SUMMARY
In Xanthomonas campestris pv. campestris (Xcc), the proteins encoded by the rpf (regulator of pathogenicity factor) gene cluster produce and sense a fatty acid signal molecule called diffusible signaling factor (DSF, 2(Z)-11-methyldodecenoic acid). RpfB was reported to be involved in DSF processing and was predicted to encode an acyl-CoA ligase. We report that RpfB activates a wide range of fatty acids to their CoA esters in vitro. Moreover, RpfB can functionally replace the paradigm bacterial acyl-CoA ligase, Escherichia coli FadD, in the E. coli β-oxidation pathway and deletion of RpfB from the Xcc genome results in a strain unable to utilize fatty acids as carbon sources. An essential RpfB function in the pathogenicity factor pathway was demonstrated by the properties of a strain deleted for both the rpfB and rpfC genes. The ΔrpfB ΔrpfC strain grew poorly and lysed upon entering stationary phase. Deletion of rpfF, the gene encoding the DSF synthetic enzyme, restored normal growth to this strain. RpfF is a dual function enzyme that synthesizes DSF by dehydration of a 3-hydroxyacyl-acyl carrier protein (ACP) fatty acid synthetic intermediate and also cleaves the thioester bond linking DSF to ACP. However, the RpfF thioesterase activity is of broad specificity and upon elimination of its RpfC inhibitor RpfF attains maximal activity and its thioesterase activity proceeds to block membrane lipid synthesis by cleavage of acyl-ACP intermediates. This resulted in release of the nascent acyl chains to the medium as free fatty acids. This lack of acyl chains for phospholipid synthesis results in cell lysis unless RpfB is present to counteract the RpfF thioesterase activity by catalyzing uptake and activation of the free fatty acids to give acyl-CoAs that can be utilized to restore membrane lipid synthesis. Heterologous expression of a different fatty acid activating enzyme, the Vibrio harveyi acyl-ACP synthetase, replaced RpfB in counteracting the effects of high level RpfF thioesterase activity indicating that the essential role of RpfB is uptake and activation of free fatty acids.
INTRODUCTION
The phytopathogenic aerobic bacterium, Xanthomonas campestris pv. campestris (Xcc), is the causal agent of black rot disease, one of the most destructive diseases of cruciferous vegetables worldwide. The rpf (regulator of pathogenicity factor) gene cluster encodes proteins that control synthesis, perception, and transduction of the diffusible signal factor (DSF) (Ryan, 2013, Dow, 2008). Xcc DSF is cis-11-methyl-2-dodecenoic acid (Ryan & Dow, 2011, Wang et al., 2004) and its synthesis requires RpfF. Previously we studied an RpfF homologue, Bcam0581, from the opportunistic human pathogen Burkholderia cenocepacia which is functionally interchangeable with RpfF and makes BDSF, a DSF homologue that lacks the terminal methyl group (Bi et al., 2012). Bcam0581 was shown to be a bifunctional enzyme that catalyzed not only dehydration of 3-hydroxydodecanoyl-acyl carrier protein (ACP) to cis-2-dodecenoyl-ACP, but also cleaved the thioester bond to give the free acid, BDSF (Bi et al., 2012). Both reactions were dependent on the same set of active site residues (Bi et al., 2012). Although dehydratase and thioesterase activities are known activities of the crotonase superfamily, Bcam0581 was the first protein shown to have both activities. Although the dehydratase activity was required to introduce the BDSF double bond and the thioesterase activity frees the acid from its linkage to ACP to allow it to diffuse from the cell, the thioesterase activity was not restricted to cis-2-dodecenoyl-ACP and cleaved a wide variety of acyl-ACPs (Bi et al., 2012). The lack of specificity argued that thioesterase activity would be detrimental to membrane lipid synthesis in BDSF/DSF producing bacteria. For this reason we argued that in vivo the dehydratase and thioesterase activities might be coupled to make the dehydration reaction irreversible and thereby avoid the wasteful cleavage of acyl-ACPs destined for membrane lipid synthesis (Bi et al., 2012). However, recent results argued that our hypothesis was incorrect and that nonspecific thioesterase activity is a hallmark and unavoidable consequence of high-level DSF synthesis. Almeida and coworkers (Almeida et al., 2012) reported that culture supernatants of wild type strains of Xcc and Xylella fastidiosa contain fatty acids whereas strains of both bacteria lacking RpfF show greatly reduced fatty acid accumulation. A similar result had been reported in another DSF-producing bacterium, Stenotrophomonas maltophilia (Huang & Wong, 2007). Although the Xcc data were qualitative and the fatty acids were not identified, inactivation of the rpfB gene located immediately upstream of rpfF in Xcc resulted in an altered thin layer chromatographic profile of fatty acid extracted from the medium (Almeida et al., 2012). RpfB is annotated as encoding a fatty acyl-CoA ligase (FCL: EC 6.2.1.3), also known as fatty acid:CoA synthetase. This well-studied enzyme family (Gulick, 2009, Black & DiRusso, 2003) plays a crucial role in intermediary metabolism by activating free fatty acids to their CoA thioesters which can be used as phospholipid acyl donors in bacteria that utilize the PlsB sn-glycerol 3-phosphate acyltransferase (Zhang & Rock, 2008). Therefore, if RpfB is indeed an FCL then its role could be to activate and thereby recycle the RpfF-released fatty acids into the lipid synthetic pathway. That is, RpfB could counteract the thioesterase activity of RpfF by restoring the thioester bond. Given that RpfB is 60% identical to E. coli FadD it seemed reasonable that RpfB encodes an FCL, although very similar enzymes are known that do not synthesize acyl-CoAs (Gulick, 2009). We report that RpfB is an authentic FCL that plays a role in fatty acid β-oxidation. However, it plays a more important role in pathogenesis by counteracting the thioesterase activity of RpfF.
RESULTS
RpfB is an FCL
The FCL catalytic mechanism proceeds in two steps (Gulick, 2009). In the activation step ATP is used to convert the substrate fatty acid to its acyl-adenylate (acyl-AMP) which is stably bound in the active site. The thiol of CoA then attacks the acyl-adenylate mixed anhydride to form the acyl-CoA plus AMP. Two highly conserved sequence elements, that comprise the ATP/AMP-binding signature motif, were identified based on sequence comparisons of adenylate forming enzymes sharing this catalytic property. Within the family of the enzymes there was a third sequence element of this signature that was less well conserved and partially overlaps the FCL signature motif. Our sequence alignments (Fig. 1B) showed that the ATP/AMP and FCL signature motifs identified for the E. coli FadD (Black & DiRusso, 2003, Gulick, 2009, Weimar et al., 2002) and an FCL of known crystal structure, that of Thermus thermophilus HB8 (Hisanaga et al., 2004) are highly conserved in the Xcc RpfB consistent with the hypothesis that RpfB is a FCL.
Figure 1. Organization of the rpf genes and the FCL motifs of RpfB.

(A) Transcriptional organization of the rpf genes. The thick arrows indicate the relative size and transcriptional direction of the genes. The rpfC and rpfG genes encode a two-component regulatory system; rpfH encodes a membrane protein having amino acid sequence similarity to the sensory input domain of RpfC; rpfF encodes a bifunctional crotonase homologue having both dehydratase and thioesterase activities. (B) Sequence alignments of the ATP/AMP and FCL motifs of the RpfB, FadD and T. thermophilus enzymes. The solid circles denote the active-site threonine and glutamate residues.
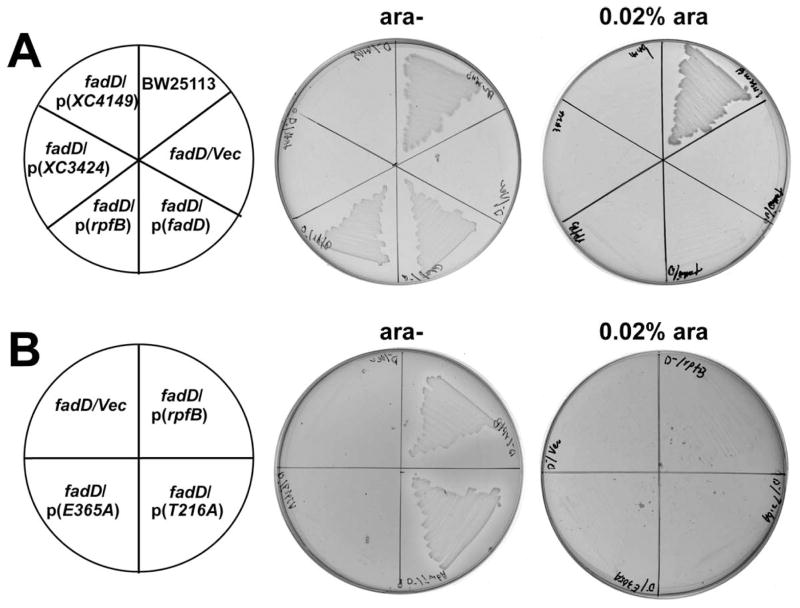
To test if RpfB can function in the uptake and activation steps of fatty acid degradation (β-oxidation) we expressed Xcc RpfB in the E. coli ΔfadD strain (JW1794) and tested for complementation. We also tested two other Xcc genes annotated as encoding FCLs, XC_3424 and XC_4149. Only rpfB functionally complemented the E. coli ΔfadD strain (Fig. 2A) and allowed growth on fatty acids as sole carbon source. As also seen in the positive control with EcFadD complementation was only seen in the absence of the arabinose inducer indicating that low level expression was sufficient for growth whereas high level expression of either enzyme was toxic (Fig. 2A).
Figure 2. Low-level expression of RpfB complements growth of an E. coli fadD strain on oleate.
Transformants of strain JW1794 (an E. coli ΔfadD ΔaraBAD strain) were grown at 37°C on minimal medium with oleate as the sole carbon source. Growth was tested in either the presence or the absence of arabinose. The strains tested were: (A) BW25113 (WT), JW1794 carrying plasmids pBHK205, pBHK206, pBHK207, or pBHK467 encoding rpfB, XC_ 3424, XC_4149 or E. coli fadD (EcfadD), respectively, or the vector plasmid, pBAD24M. (B) Strain JW1794 carrying plasmids pBHK205, pBHK285 or pBHK286 encoding rpfB, T216A or the E365A rpfB mutant, respectively, or the vector plasmid, pBAD24M.
In previous work residue substitutions within the ATP/AMP signature motif of E. coli FadD identified specific residues critical for catalytic activity (Weimar et al., 2002). To test this for RpfB, we also substituted alanine residues for RpfB residues Thr-216 and Glu-365 of the ATP/AMP signature motif by site-directed mutagenesis to give plasmids pBHK285 (RpfB T216A) and pBHK286 (RpfB E365A). The growth of E. coli strain JW1794 carrying plasmids encoding the mutant proteins was tested on oleate (Fig. 2B). The strain expressing the T216A mutant protein grew in the absence of arabinose, whereas the strain expressing the E365A mutant protein failed to grow either in the presence or in the absence of arabinose. These results demonstrated that Glu-365 is required for RpfB FCL activity in agreement with previous result that substitution of this glutamate to alanine in FadD results in complete loss of enzyme activity (Weimar et al., 2002). Note that T216 is not strictly conserved in the family of acyl-adenylate forming enzymes (Gulick, 2009). These data argue that RpfB has sufficient activity to accumulate high levels of activated fatty acids derived from the culture medium.
The effect of deletion of rpfB on growth on fatty acids
Since RpfB functionally replaced the E. coli FadD β-oxidation protein we asked if RpfB could function in Xcc fatty acid utilization. We generated an ΔrpfB strain (Fig. S1) and tested for growth of the strain on various fatty acids as sole carbon sources (Table 1). The ΔrpfB strain grew well on glucose, glycerol or acetate, but completely failed to grow on any fatty acid tested. The strain complemented with an rpfB plasmid had growth phenotype similar to that of the wild type Xcc strain 8004 which grew well on fatty acids of chain length C12:0 or longer. As expected, the ΔrpfB ΔrpfC double mutant and the ΔrpfB ΔrpfC ΔrpfF strains were also completely defective in fatty acid utilization. In contrast, the ΔrpfC and ΔrpfF strains grew well on the fatty acids tested as well as on the other carbon sources (Table 1). These results indicated that RpfB is required for the utilization of fatty acids as a sole carbon and energy source in addition to its role in the regulator of pathogenicity factor pathway.
Table 1.
Aerobic growth of Xcc strains on fatty acids of varying chain length.
| C-source | WT | ΔrpfB | ΔrpfC | ΔrpfF | ΔrpfB ΔrpfC | ΔrpfB ΔrpfC ΔrpfF | ΔrpfB ΔrpfC/p(rpfB) | ΔrpfB/p(rpfB) |
|---|---|---|---|---|---|---|---|---|
| GLU | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ |
| GLY | +++ | ++ | + | ++ | ++ | + | ++ | +++ |
| ACE | ++ | + | + | + | + | + | + | + |
| 8:0 | − | − | − | − | − | − | − | − |
| 10:0 | − | − | − | − | − | − | − | − |
| 12:0 | + | − | + | + | − | − | + | + |
| 14:0 | + | − | + | + | − | − | + | + |
| 18:1 | ++ | − | ++ | + | − | − | ++ | ++ |
Xcc strains were grown at 30 °C on XVM2 plates supplemented with 0.2% glucose (Glu), 0.4% glycerol (Glu), 0.4% acetate (Ace) or 1 g l−1 fatty acid. The fatty acids were octanoate (8:0), decanoate (10:0), dodecanoate (12:0), tetradecanoate (14:0) or oleate (18:1). Growth was usually obvious after 2–4 days of aerobic growth. Growth was scored + if growth after 4 days was obvious or minus when no colonies were apparent after 6 days of incubation. Aerobic growth (after 2 days) on a given carbon source was given a score of +++. IPTG (0.5 mM) was added into the plates of plasmid (pYH-5 expressing RpfB)-containing strains for induction. The ΔrpfB ΔrpfC strain carrying plasmid pBHK476 encoding AasS failed to grow on any fatty acid as expected from its synthesis of ACP thioesters rather than the CoA thioesters required for β-oxidation.
RpfB purification and properties
A version of RpfB with an N-terminal hexahistidine tag was readily expressed in E. coli and purified to homogeneity by affinity chromatography using a nickel-chelating column followed by size exclusion chromatography where the RpfB elution profile showed that RpfB exists in three forms in solution (monomer, trimer and hexamer) (Fig. S1A & B). The purified proteins collected from each form had the same apparent monomeric molecular mass of 64 kDa (Fig. S1C). Liquid chromatography mass spectrometry of tryptic peptides validated the identification of the recombinant protein with 78% coverage of the peptides predicted from the DNA sequence (Fig. S1D).
RpfB enzymatic activity was monitored by use of radiolabelled octanoate or oleate (Fig. 3A). In the absence of enzyme no products were formed. When [1-14C]octanoate was the substrate in the absence of CoA RpfB formed a barely detectable product having an retention factor (Rf) value of 0.54 as expected for an acyl-adenylate (Trivedi et al., 2004) the production of which is essentially stoichiometric with enzyme active sites. When CoA was added to the reaction mixture, the Rf of the product shifted to 0.43, the migration position of an acyl-CoA (Trivedi et al., 2004). Similarly, when [1-14C]oleate was provided as a fatty acid substrate, RpfB catalyzed formation of a product having an Rf value of 0.54 in the presence of CoA, although in the absence of CoA, RpfB reactions failed to accumulate detectable levels of oleoyl-AMP (Fig. 3A). These data indicate that the RpfB FCL reaction proceeds by the canonical acyl-AMP intermediate. The products obtained upon addition of CoA were analyzed by liquid chromatography (LC) and were identified as octanoyl-CoA and oleoyl-CoA by electrospray ionization mass spectrometry (Fig. 3B and 3C).
Figure 3. Chemical characterization of the RpfB products.
(A) Thin layer chromatographic analysis of the products of purified RpfB. The fatty acid substrates were [1-14C]octanoate (left plate) or [1-14C]oleate (right plate) and assays were done in the presence or absence of CoA. Lane 1 and 4 lacked enzyme and CoA, whereas lanes 2 and 5 lacked CoA. (B) Electrospray ionization mass spectrometry spectrum for the RpfB product formed from oleic acid. The mass/charge (m/z) peak at 1030.5 corresponds to the molecular ion peak [M-H]−1 of oleoyl-CoA. (C) Electrospray ionization mass spectrometry spectrum for the RpfB product formed from octanoic acid. The mass/charge (m/z) peak at 892.4 corresponds to the molecular ion peak [M-H]−1 of octanoyl-CoA.
Fatty acid substrate specificity of RpfB
FadD has been shown to be a medium- and long-chain (C10–C18) FCL (Black & DiRusso, 2003, Morgan-Kiss & Cronan, 2004, Weimar et al., 2002, Overath et al., 1969). To determine the substrate specificity of RpfB, we assayed FCL activity by the loss of the free CoA thiol. RpfB had high activity on short (C8), medium and long chain fatty acids (Fig. 4), indicating that this is a broad chain-length FCL. In contrast, RpfB FCL activity was very low with DSF fatty acid substrates (Fig. 4). Indeed, DSF plate bioassays (Barber et al., 1997, Bi et al., 2012, Ionescu et al., 2013, Jiang et al., 2006) showed that the ΔrpfB strain or RpfB-overproduction strains did not show any detectable change in DSF production (data not shown). Therefore, RpfB is not involved in DSF metabolism.
Figure 4. Fatty acyl chain-length specificity of RpfB.
Enzyme activity (nmol of product formed per min/mg of protein) was assayed as described under Experimental procedures. Values represent the mean ± S.D. (n=4). C18:1 is oleic acid and the Me acids are iso-branched chain fatty acids.
The kinetic properties of RpfB for oleate and octanoate were also examined. RpfB had Vmax values of 83.3 and 71.4 nmol acyl-CoA formed/min/mg of protein for octanoic and oleic acids, respectively, whereas the respective Km values were 60.1 and 49.2 μM. In agreement with the fadD complementation results (Fig. 2B) the E365A RpfB had barely detectable Vmax values (<0.4% of the wild type protein) on the two substrates and thus Km values could not be obtained. We also tested the ability of Xcc ACP to replace CoA as the acyl acceptor and found ACP to be completely inactive as a RpfB substrate (Fig. S4) and thus RpfB cannot directly reverse the RpfF thioesterase activity by conversion of fatty acids back to acyl-ACPs.
The ΔrpfB ΔrpfC strain shows a severe defect in growth and viability upon entry into stationary phase which is relieved by deletion of rpfF
The growth phenotypes of the rpf deletion strains were tested using minimal media to examine the role of rpfB in fatty acid β-oxidation. We also grew these mutant cells in NYG medium to analyze growth phenotypes (Fig. 5A). The singly deleted ΔrpfB, ΔrpfC or ΔrpfF mutant strains grew similarly to the wild type strain. However when the various double deletion strains were assayed, the optical density of the ΔrpfB ΔrpfC strain began to decrease after 24 h of growth and by 48 h the cultures appeared to be completely lysed. Viable counts showed that virtually all of the cells of these cultures were unable to form colonies (Fig. 5B). The defective growth and loss of viability of the ΔrpfB ΔrpfC strain in liquid culture was completely rescued by rpfB expression. The growth of the strain carrying the plasmid pYH-5 (expressing rpfB) was indistinguishable from that of the ΔrpfC mutant strain. Moreover, the ΔrpfB ΔrpfC ΔrpfF triple deletion strain grew similarly to the wild type strain, suggesting that the ΔrpfF mutation suppressed the growth and viability defects of the ΔrpfB ΔrpfC strain observed upon entry into stationary phase (Fig. 5A and 5B). Thus high levels of RpfF thioesterase activity engendered by the lack of RpfC seemed likely to trigger the severe growth defect of the ΔrpfB ΔrpfC strain. Note that inactivation of RpfC gives a 16-fold increase in DSF production (Deng et al., 2011). Note also that the mechanism whereby RpfC blocks RpfF activity remains unsettled because RpfC is a complex protein containing several sensory domains (Ryan & Dow, 2011). However, overexpression of only the REC domain of RpfC abolishes DSF biosynthesis (He et al., 2006) and a high-resolution crystal structure of the RpfF-REC domain complex has been obtained (Cheng et al., 2010). An illustration of the complexity of this regulatory system is a recent report that deletion of rpfC results in increased transcription of rpfF (An et al., 2013). RpfF has a high level of non-specific thioesterase activity active on acyl-ACPs (Bi et al., 2012). This suggested that when the activity of RpfF is high, such as in the ΔrpfC mutant background, the absence of RpfB would lead to the accumulation of free fatty acids that could not be activated to their acyl-CoA thioesters. These events could offer a possible explanation for the inhibition of cell growth and cell lysis that occurs in the ΔrpfB ΔrpfC strain. In order to test this hypothesis we labelled newly synthesized fatty acids with [1-14C]acetate to test if free fatty acids released by the thioesterase coupled with failure of activation to their acyl-CoAs (due to loss of RpfB) could explain the inhibition of cell growth and cell lysis. After growth of the wild type and Δrpf strains in the presence of [14C]acetate the medium was collected from log and stationary phase cultures and the free fatty acids were extracted. After conversion of the acids to their methyl esters, the esters were analyzed by argentation thin-layer chromatography (Fig. 5C and 5D). We found that the level of de novo synthesized fatty acids including saturated, branched and unsaturated fatty acids secreted to the culture medium was much higher in the ΔrpfB ΔrpfC strain than in the wild type strain and the ΔrpfB or ΔrpfC strains (Fig. 5C and 5D). Moreover, introduction of the ΔrpfF mutation into the ΔrpfB ΔrpfC strain greatly diminished the levels of free fatty acids released to the medium as also seen in the strain carrying the plasmid pYH-5 expressing RpfB. No detectable accumulation of free fatty acids was observed from the medium of the mutant strain containing only the ΔrpfF deletion (Fig. 5C & 5D). Expression of an inactive form of RpfB (the E365A RpfB mutant protein discussed above) in the ΔrpfB ΔrpfC strain failed to decrease the levels of free fatty acids released to the medium (Fig. 5D). These data indicated that the growth defect of the ΔrpfB ΔrpfC strain upon entry into the stationary phase seemed likely to result from the high level of free fatty acids released to the medium by robust RpfF thioesterase activity in the absence of the RpfB FCL activity. We also analyzed the profile of free fatty acids secreted into the culture medium of the ΔrpfB ΔrpfC strain by gas chromatography-mass spectroscopy and found the main components to be three long-chain acids, Me-C14:0, C16:1 and C16:0 (Fig. S3). Note that the ΔrpfB strain is not sensitive to the presence of fatty acids in the medium. The ΔrpfB strain grew normally in NYG medium containing 5 mM oleic acid (data not shown).
Figure 5. Growth of the Xcc Δrpf mutant strains on NYG medium and argentation thin-layer chromatographic analysis of [1-14C]acetate-labelled esters isolated from the medium of Xcc Δrpf mutant strains.
(A) Each growth assay was carried out in triplicate in NYG medium at 30°C and the averages are given. Cultures of the ΔrpfB ΔrpfC strain carrying the plasmids pBHK476, pYH-5 or pBHK497 which express Vibrio harveyi AasS, RpfB or the E365A mutant of RpfB, respectively, were induced with IPTG (0.5 mM). (B) Viability of the strains of (A) sampled through the growth curve by plating serial dilutions of the cultures on NYG plates (the data are the mean of results from three independent experiments). The colour codes of (A) and (B) are identical. The hash tag indicates no viable cells were present in the sample. Samples (2 ml) of the cultures grown in NYG medium to log phase (C) or stationary phase (D) were taken and the fatty acid methyl esters were obtained from the medium as described in Experimental procedures. The methyl esters were then separated by argentation thin-layer chromatography followed by autoradiography. The migration positions of the fatty acid species are shown. Line 1 in C and D indicate the phospholipid-derived fatty acid methyl esters from E. coli strain YYC1273. Sat, saturated fatty acid esters. Note that acetate labeling is insufficiently sensitive to detect DSF. The labelled compound at the top of the plate is the extremely hydrophobic isoprenoid xanthomonadin (Xmon), the C28 brominated pigment characteristic of the Xanthomonads. The appearance of Xanthomonadin in our lipid extractions is idiosyncratic. The pigment seems to turn over rapidly and may partition into fatty acid micelles.
RpfB counteracts the thioesterase activity of RpfF
To further test the hypothesis that RpfB FCL activity acts to counter RpfF thioesterase activity by allowing maintenance of cell membrane integrity, we constructed a plasmid (pYH-8) carrying rpfF under control of an IPTG-inducible promoter and transformed it into the wild-type and ΔrpfB mutant strains to compare the growth phenotypes (Fig. 6A). Both strains carrying plasmid pYH-8 grew well and showed no growth defect. Although expression of rpfF from the plasmid in the ΔrpfB mutant failed to produce the extreme stationary phase growth defect seen in the ΔrpfB ΔrpfC strain, it remained possible that sufficient RpfC was present to neutralize the plasmid-encoded RpfF. To exclude this possibility we transformed plasmid pYH-8 into both the ΔrpfC and ΔrpfB ΔrpfC strains and analyzed the growth phenotypes of the transformants. We found that the ΔrpfC strain carrying plasmid pYH-8 grew fairly well and retained greater viability in stationary phase relative to the ΔrpfB ΔrpfC strain containing the plasmid pYH-8 which showed a severe growth defect and massive loss of cell viability upon entry into the stationary phase (Fig. 6A & 6B) indicating that RpfB is required to counteract RpfF thioesterase activity.
Figure 6. Overexpression of RpfF in various Xcc strains.
(A) Growth of the Xcc wild type and Δrpf mutant carrying the plasmid pYH-8 was carried out in triplicate in NYG medium at 30°C and the mean is given. Cultures of plasmid-containing strains were induced with IPTG (0.5 mM). (B) Viability of the strains of (A) sampled through the growth curve by plating serial dilutions of the cultures on NYG plates (the data are the mean of results from three independent experiments). The colour codes of (A) and (B) are identical. The hash tags indicate that no viable cells were present in the sample. [1-14C]Acetate-labelled fatty acyl esters derived from the phospholipids (C) or present in the medium (D) of Xcc strains YH2 and YH3 carrying plasmid pYH-8 were analyzed by argentation thin-layer chromatography. Samples (2 ml) of the cultures were taken at different time points. In Fig. 6D as discussed in the text the fatty acids of lane 5 result from cleavage of nascent acyl-ACPs labelled prior to re-suspension in unlabelled medium whereas the fatty acids of lanes 6–8 result from the PldA-catalyzed cleavage of the phospholipids of lysed cells. The fatty acid methyl esters were obtained as described in Experimental procedures and separated by argentation thin-layer chromatography followed by autoradiography. The migration positions of the fatty acid species are shown. Sat, saturated fatty acid esters; UFA, unsaturated fatty acid esters.
To monitor phospholipid synthesis and free fatty acid release, the ΔrpfC and ΔrpfB ΔrpfC strains carrying plasmid pYH-8 were grown with [1-14C]acetate labeling from 10 to 30 h of the growth curve at which time the cells were collected, washed with NYG medium, suspended in the same volume of fresh nonradioactive NYG medium and allowed to resume growth. The cultures were sampled at different time points (38, 48 and 58 h) in the growth curve. The phospholipids were extracted from the cell pellets whereas the free fatty acids were extracted from the medium. After conversion to their fatty acid methyl esters, the labelled species were resolved by thin layer chromatography TLC, and visualized by autoradiography (Fig. 6C & 6D). In the ΔrpfC strain expressing the plasmid borne RpfF there was no detectable accumulation of fatty acids in the medium whereas in the strain lacking RpfB labelled fatty acids were found in the medium (Fig 6D). However, the fatty acids in the medium resulted from two different processes, cleavage of labelled nascent acyl-ACPs which was seen only immediately after resuspension in unlabelled medium (30 h time point, lane 5 of Fig. 6D) and phospholipid hydrolysis caused by lytic damage to the outer membrane. The outer membranes of highly diverse gram-negative bacteria contain a phospholipase called PldA (Brok et al., 1998). This is one of several hydrolytic enzymes that become active only upon membrane damage (Bishop, 2008). PldA is an extraordinarily robust enzyme that cleaves both fatty acid moieties from the phospholipid backbone. Since a locus (XC_2818) of the Xcc 8004 genome encodes a 41% identical homologue of E. coli PldA containing all of the active site residues defined by the crystal structure of the E. coli enzyme (Bishop, 2008), we expected that phospholipid hydrolysis would occur upon lysis of Xcc. Indeed, the later time points of Fig. 6D (lanes 6–8) show that labelled fatty acids accumulate in the medium in the absence of [1-14C]acetate indicating that the acids must come from previously labelled products which a comparison of lane 8 of Fig. 6C with lane 8 of Fig. 6D clearly demonstrates to be the membrane phospholipids. Labelled fatty acids are only barely detectable at the 38 h time point when the cultures have just entered stationary phase and only a few cells have lysed whereas by 58 h, when lysis is essentially complete, the most of the phospholipids had been hydrolyzed.
Vibro harveyi AasS supports normal growth of the ΔrpfB ΔrpfC strain by activation of free fatty acids
Based on the above data, we expected that the growth recovery of the ΔrpfB ΔrpfC strain in stationary phase depended on the uptake and utilization of the free fatty acids released to the medium. This hypothesis was tested by expression of V. harveyi acyl-ACP synthetase (AasS). Conversion of the released free fatty acids to acyl-ACPs would allow these acyl chains to enter the Xcc lipid synthetic pathway as previously observed in E. coli (Jiang et al., 2006, Jiang et al., 2010). Labeling with [1-14C]acetate showed no detectable accumulation of free fatty acids in the medium of the ΔrpfB ΔrpfC strain expressing AasS (Fig. 5C & 5D). This strain grew similarly to the wild type strain (Fig. 5A) indicating that the recovery of normal growth of the ΔrpfB ΔrpfC strain resulted from Vibro AasS expression. Hence, activation of the free fatty acids to their acyl-ACP esters resulted in restoration of membrane lipid synthesis. Note that unlike acyl-CoAs, acyl-ACPs are not substrates of the β-oxidation pathway.
DISCUSSION
In our prior studies of BDSF and DSF synthesis (Bi et al., 2012) we proposed that the thioesterase activity observed with the two synthetic enzymes, Bcam0581 and RpfF, might be an artifact of our in vitro conditions. However, the report of Almeida and coworkers (Almeida et al., 2012) indicated that RpfF activity results in the appearance of free fatty acids in the culture medium of the producing bacterium. Hence, DSF synthesis concomitantly results in thioesterase-mediated release of fatty acids in addition to DSF. It seems that this bacterium is willing to tolerate marked disturbance of its membrane lipid synthetic pathways in order to produce the DSF regulatory fatty acid. If the thioesterase was specific for the ACP thioester of DSF, this disturbance would be avoided. However, this is not the case; the RpfF and Bcam0581 thioesterase activities cleave a broad range of acyl-ACP substrates (Bi et al., 2012).
We report that RpfB is an FCL that counteracts RpfF thioesterase activity by allowing Xcc to recapture fatty acids from the medium. This is energetically costly because acyl-CoA synthesis requires ATP hydrolysis, but the cost is much less than loss of a long chain fatty acid (synthesis of an sixteen carbon fatty acid requires hydrolysis of 15 ATP equivalents). Moreover, the bulk of the recaptured acyl chains are sufficiently long to be directly incorporated into membrane phospholipids by the PlsB/PlsC pathway of gram negative bacteria which can utilize either acyl-CoA or acyl-ACP thioesters (Zhang & Rock, 2008). Moreover, a foreign activating enzyme having a different thiol specificity, the Vibrio harveyi acyl-ACP synthetase (AasS), replaced the function of RpfB in counteracting RpfF. Note that both DSF and BDSF are essentially inactive as RpfB substrates (Fig. 4) indicating that the cis-2 double bond precludes recapture of these acids. Hence, Xcc avoids cancelling the role of the RpfF thioesterase activity in release of the free acids by converting them back to thioesters.
It is clear that RpfB is an efficient scavenger of exogenous fatty acids because virtually no long chain fatty acids are found in the medium when the enzyme is active. Moreover, the enzyme is sufficiently robust to provide the activation step required for growth on fatty acids as sole carbon sources. The Xcc genome encodes homologues (30–55% identity) of each of E. coli proteins required for β-oxidation of long chain fatty acids. These genes are scattered around the genome as is the case in E. coli, although it is interesting that RpfB supports β-oxidation despite being encoded in the pathogenicity factor gene cluster. Although it seems possible that rpfB was recruited from elsewhere in the genome to offset the RpfF thioesterase, there are no evidence that co-localization with rpfF is important. Indeed, in Xylella fastidiosa rpfB is not encoded within the pathogenicity factor gene cluster (Almeida et al., 2012).
The pattern of fatty acids utilized as sole carbon sources by Xcc is very similar to that of E. coli (Campbell & Cronan, 2002, Campbell et al., 2003). In E. coli the pattern is dictated by the specificity of acyl-CoA binding by the FadR transcription factor which represses transcription of the β-oxidation regulon genes. However, Xcc lacks a FadR homologue as well as homologues of other fatty acid metabolism regulatory genes (Kazakov et al., 2009). Hence, the mechanism responsible for the fatty acid specificity of the Xcc β-oxidation pathway remains to be determined.
The most significant finding of this work is that RpfB was shown to have an essential role in lipid synthesis when Xcc enters the stationary phase of growth. RpfB activates and thereby traps the free fatty acids released by RpfF thioesterase activity by conversion to their CoA esters. These acyl-CoA esters serve as a required supplement to the thioesterase-compromised de novo fatty acid pathway (Fig. 7). The resulting acyl-CoAs are incorporated into membrane phospholipids or possibly degraded to acetyl-CoA by β-oxidation (although if higher status carbon source are available, the β-oxidation pathway is probably not expressed). Loss of RpfB function together with increased RpfF activity results in defective growth and lysis in stationary phase (Fig. 5 & 6). The cell lysis observed in stationary phase is an expected consequence of defective membrane lipid synthesis based on studies with other gram-negative bacteria. A plausible explanation for the lysis that occurs in stationary phase is that lipid synthesis (as well as most other synthetic pathways) necessarily slows as cultures enter stationary phase and thus the fatty acids lost to the medium could become a more serious drain.
Figure 7. Proposed model for the interplay of RpfB and RpfF.

The free fatty acid RpfB substrates are produced by the RpfF thioesterase activity and may be transported through the outer membrane by a FadL-like protein. RpfB then convert these acids to their CoA esters to serve as a supplement to those fatty acyl-ACPs synthesized which escape thioesterase action. Note that either acyl-CoAs or acyl-ACPs can be utilized by the PlsB and PlsC acyltransferases for acylation of sn-glycerol-3-phosphate (G3P) to phosphatidic acid, the key precursor of membrane phospholipids (Zhang & Rock, 2008). The efflux pump(s) responsible for efflux of DSF and other fatty acids are unknown. Studies in E. coli indicate that multiple pumps may be utilized (Lennen et al., 2013).
We also expressed Vibrio harveyi acyl-ACP synthetase (AasS) to test if it could recapture fatty acid from the medium and restore normal growth. This was successful indicating that the differing products of RpfB and AasS (acyl-CoAs or acyl-ACPs) are without consequence because either species can be utilized by the acyltransferases which catalyze the acylation steps of phospholipid biosynthesis (Zhang & Rock, 2008). Thus, RpfB provides essential fatty acyl-CoA esters as the substrates for phospholipid biosynthesis to maintain cell growth especially when cells grow into the stationary phase. In addition, we analyzed the profile of fatty acid composition from Xcc wild type and Δrpf mutant strains and found that Rpf expression does not affect Xcc fatty acid composition (Table S3).
Our results confirmed prior indications that RpfB is not directly involved in DSF metabolism. Our current scenario is that as Xcc grows, most of the free fatty acids released by RpfF thioesterase activity are activated by RpfB to restore membrane biogenesis whereas the DSF molecules released by the combined dehydratase and thioesterase activities of RpfF are ignored by RpfB and accumulate as the free acids required for cell signaling.
Experimental procedures
Materials
Sodium [1-14C]acetate, [1-14C]octanoic acid and [1-14C]oleate were purchased from Moravek. Advanced Synthesis Technologies and Cayman Chemicals synthesized cis-2-dodecenoic and cis-11-methyl-2-dodecenoic acids, respectively. Antibiotics and most other chemicals were purchased from Sigma. Qiagen provided plasmid isolation and PCR product purification kits plus Fast T4 DNA ligase. Oligonucleotide primers were synthesized by Integrated DNA Technologies and DNA sequencing was done by AGCT. Invitrogen provided Ni2+-agarose columns.
Bacterial strains, plasmids and growth conditions
Bacterial strains and plasmids used in this study are listed in Table S1. The Xcc strain was X. campestris pv. campestris 8004. E. coli K-12 strains were grown at 37°C in Luria–Bertani (LB) medium (tryptone, 10 g l−1; yeast extract, 5 g l−1; NaCl, 10 g l−1; pH 7.0). Xcc was grown at 30°C in NYG medium (Daniels et al., 1984). For testing the utilization of fatty acids, Xcc strains were grown on a minimal medium based on XVM2 medium (Wengelnik et al., 1996) and having the following composition: 20 mM NaCl, 10 mM (NH4)2SO4, 5 mM MgSO4, 1 mM CaCl2, 0.16 mM KH2PO4, 0.32 mM K2HPO4, 0.01 mM FeSO4, 0.01% Casamino Acids and fatty acids of different chain-lengths at 1 g l−1. When required, antibiotics were added as follows (in μg ml−1): sodium ampicillin, 100, kanamycin sulfate, 50 and tetracycline HCl, 15 for E. coli; rifampicin, 50, kanamycin sulfate, 20 and tetracycline HCl, 5 for Xcc. Bacterial growth was determined by measuring optical density at 600 nm.
The pMD19 vector (Takara) was used for cloning PCR products and Xcc rpfB was inserted into this vector to give plasmid pYH-6. The pYH-6 NdeI (CATATG) site in rpfF was mutated to CACATG (synonymous mutation) to obtain pYH-7 by use of the QuickChange site-directed mutagenesis kit (Stratagene). The NdeI-HindIII fragment of pYH-7 was inserted to pSRKKm digested with the same enzymes to give pYH-8. To construct the plasmid pBHK486, Vibrio harveyi aasS from pYFJ84 digested with BamHI and NdeI was inserted into pSRKKm at the same sites.
Complementation of a E. coli ΔfadD strain
The fadD mutant strain (JW1794) was used for the complementation. To construct the E. coli complementation vectors, coding regions of Xcc rpfB, XC_3424 and XC_4149 were amplified from Xcc 8004 chromosomal DNA using primer sets rpfB-f/rpfB-r, XC3424-f/XC3424-r and XC4149-f/Xc4149-r, respectively. PCR products were then digested with NdeI and HindIIII and ligated individually into pBAD24M (Zhu et al., 2010), digested with the same enzymes, yielding plasmids pBHK205, pBHK206 and pBHK207. As a control for the complementation study, the E. coli fadD gene was also amplified from strain K12 chromosomal DNA using primer set fadD-r/fadD-r. The 1.8-kb PCR product was digested with EcoRI and HindIII, and ligated with pBAD24 digested with the same enzymes, yielding pBHK467. These four recombinant vectors above were introduced into E. coli strain JW1794 for complementation. JW1794, harboring each complementation vector, was patched on M9 minimal medium supplemented with 0.1% oleate and growth was determined after 3 days incubation at 37 °C (Fig. 2).
Site-directed mutagenesis of rpfB
Plasmids pBHK285 and pBHK286 carrying a single mutation within the RpfB coding sequence were obtained using the QuickChange mutagenesis kit with pBHK205 as the PCR template. The primers used in PCR and in mutagenesis are listed in Table 1. The constructed plasmids were transformed into E. coli DH5α by CaCl2 treatment. The mutations were verified by DNA sequencing. These two RpfB mutant plasmids were then introduced into E. coli strain JW1794 for complementation study.
Deletion of Xcc rpf genes
To delete Xcc rpfB a suicide plasmid was constructed. The 500 bp regions upstream and downstream of rpfB (called Up rpfB and Down rpfB, respectively) were amplified with Pfu DNA polymerase using Xcc strain 8004 genome DNA as the template and either XcrpfB1and XcrpfB2 (for Up rpfB) or XcrpfB3 and XcrpfB4 (for Down rpfB) as the primers (Table S2). The products of these PCR reactions were purified, and overlapping PCR was carried out using XcrpfB1and XcrpfB4 as the primers. A 1000 bp DNA fragment digested with BamHI and HindIII was inserted between the same sites of pK18mobsacB to yield pYH-1 (carrying the rpfB deletion cassette).
Following mating of derivatives of E. coli strain S17-1 carrying pYH-1 with Xcc strain 8004 on NYG plates for 36 h at 30°C, the cells were suspended in NYG medium, and appropriate dilutions were spread onto NYG plates containing rifampicin (to select against the donor strain) and kanamycin to select for the integration of the non replicating plasmid into the chromosome of the recipient. Several colonies were inoculated into NYG medium and the cultures were incubated at 30°C for 36 h after which appropriate dilutions were spread onto NYG plates containing 10% sucrose. Colonies sensitive to kanamycin and gentamicin were screened by colony PCR utilizing the primers listed in Table S2. Finally, mutant strain YH1 (ΔrpfB) was obtained. Using parallel methods we also obtained strains YH2 (ΔrpfC), YH3 (ΔrpfB rpfC) and YH4 (ΔrpfB ΔrpfC ΔrpfF). All deletion events were confirmed by PCR using appropriate primers followed by sequencing of the PCR products.
Protein expression and purification
The rpfB gene amplified from Xcc 8004 genomic DNA with a N-terminal hexahistidine (His)-tag was inserted into vector pET-28b to give plasmid pBHK268. RpfB was expressed in E. coli Tuner (BL21) grown at 37°C in LB medium. At an OD600 of 0.8, the cultures were induced with 0.1 mM isopropyl-β-D-thio-D-galactoside (IPTG) and grown at 18°C for an additional 10 h prior to harvest. The cells were collected, resuspended in lysis buffer (50 mM sodium phosphate, 300 mM NaCl, 10 mM imidazole, 1 mM dithiothreitol, pH 7.4), lysed by French pressure cell treatment and centrifuged. The clarified bacterial supernatant was loaded onto a nickel-ion affinity column (Qiagen). The column was washed with wash buffer (50 mM NaH2PO4, 300 mM NaCl, 40 mM imidazole, 1 mM dithiothreitol, pH 7.4) to remove contaminant proteins and the His-tagged RpfB protein was eluted in the same buffer (elution buffer) containing 200 mM imidazole. The protein was concentrated by ultrafiltration (10 kDa cutoff) and exchanged into sodium phosphate buffer (50 mM NaH2PO4, 150 mM NaCl, 1 mM dithiothreitol, pH7.4). The purity of the samples was monitored by SDS-PAGE. The solution structure of RpfB was analyzed by size exclusion chromatography on a Superdex 200 10/300 GL column (GE Healthcare) using an AKTA purifier10 at 0.4 ml/min in phosphate running buffer (135 mM NaCl, 2.7 mM KCl, 1.5 mM Na2HPO4, and 8 mM K2HPO4, 10% glycerol, pH 7.4). The Xcc holo-ACP was expressed in BL21(Tuner) transformed with pBHK473 carrying the Xcc acpP gene and pBHK475 carrying the E. coli acpS gene and purified as described previously (Cronan & Thomas, 2009).
Labelling and analysis of fatty acids
The Xcc strains were grown at 30°C in NYG medium to log phase or stationary phase. [1-14C]Acetate (2 μCi/ml culture, 50–60 mCi/mmol) was added to the medium to label the newly synthesized fatty acids. Cultures of plasmid-containing strains were induced with IPTG (0.5mM). The cultures were incubated at 30°C with shaking for 10 h and 28 h for log phase and stationary phase analysis, respectively. Then the cells from log or stationary phase were pelleted at 4750 × g for 10 min and each 2 ml supernatant was decanted into a second tube. The free fatty acids present at the supernatant were extracted and then methyl esters were prepared as described previously (Feng & Cronan, 2009). The resulting methyl esters were separated by argentation thin-layer chromatography and analyzed by autoradiography (Feng & Cronan, 2009).
Measurement of FCL activity
FCL activity was assayed using Ellman’s reagent (5,5′-dithiobis(2-nitrobenzoic acid), as previously described (Bar-Tana et al., 1971, Kang et al., 2010) to detect the decrease in the concentration of CoA thiol groups resulting from acyl-CoA synthesis. The reaction mixtures contained 150 mM Tris-HCl (pH 7.2), 10 mM MgCl2, 2 mM EDTA, 0.1% Triton X-100, 5 mM ATP, 0.5 mM reduced CoA) and fatty acid substrate (30 to 300 μM), Purified RpfB 15 μg was added in a total volume of 0.45 ml. Briefly, to perform the reaction, each mixture was assembled containing all components above (excluding CoA) and the 405 μl mixture was pre-incubated at 30°C for 3 min. The reaction was then initiated with the addition of 45 μl of 0.5 mM reduced CoA that was pre-incubated at 30°C for 3 min, quickly mixed and incubated at 30°C during the course of the reaction. Immediately after mixing, a time zero point was taken by removing 75 μl from the 450 ml reaction mix and adding it to 600 ml of 0.4 mM 5,5′-dithiobis(2-nitrobenzoic acid), dissolved in 0.1 M potassium phosphate at pH 8.0) and the A412 were measured. Subsequent 75 μl aliquots of the reaction were taken at 30-sec intervals and mixed with 5,5′-dithiobis(2-nitrobenzoic acid for additional measurements. Addition of enzyme at the termination of the reaction served as a blank. The molar extinction coefficient given by CoASH under these conditions was assumed to be 1.36×104 cm−1. Reactions with RpfB were repeated to obtain triplicate data for each fatty acid at each concentration. For each fatty acid substrate, decreases in A412 values (loss of reduced CoA) over time were used to calculate the initial velocity (V0) for each fatty acid concentration. The maximum velocity (Vmax) of the enzymes and affinity for the different substrates (Michaelis constant, Km) were then determined using Hanes-Woolf plots.
Fatty acid activation by RpfB was also analyzed by radiolabelled TLC. The reaction mixture was essentially as described above except that the reaction volume was scaled down to 15 μl and [1-14C]octanoic acid or [1-14C]oleate (each at 20 μM, specific activity of 50–55 mCi/mmol,) was used as the fatty acid substrate. Following incubation of the reaction mixture for 15 min at 30 °C, the reactions were quenched with 5% acetic acid and directly spotted on silica gel TLC plates. Products were resolved by TLC using n-butanol/acetic acid/water (80:25:40) as the solvent system at 4 °C. The radiolabelled products were visualized by exposing the TLC plate to Kodak X-AR film for 1 to 2 days.
Assay of acyl-ACP ligase activity
The assay was performed as described previously (Cronan & Thomas, 2009). Briefly, a typical reaction mixture consists of 20 mM ACP, 200 mM fatty acid and 170 nM AasS or RpfB in a buffer containing 100 mM Tris-HCl (pH 7.8), 10 mM MgCl2, 1 mM TCEP, and 10 mM ATP (or GTP, CTP, UTP) in a reaction volume of 40 μl. The reaction is allowed to proceed for 4 h at 30 °C and the reaction products were resolved by conformationally sensitive gel electrophoresis on 18% polyacrylamide gels containing a concentration of urea optimized for the separation (Cronan & Thomas, 2009, Post-Beittenmiller et al., 1991). The gels were stained with Coomassie Brilliant Blue R250.
Mass spectrometric analysis of RpfB reaction products
The RpfB reactions were scaled up for HPLC (Shimadzu) analysis. After incubation, the reactions were quenched and samples were analyzed with the Agilent LC/MS system (MSD Trap XCT Plus) with a 1100 series HPLC system (Agilent Technologies) including a degasser, an autosampler, and a binary pump. The LC separation was performed on a Phenomenex Snergy 4μ Fusion RP 80A column (4.6 × 100mm) with mobile phase A (15 mM ammonia formate in water) and mobile phase B (90 % methanol and 10 % 15 mM ammonium formate). The flow rate was 0.35 ml/min. The linear gradient was as follows: 0–1 min, 95%A; 7–20 min, 5%A. The autosampler was set at 5°C. The injection volume was 5 μl. Mass spectra were acquired with negative electrospray ionization (ESI). The dry temperature, nebulizer and dry gas were 350 °C, 35 psi, and 8L/min, respectively.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant AI15650 from the National Institute of Allergy and Infectious Diseases. We thank Dr. Peter Yau, Dr. Alexander Ulanov and Dr. Lucas Li of the Roy J. Carver Biotechnology Center, University of Illinois at Urbana-Champaign for help with protein identification and gas chromatography-mass spectrometry analysis.
References
- Almeida RP, Killiny N, Newman KL, Chatterjee S, Ionescu M, Lindow SE. Contribution of rpfB to cell-to-cell signal synthesis, virulence, and vector transmission of Xylella fastidiosa. Mol Plant Microbe Interact. 2012;25:453–462. doi: 10.1094/MPMI-03-11-0074. [DOI] [PubMed] [Google Scholar]
- An SQ, Febrer M, McCarthy Y, Tang DJ, Clissold L, Kaithakottil G, Swarbreck D, Tang JL, Rogers J, Dow JM, Ryan RP. High-resolution transcriptional analysis of the regulatory influence of cell-to-cell signalling reveals novel genes that contribute to Xanthomonas phytopathogenesis. Mol Microbiol. 2013;88:1058–1069. doi: 10.1111/mmi.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Tana J, Rose G, Shapiro B. The purification and properties of microsomal palmitoyl-coenzyme A synthetase. Biochem J. 1971;122:353–362. doi: 10.1042/bj1220353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber CE, Tang JL, Feng JX, Pan MQ, Wilson TJ, Slater H, Dow JM, Williams P, Daniels MJ. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol Microbiol. 1997;24:555–566. doi: 10.1046/j.1365-2958.1997.3721736.x. [DOI] [PubMed] [Google Scholar]
- Bi H, Christensen QH, Feng Y, Wang H, Cronan JE. The Burkholderia cenocepacia BDSF quorum sensing fatty acid is synthesized by a bifunctional crotonase homologue having both dehydratase and thioesterase activities. Mol Microbiol. 2012;83:840–855. doi: 10.1111/j.1365-2958.2012.07968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop RE. Structural biology of membrane-intrinsic beta-barrel enzymes: sentinels of the bacterial outer membrane. Biochim Biophys Acta. 2008;1778:1881–1896. doi: 10.1016/j.bbamem.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black PN, DiRusso CC. Transmembrane movement of exogenous long-chain fatty acids: proteins, enzymes, and vectorial esterification. Microbiol Mol Biol Rev. 2003;67:454–472. doi: 10.1128/MMBR.67.3.454-472.2003. table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brok RG, Boots AP, Dekker N, Verheij HM, Tommassen J. Sequence comparison of outer membrane phospholipases A: implications for structure and for the catalytic mechanism. Res Microbiol. 1998;149:703–710. doi: 10.1016/s0923-2508(99)80017-5. [DOI] [PubMed] [Google Scholar]
- Campbell JW, Cronan JE., Jr The enigmatic Escherichia coli fadE gene is yafH. J Bacteriol. 2002;184:3759–3764. doi: 10.1128/JB.184.13.3759-3764.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JW, Morgan-Kiss RM, Cronan JE., Jr A new Escherichia coli metabolic competency: growth on fatty acids by a novel anaerobic beta-oxidation pathway. Mol Microbiol. 2003;47:793–805. doi: 10.1046/j.1365-2958.2003.03341.x. [DOI] [PubMed] [Google Scholar]
- Cheng Z, He YW, Lim SC, Qamra R, Walsh MA, Zhang LH, Song H. Structural basis of the sensor-synthase interaction in autoinduction of the quorum sensing signal DSF biosynthesis. Structure. 2010;18:1199–1209. doi: 10.1016/j.str.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Cronan JE, Thomas J. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 2009;459:395–433. doi: 10.1016/S0076-6879(09)04617-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJ, Barber CE, Turner PC, Sawczyc MK, Byrde RJ, Fielding AH. Cloning of genes involved in pathogenicity of Xanthomonas campestris pv. campestris using the broad host range cosmid pLAFR1. EMBO J. 1984;3:3323–3328. doi: 10.1002/j.1460-2075.1984.tb02298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Y, Wu J, Tao F, Zhang LH. Listening to a new language: DSF-based quorum sensing in Gram-negative bacteria. Chem Rev. 2011;111:160–173. doi: 10.1021/cr100354f. [DOI] [PubMed] [Google Scholar]
- Dow M. Diversification of the function of cell-to-cell signaling in regulation of virulence within plant pathogenic xanthomonads. Sci Signal. 2008;1:pe23. doi: 10.1126/stke.121pe23. [DOI] [PubMed] [Google Scholar]
- Feng Y, Cronan JE. Escherichia coli unsaturated fatty acid synthesis: complex transcription of the fabA gene and in vivo identification of the essential reaction catalyzed by FabB. J Biol Chem. 2009;284:29526–29535. doi: 10.1074/jbc.M109.023440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick AM. Conformational dynamics in the Acyl-CoA synthetases, adenylation domains of non-ribosomal peptide synthetases, and firefly luciferase. ACS Chem Biol. 2009;4:811–827. doi: 10.1021/cb900156h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He YW, Wang C, Zhou L, Song H, Dow JM, Zhang LH. Dual signaling functions of the hybrid sensor kinase RpfC of Xanthomonas campestris involve either phosphorelay or receiver domain-protein interaction. J Biol Chem. 2006;281:33414–33421. doi: 10.1074/jbc.M606571200. [DOI] [PubMed] [Google Scholar]
- Hisanaga Y, Ago H, Nakagawa N, Hamada K, Ida K, Yamamoto M, Hori T, Arii Y, Sugahara M, Kuramitsu S, Yokoyama S, Miyano M. Structural basis of the substrate-specific two-step catalysis of long chain fatty acyl-CoA synthetase dimer. J Biol Chem. 2004;279:31717–31726. doi: 10.1074/jbc.M400100200. [DOI] [PubMed] [Google Scholar]
- Huang TP, Wong AC. Extracellular fatty acids facilitate flagella-independent translocation by Stenotrophomonas maltophilia. Res Microbiol. 2007;158:702–711. doi: 10.1016/j.resmic.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Ionescu M, Baccari C, Da Silva AM, Garcia A, Yokota K, Lindow SE. Diffusible signal factor (DSF) synthase RpfF of Xylella fastidiosa is a multifunction protein also required for response to DSF. J Bacteriol. 2013;195:5273–5284. doi: 10.1128/JB.00713-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Chan CH, Cronan JE. The soluble acyl-acyl carrier protein synthetase of Vibrio harveyi B392 is a member of the medium chain acyl-CoA synthetase family. Biochemistry. 2006;45:10008–10019. doi: 10.1021/bi060842w. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Morgan-Kiss RM, Campbell JW, Chan CH, Cronan JE. Expression of Vibrio harveyi acyl-ACP synthetase allows efficient entry of exogenous fatty acids into the Escherichia coli fatty acid and lipid A synthetic pathways. Biochemistry. 2010;49:718–726. doi: 10.1021/bi901890a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y, Zarzycki-Siek J, Walton CB, Norris MH, Hoang TT. Multiple FadD acyl-CoA synthetases contribute to differential fatty acid degradation and virulence in Pseudomonas aeruginosa. PLoS One. 2010;5:e13557. doi: 10.1371/journal.pone.0013557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazakov AE, Rodionov DA, Alm E, Arkin AP, Dubchak I, Gelfand MS. Comparative genomics of regulation of fatty acid and branched-chain amino acid utilization in proteobacteria. J Bacteriol. 2009;191:52–64. doi: 10.1128/JB.01175-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lennen RM, Politz MG, Kruziki MA, Pfleger BF. Identification of transport proteins involved in free fatty acid efflux in Escherichia coli. J Bacteriol. 2013;195:135–144. doi: 10.1128/JB.01477-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan-Kiss RM, Cronan JE. The Escherichia coli fadK (ydiD) gene encodes an anerobically regulated short chain acyl-CoA synthetase. J Biol Chem. 2004;279:37324–37333. doi: 10.1074/jbc.M405233200. [DOI] [PubMed] [Google Scholar]
- Overath P, Pauli G, Schairer HU. Fatty acid degradation in Escherichia coli. An inducible acyl-CoA synthetase, the mapping of old-mutations, and the isolation of regulatory mutants. Eur J Biochem. 1969;7:559–574. [PubMed] [Google Scholar]
- Post-Beittenmiller D, Jaworski JG, Ohlrogge JB. In vivo pools of free and acylated acyl carrier proteins in spinach. Evidence for sites of regulation of fatty acid biosynthesis. J Biol Chem. 1991;266:1858–1865. [PubMed] [Google Scholar]
- Ryan RP. Cyclic di-GMP signalling and the regulation of bacterial virulence. Microbiology. 2013;159:1286–1297. doi: 10.1099/mic.0.068189-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan RP, Dow JM. Communication with a growing family: diffusible signal factor (DSF) signaling in bacteria. Trends Microbiol. 2011;19:145–152. doi: 10.1016/j.tim.2010.12.003. [DOI] [PubMed] [Google Scholar]
- Trivedi OA, Arora P, Sridharan V, Tickoo R, Mohanty D, Gokhale RS. Enzymic activation and transfer of fatty acids as acyl-adenylates in mycobacteria. Nature. 2004;428:441–445. doi: 10.1038/nature02384. [DOI] [PubMed] [Google Scholar]
- Wang LH, He Y, Gao Y, Wu JE, Dong YH, He C, Wang SX, Weng LX, Xu JL, Tay L, Fang RX, Zhang LH. A bacterial cell-cell communication signal with cross-kingdom structural analogues. Mol Microbiol. 2004;51:903–912. doi: 10.1046/j.1365-2958.2003.03883.x. [DOI] [PubMed] [Google Scholar]
- Weimar JD, DiRusso CC, Delio R, Black PN. Functional role of fatty acyl-coenzyme A synthetase in the transmembrane movement and activation of exogenous long-chain fatty acids. Amino acid residues within the ATP/AMP signature motif of Escherichia coli FadD are required for enzyme activity and fatty acid transport. J Biol Chem. 2002;277:29369–29376. doi: 10.1074/jbc.M107022200. [DOI] [PubMed] [Google Scholar]
- Wengelnik K, Marie C, Russel M, Bonas U. Expression and localization of HrpA1, a protein of Xanthomonas campestris pv. vesicatoria essential for pathogenicity and induction of the hypersensitive reaction. J Bacteriol. 1996;178:1061–1069. doi: 10.1128/jb.178.4.1061-1069.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YM, Rock CO. Thematic review series: Glycerolipids. Acyltransferases in bacterial glycerophospholipid synthesis. J Lipid Res. 2008;49:1867–1874. doi: 10.1194/jlr.R800005-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L, Lin J, Ma J, Cronan JE, Wang H. Triclosan resistance of Pseudomonas aeruginosa PAO1 is due to FabV, a triclosan-resistant enoyl-acyl carrier protein reductase. Antimicrob Agents Chemother. 2010;54:689–698. doi: 10.1128/AAC.01152-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.