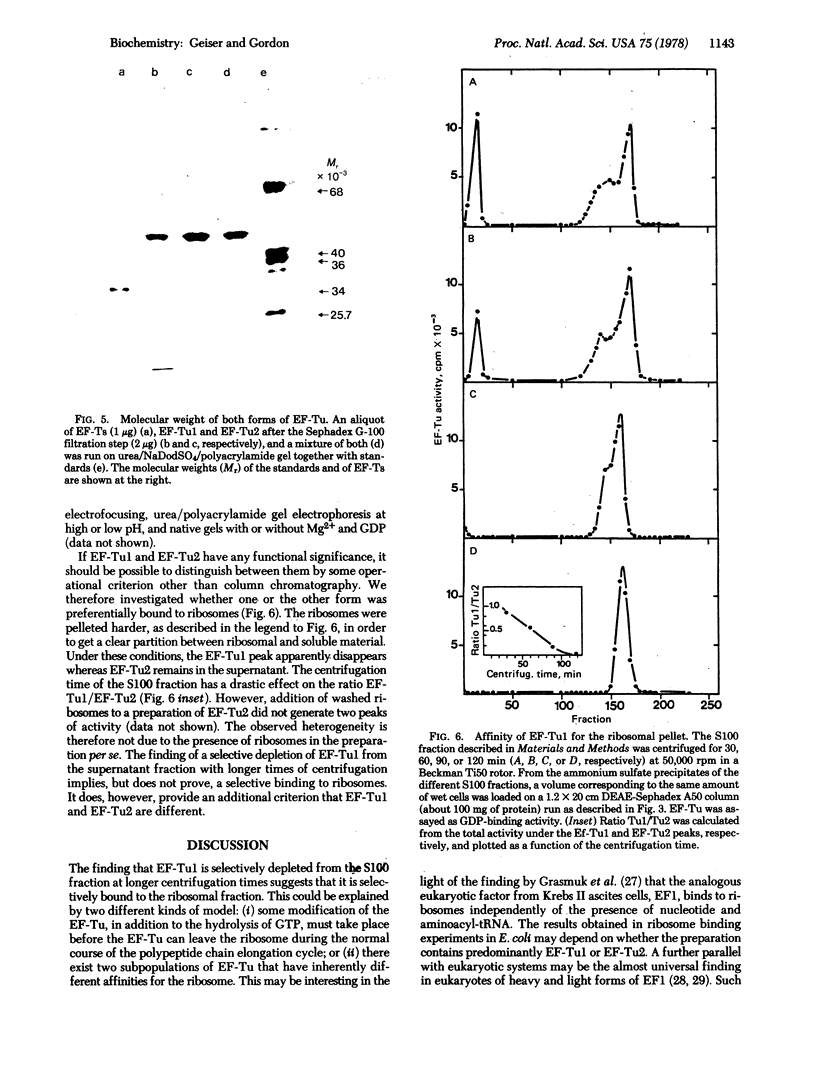
Two forms of the elongation factor Tu from Escherichia coli have been separated by chromatography on DEAE-Sephadex A50. Obvious chromatographic artifacts have been ruled out by investigation of the elution profile of GDP (a component of the column buffer as well as a ligand of Tu) and by rechromatography of the two components, either separately to give the component peaks or together to give a double peak. The two components have been confirmed as Tu by the poly(uridylic)-dependent polyphenylalanine synthesis and by the distribution of the Tu protein as quantitated from sodium dodecyl sulfate/polyacrylamide gel electrophoresis. Complexes with the elongation factors Ts and G have also been ruled out by activity profiles and by quantitation of the protein distribution, again on gels. The distribution of the two forms between ribosomal and supernatant fractions has been examined: one is bound preferentially to the ribosomal fraction and the other is found in the supernatant fraction. The possible significance of this is discussed.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Arai K., Nakamura S., Arai T., Kawakita M., Kaziro Y. Limited hydrolysis of the polypeptide chain elongation factor Tu by trypsin. Isolation and characterization of the polypeptide fragments. J Biochem. 1976 Jan;79(1):69–83. doi: 10.1093/oxfordjournals.jbchem.a131060. [DOI] [PubMed] [Google Scholar]
- Blumenthal T., Landers T. A., Weber K. Bacteriophage Q replicase contains the protein biosynthesis elongation factors EF Tu and EF Ts. Proc Natl Acad Sci U S A. 1972 May;69(5):1313–1317. doi: 10.1073/pnas.69.5.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cashel M. Preparation of guanosine tetraphosphate (ppGpp) and guanosine pentaphosphate (pppGpp) from Escherichia coli ribosomes. Anal Biochem. 1974 Jan;57(1):100–107. doi: 10.1016/0003-2697(74)90056-6. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Ehresmann B., Imbault P., Weil J. H. Spectrophotometric determination of protein concentration in cell extracts containing tRNA's and rRNA's. Anal Biochem. 1973 Aug;54(2):454–463. doi: 10.1016/0003-2697(73)90374-6. [DOI] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. The elongation factor Tu coded by the tufA gene of Escherichia coli K-12 is almost identical to that coded by the tufB gene. J Biol Chem. 1977 Mar 25;252(6):2154–2157. [PubMed] [Google Scholar]
- Gordon J. Hydrolysis of guanosine 5'-triphosphate associated wh binding of aminoacyl transfer ribonucleic acid to ribosomes. J Biol Chem. 1969 Oct 25;244(20):5680–5686. [PubMed] [Google Scholar]
- Grasmuk H., Nolan R. D., Drews J. A new concept of the function of elongation factor 1 in peptid chain elongation. Eur J Biochem. 1976 Dec;71(1):271–279. doi: 10.1111/j.1432-1033.1976.tb11113.x. [DOI] [PubMed] [Google Scholar]
- Hollis V. W., Jr, Furano A. V. The fractionation of transfer factors in the presence of a proteolytic inhibitor. J Biol Chem. 1968 Sep 25;243(18):4926–4930. [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. A functionally active tryptic fragment of Escherichia coli elongation factor Tu. Biochemistry. 1976 Nov 16;15(23):5105–5110. doi: 10.1021/bi00668a024. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Rosenbusch J. P. Abundance and membrane association of elongation factor Tu in E. coli. Nature. 1976 May 6;261(5555):23–26. doi: 10.1038/261023a0. [DOI] [PubMed] [Google Scholar]
- Jacobson G. R., Takacs B. J., Rosenbusch J. P. Properties of a major protein released from Escherichia coli by osmotic shock. Biochemistry. 1976 Jun 1;15(11):2297–2303. doi: 10.1021/bi00656a008. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nagate S., Motoyoshi K., Iwasaki K. Exchange of free GTP with EF-1alpha-GDP complex promoted by a factor EF-1beta from pig liver. Biochem Biophys Res Commun. 1976 Aug 23;71(4):933–938. doi: 10.1016/0006-291x(76)90745-2. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Rohrbach M. S., Dempsey M. E., Bodley J. W. Preparation of homogeneous elongation factor G and examination of the mechanism of guanosine triphosphate hydrolysis. J Biol Chem. 1974 Aug 25;249(16):5094–5101. [PubMed] [Google Scholar]
- Slobin L. I., Möller W. The heavy form of elongation factor 1 in artemia salina embryos is functionally analogous to a complex of bacterial factors EF-Tu and EF-Ts. Biochem Biophys Res Commun. 1977 Jan 24;74(2):356–365. doi: 10.1016/0006-291x(77)90312-6. [DOI] [PubMed] [Google Scholar]
- Thomas G., Sweeney R., Chang C., Noller H. F. Identification of proteins functionally altered by chemical modification of the transfer RNA and polyuridylic acid binding sites of 30 S ribosomal subunits. J Mol Biol. 1975 Jun 15;95(1):91–102. doi: 10.1016/0022-2836(75)90338-1. [DOI] [PubMed] [Google Scholar]
- Travers A. A., Kamen R. I., Schleif R. F. Factor necessary for ribosomal RNA synthesis. Nature. 1970 Nov 21;228(5273):748–751. doi: 10.1038/228748a0. [DOI] [PubMed] [Google Scholar]
- Travers A. Control of ribosomal RNA synthesis in vitro. Nature. 1973 Jul 6;244(5410):15–18. doi: 10.1038/244015a0. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]